Molecular Dynamics Study of the Influence of Nano SiO2 on the Thermodynamic Properties of PMIA Composites
Abstract
:1. Introduction
2. Modeling and Dynamic Optimization
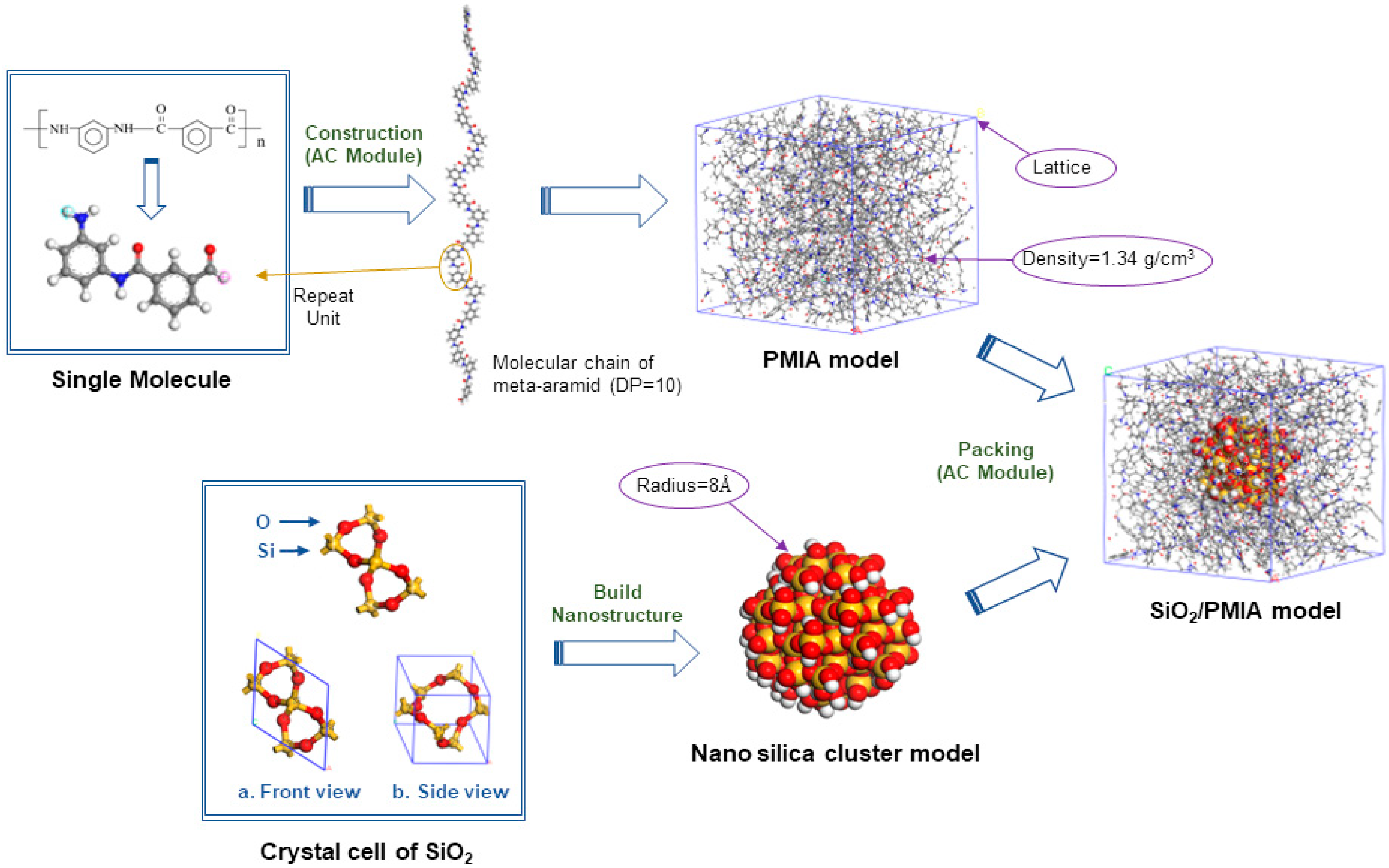
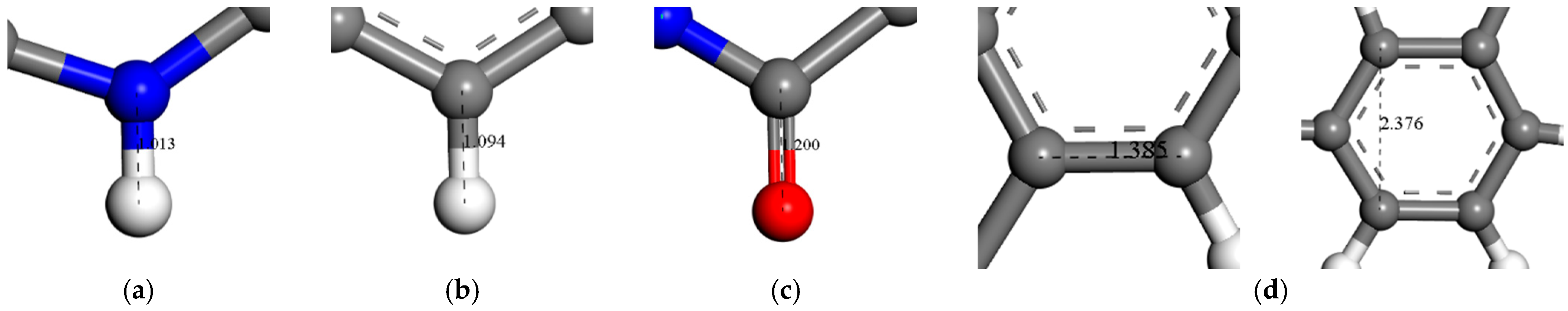
2.1. Establishment of PMIA Monomer Model
2.2. Establishment of Nano-SiO2 Model
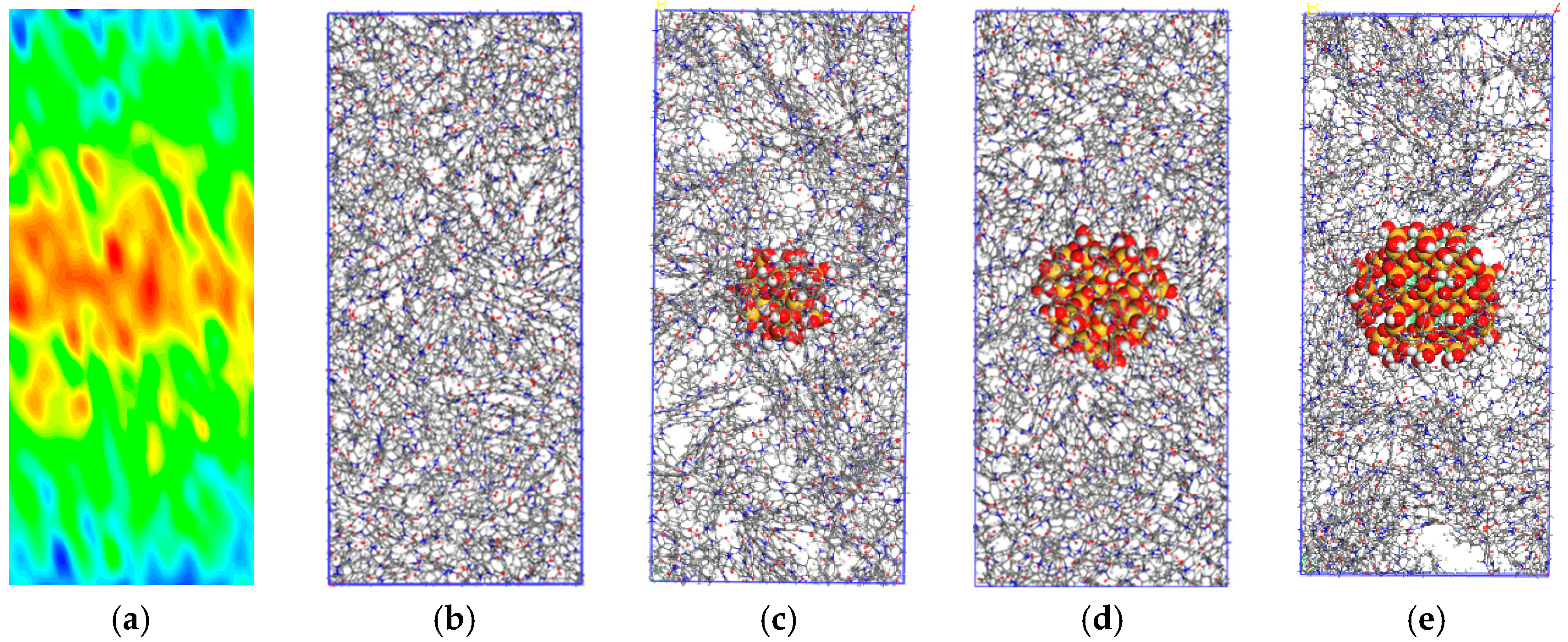
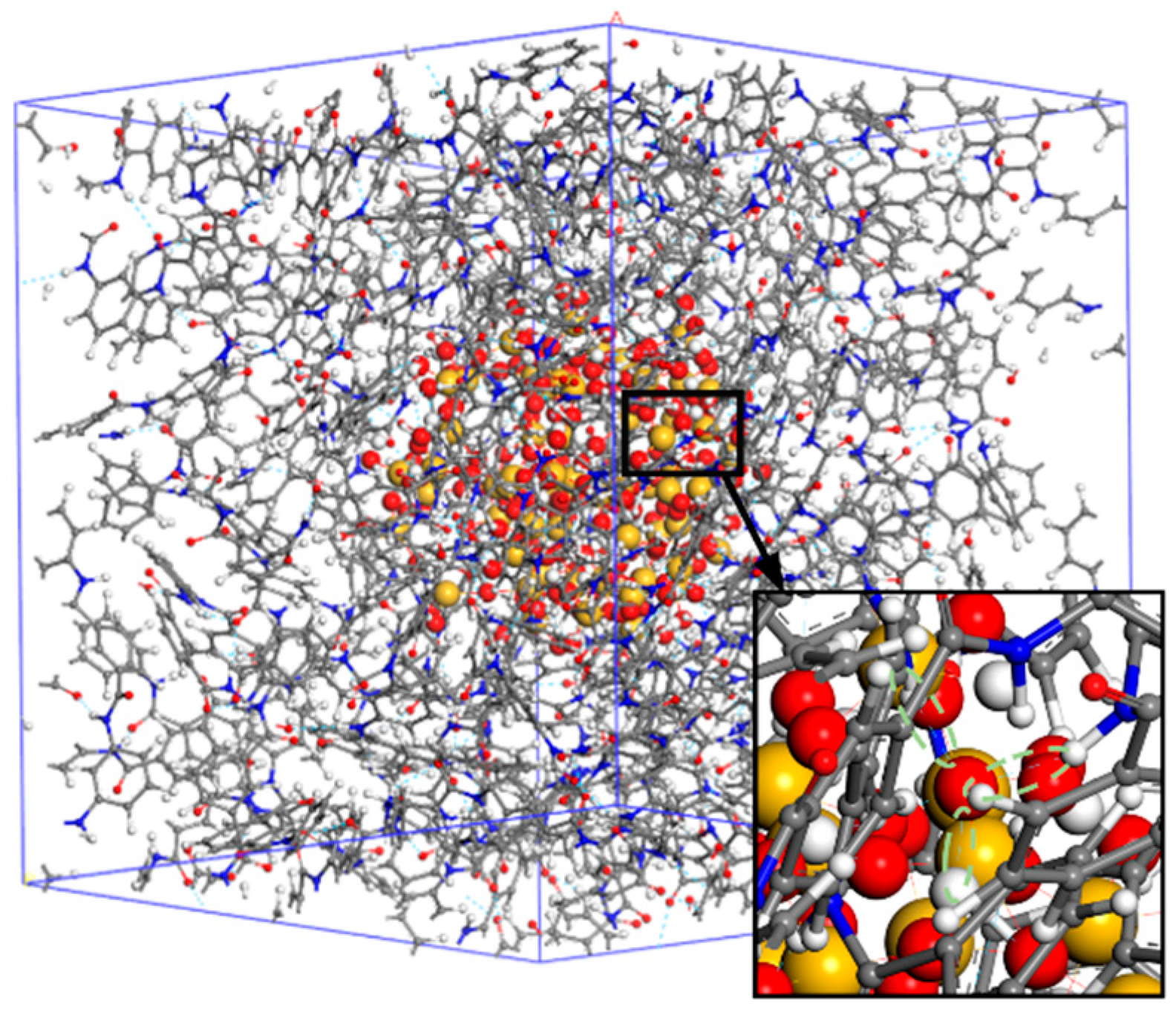
2.3. Establishment of PMIA/SiO2 Composite Model
2.4. Geometric Optimization and Annealing Processes
3. Calculation of Thermodynamic Performance
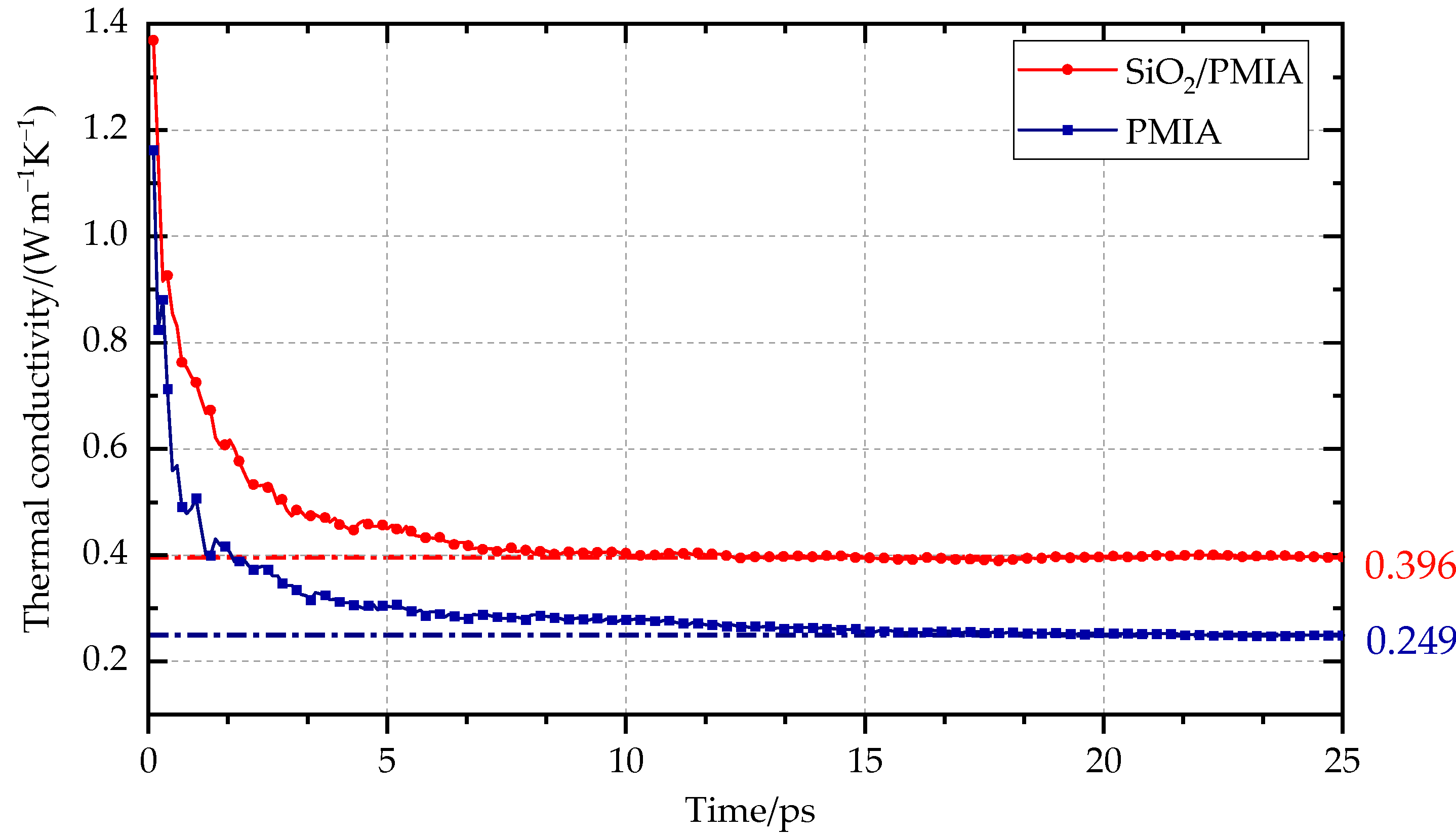
3.1. Calculation of Thermal Conductivity
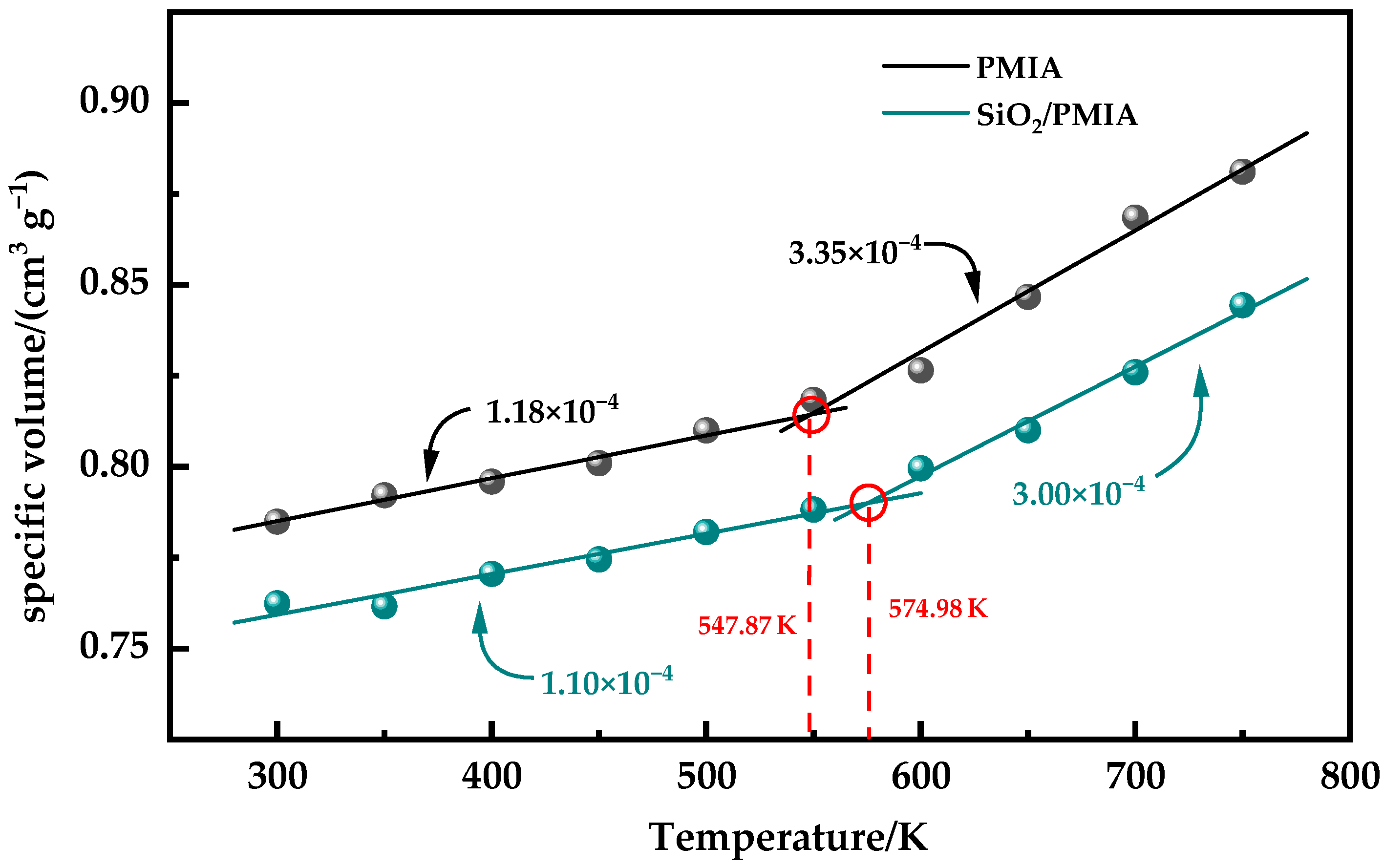
3.2. Coefficient of Thermal Expansion
3.3. Mechanical Parameters
4. Calculation of Structural Parameters
4.1. Proportion of Free Volume
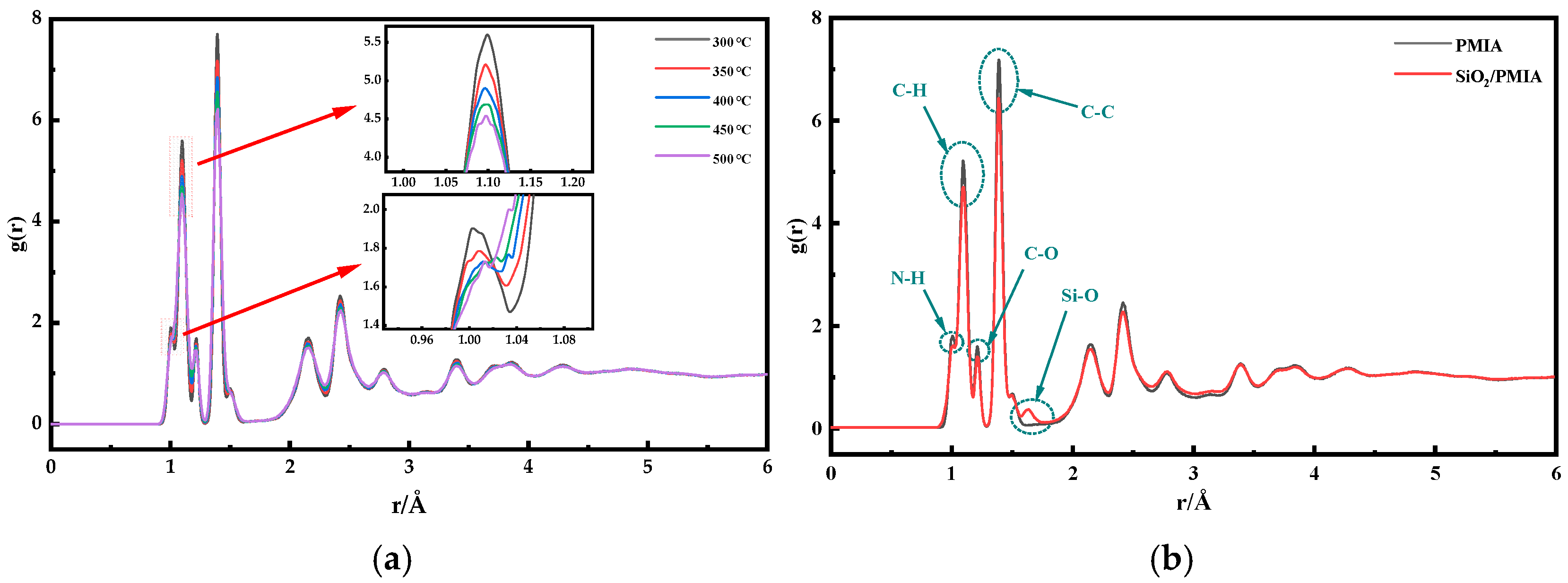
4.2. Radial Distribution Function
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, R.J.; Yang, L.J.; Li, J.; Grzybowski, S. Ageing condition assessment of transformer oil-paper insulation model based on partial discharge analysis. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 303–311. [Google Scholar] [CrossRef]
- Liao, Z.L. The Market Demand of Aramid Fiber and Application of Aramid Fiber Pulp. Hi-Tech Fiber Appl. 2008, 33, 36–39. [Google Scholar]
- Tian, W.; Qiu, T.; Shi, Y.; He, L.; Tuo, X. The facile preparation of aramid insulation paper from the bottom-up nanofiber synthesis. Mater. Lett. 2017, 202, 158–161. [Google Scholar] [CrossRef]
- Bourbigot, S.; Flambard, X. Heat resistance and flammability of high performance fibres: A review. Fire Mater. 2006, 26, 155–168. [Google Scholar] [CrossRef]
- Maity, J.; Jacob, C.; Das, C.K.; Kharitonov, A.P.; Singh, R.P.; Alam, S. Fluorinated aramid fiber reinforced polypropylene composites and their characterization. Polym. Compos. 2007, 28, 462–469. [Google Scholar] [CrossRef]
- Mukherjee, M.; Das, C.K.; Kharitonov, A.P.; Banik, K.; Mennig, G.; Chung, T.N. Properties of syndiotactic polystyrene composites with surface modified short Kevlar fiber. Mater. Sci. Eng. A 2006, 441, 206–214. [Google Scholar] [CrossRef]
- Liao, R.; Liu, H.; Bai, G.; Hao, J.; Lin, Y. Space charge properties and dielectric properties of nano-SiO2/aramid insulating paper composites. J. Electr. Eng. Technol. 2016, 31, 40–48. [Google Scholar]
- Liao, R.; Cheng, L.; Yang, L.; Zhang, Y.; Chao, T. The insulation properties of oil-impregnated insulation paper reinforced with nano-TiO2. J. Nanomater. 2013, 1, 1. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Wang, X.; Wang, W.; Fu, Y.; Xie, Q. Atomic-scale insights into interface thermal resistance between epoxy and boron nitride in nanocomposites. Int. J. Heat Mass Transf. 2020, 159, 120105. [Google Scholar] [CrossRef]
- Wang, L.; Tang, C.; Wang, X.; Zheng, W. Molecular dynamics simulation on the thermodynamic properties of insulating paper cellulose modified by silane coupling agent grafted nano-SiO2. AIP Adv. 2019, 09, 125134. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Lv, Q.; Chen, S.; Li, C.; Sun, S.; Hu, S. Effect of interfacial bonding on interphase properties in SiO2/epoxy nanocomposite: A molecular dynamics simulation study. ACS Appl. Mater. Interfaces 2016, 8, 7499–7508. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, J.; Yang, S.; Yu, S.; Cho, M. Influence of crosslink density on the interfacial characteristics of epoxy nanocomposites. Polymer 2015, 60, 186–197. [Google Scholar] [CrossRef]
- Chao, T.; Xu, L.; Fei, Y.; Jian, H. The performance improvement of aramid insulation paper by nano-SiO2 modification. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 2400–2409. [Google Scholar]
- Zheng, W.; Xie, J.; Zhang, J.; Tang, C.; Zhao, Z. Influence of polymethylsilsesquioxane content to the thermal stability of meta-aramid fiber insulation paper. Materials 2018, 11, 2317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, C.; Zheng, W.; Wang, L.; Xie, J. Thermal stability of polyphenylsilsesquioxane-modified meta-aramid insulation paper. High Volt. 2020, 5, 264–269. [Google Scholar] [CrossRef]
- Jain, A.; Vijayan, K. Thermally induced structural changes in Nomex fibres. Bull. Mater. Sci. 2002, 25, 341–346. [Google Scholar] [CrossRef]
- Mazeau, K.; Heux, L. Molecular dynamics simulations of bulk native crystalline and amorphous structures of cellulose. J. Phys. Chem. B 2003, 107, 2394–2403. [Google Scholar] [CrossRef]
- Wang, X.; Tang, C.; Wang, Q.; Li, X.; Hao, J. Selection of Optimal Polymerization Degree and Force Field in the Molecular Dynamics Simulation of Insulating Paper Cellulose. Energies 2017, 10, 1377. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.; Liang, C.; Landry, M.; Nelson, J.; Schadler, L. The mechanisms leading to the useful electrical properties of polymer nanodielectrics. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 187–196. [Google Scholar] [CrossRef]
- Li, Z.W.; Zhu, Y.F. Surface-modification of SiO2 nanoparticles with oleic acid. Appl. Surf. Sci. 2003, 211, 315–320. [Google Scholar] [CrossRef]
- Zhang, H. Molecular Dynamics Simulation of Mechanical Properties of Modified Nanoparticles Reinforced Polymer Matrix Composites; Jinan University: Shangdong, China, 2014. (In Chinese) [Google Scholar]
- Sun, H. COMPASS: An ab Initio Force-Field Optimized for Condensed-Phase Applications Overview with Details on Alkane and Benzene Compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Muller-Plathe, F. A simple nonequilibrium molecular dynamics method for calculating the thermal conductivity. J. Phys. Chem. 1997, 106, 6082–6085. [Google Scholar] [CrossRef]
- Plimpton, S.J. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Müller-Plathe, F. Increasing the thermal conductivity of graphene-polyamide-6,6 nanocomposites by surface-grafted polymer chains: Calculation with molecular dynamics and effective-medium approximation. J. Phys. Chem. B 2016, 120, 1336–1346. [Google Scholar] [CrossRef]
- Li, C.; Medvedev, G.A.; Lee, E.-W.; Kim, J.; Caruthers, J.M. Molecular dynamics simulations and experimental studies of the thermomechanical response of an epoxy thermoset polymer. Polymer 2012, 53, 4222–4230. [Google Scholar] [CrossRef]
- Xie, Q.; Fu, K.; Liang, S.; Liu, B.; Lu, L.; Yang, X.; Huang, Z.; Lü, F. Micro-Structure and Thermomechanical Properties of Crosslinked Epoxy Composite Modified by Nano-SiO2: A Molecular Dynamics Simulation. Polymers 2018, 10, 801. [Google Scholar] [CrossRef] [Green Version]
- Hill, R. Elastic Properties of Reinforced Solids: Some Theoretical Principles. J. Mech. Phys. Solids 1963, 11, 357–372. [Google Scholar] [CrossRef]
- Fox, T.G.; Flory, P.J. Second-Order Transition Temperatures and Related Properties of Polystyrene. I. Influence of Molecular Weight. J. Appl. Phys. 1950, 21, 581–591. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, Y.; Wang, Y. A molecular dynamic simulation method to elucidate the interaction mechanism of nano-SiO2 in polymer blends. J. Mater. Sci. 2017, 21, 12889–12901. [Google Scholar] [CrossRef]
- Wu, C. Atomistic Molecular Simulation of Crosslinked Epoxy Resin; Hunan University: Hunan, China, 2007. (In Chinese) [Google Scholar]

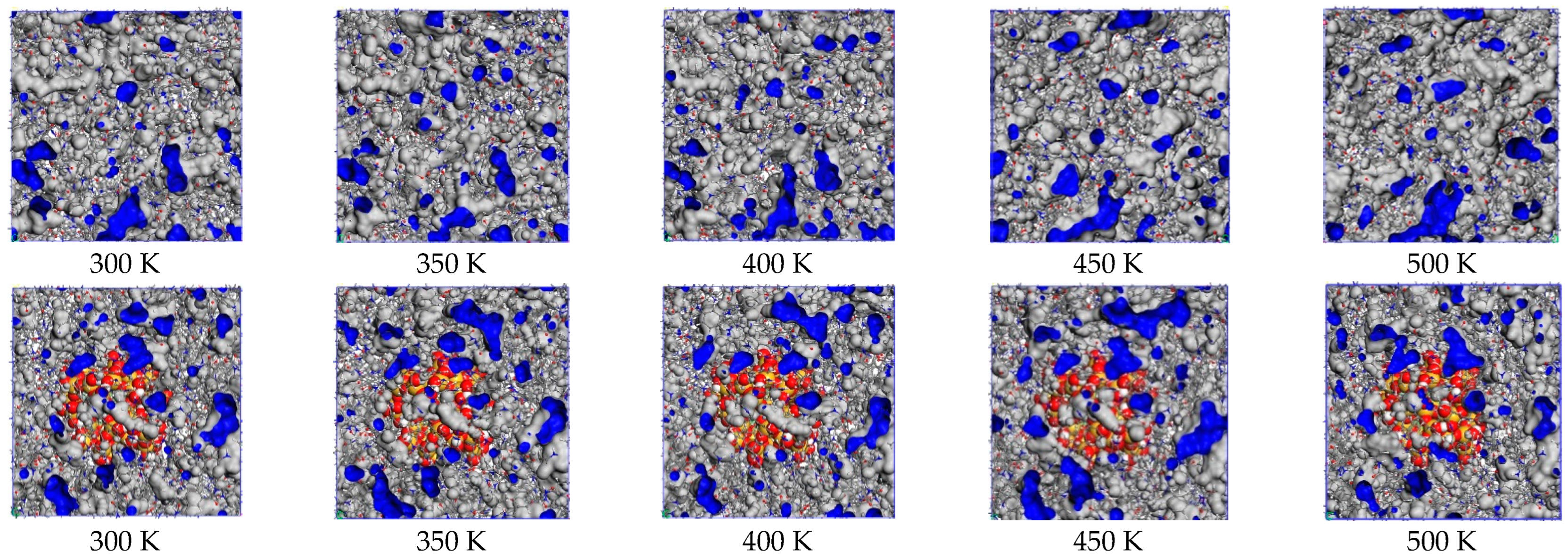
| Radius of Nano-SiO2 | 0 Å | 4 Å | 8 Å | 12 Å |
|---|---|---|---|---|
| Thermal conductivity/(W m−1 K−1) | 0.249 | 0.286 | 0.396 | 0.387 |
| 300 K | 350 K | 400 K | 450 K | 500 K | |
|---|---|---|---|---|---|
| Vo/Å3 | 39,409.69 | 39,756.95 | 39,717.33 | 39,661.39 | 39,599.70 |
| Vf/Å3 | 6356.44 | 6517.82 | 6781.41 | 7738.20 | 8201.36 |
| FFVPMIA | 0.139 | 0.141 | 0.146 | 0.163 | 0.172 |
| Vo/Å3 | 41,472.52 | 41,421.98 | 41,609.79 | 41,510.58 | 41,735.27 |
| Vf/Å3 | 5537.94 | 6134.32 | 5977.88 | 6386.57 | 6318.73 |
| FFVSiO2 | 0.118 | 0.123 | 0.126 | 0.133 | 0.135 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Lv, F.; Fan, X.; Li, Y.; Jiang, B. Molecular Dynamics Study of the Influence of Nano SiO2 on the Thermodynamic Properties of PMIA Composites. Polymers 2022, 14, 3134. https://doi.org/10.3390/polym14153134
Liu B, Lv F, Fan X, Li Y, Jiang B. Molecular Dynamics Study of the Influence of Nano SiO2 on the Thermodynamic Properties of PMIA Composites. Polymers. 2022; 14(15):3134. https://doi.org/10.3390/polym14153134
Chicago/Turabian StyleLiu, Bowen, Fangcheng Lv, Xiaozhou Fan, Yuxuan Li, and Bowen Jiang. 2022. "Molecular Dynamics Study of the Influence of Nano SiO2 on the Thermodynamic Properties of PMIA Composites" Polymers 14, no. 15: 3134. https://doi.org/10.3390/polym14153134






