Effect of MWNT Functionalization with Tunable-Length Block Copolymers on Dispersity of MWNTs and Mechanical Properties of Epoxy/MWNT Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of Block Copolymers
2.3. Preparation of PGMA-b-PHMA@fMWNTs
2.4. The Solubility of bc@fMWNTs in Organic Solvents
2.5. Fabrication of Epoxy Nanocomposites
2.6. Characterizations
2.7. Mechanical Test
3. Results
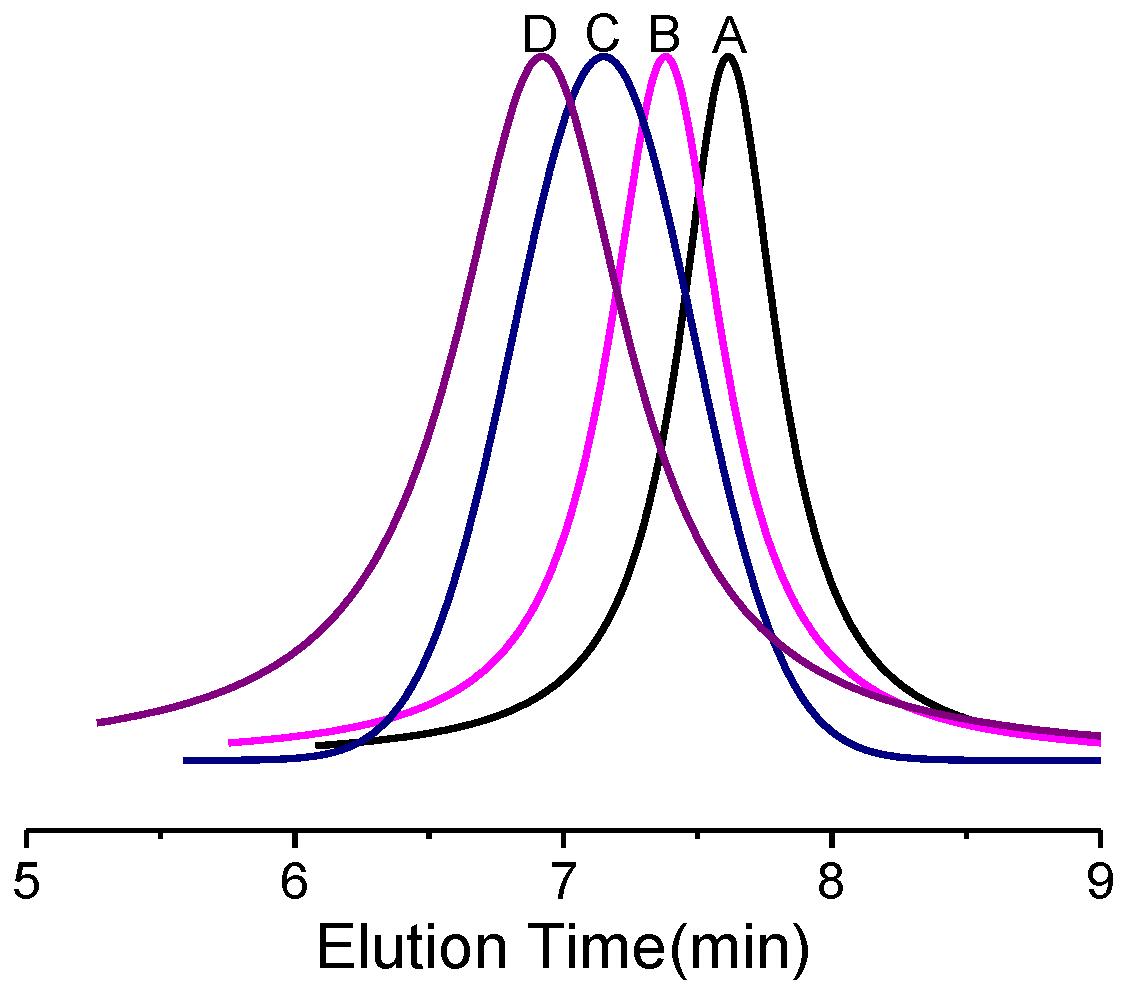
3.1. Synthesis of Block Copolymers
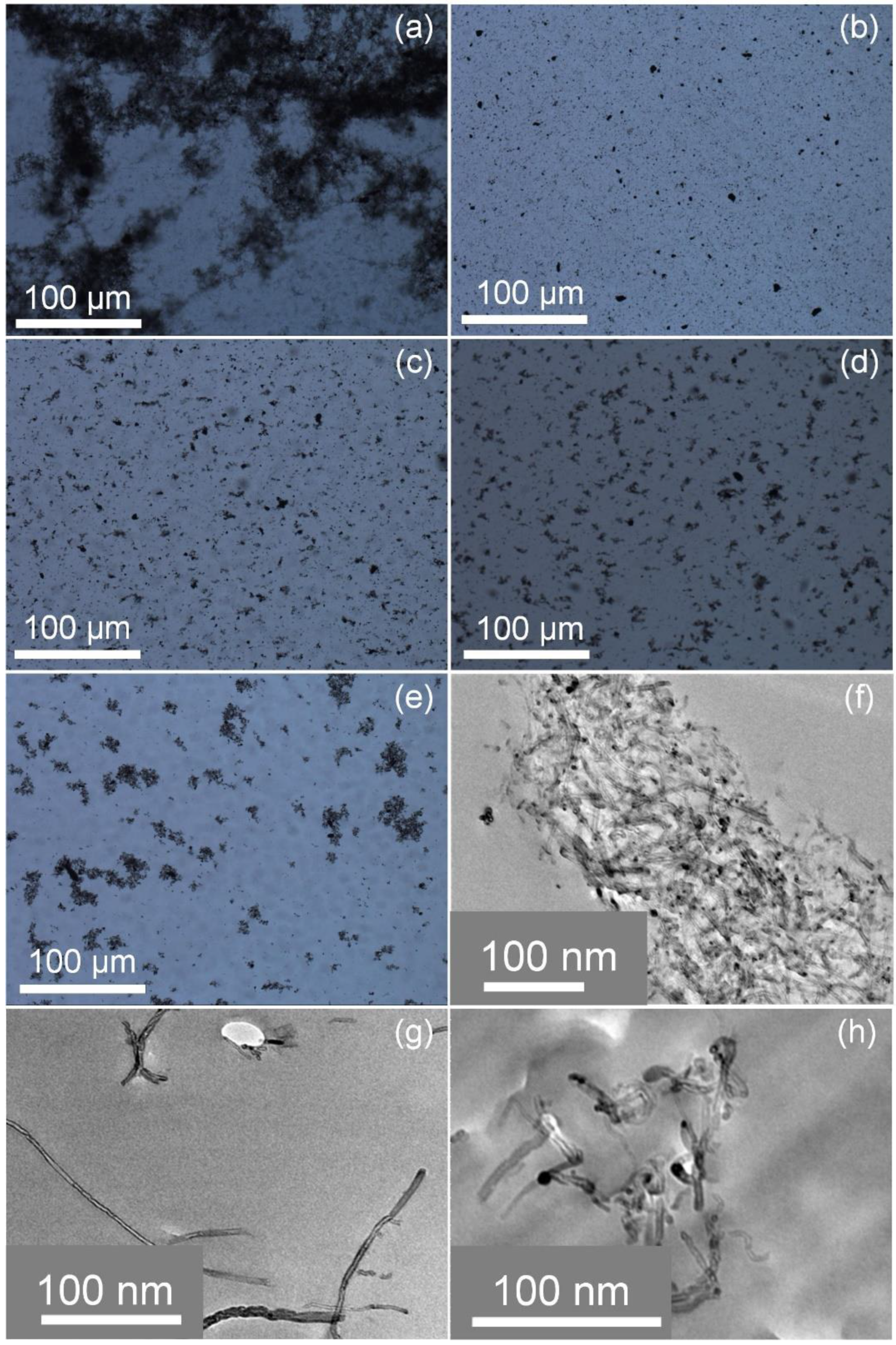
3.2. Preparation of bc@fMWNTs
3.3. Solubility of bc@fMWNTs
3.4. Dispersity of bc@fMWNTs in Epoxy
3.5. Dynamic Mechanical Properties of Epoxy/bc@fMWNTs
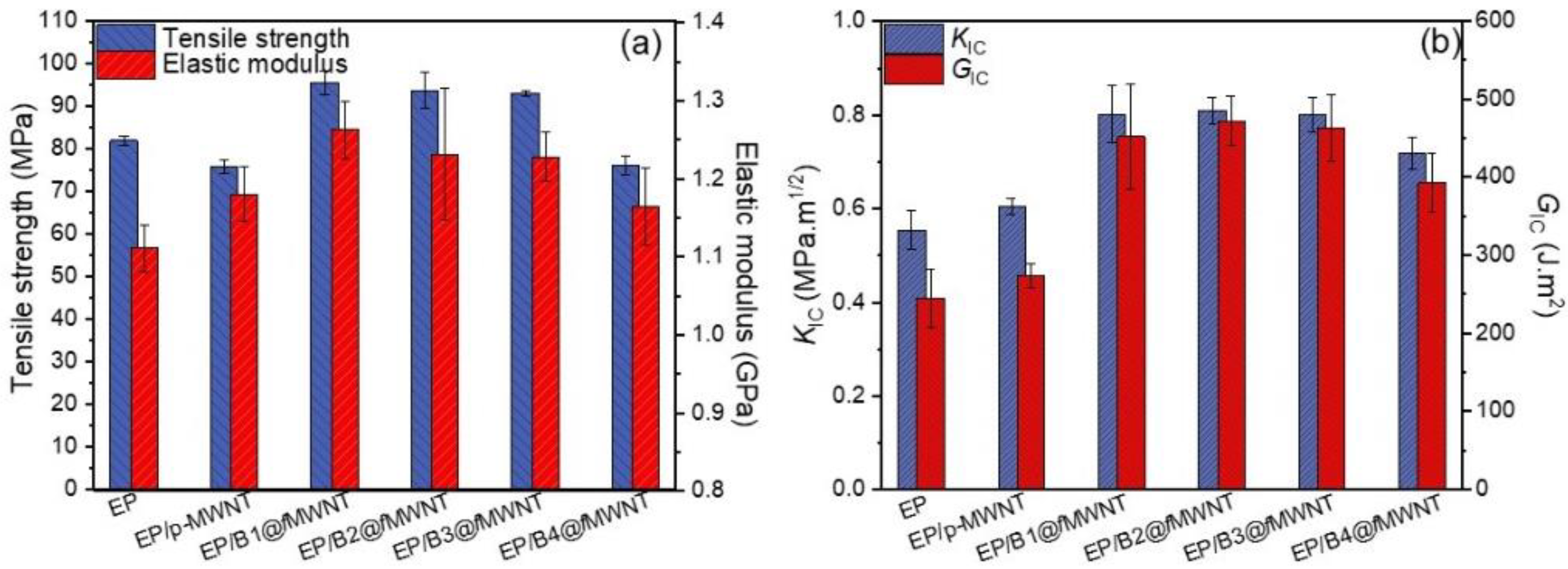
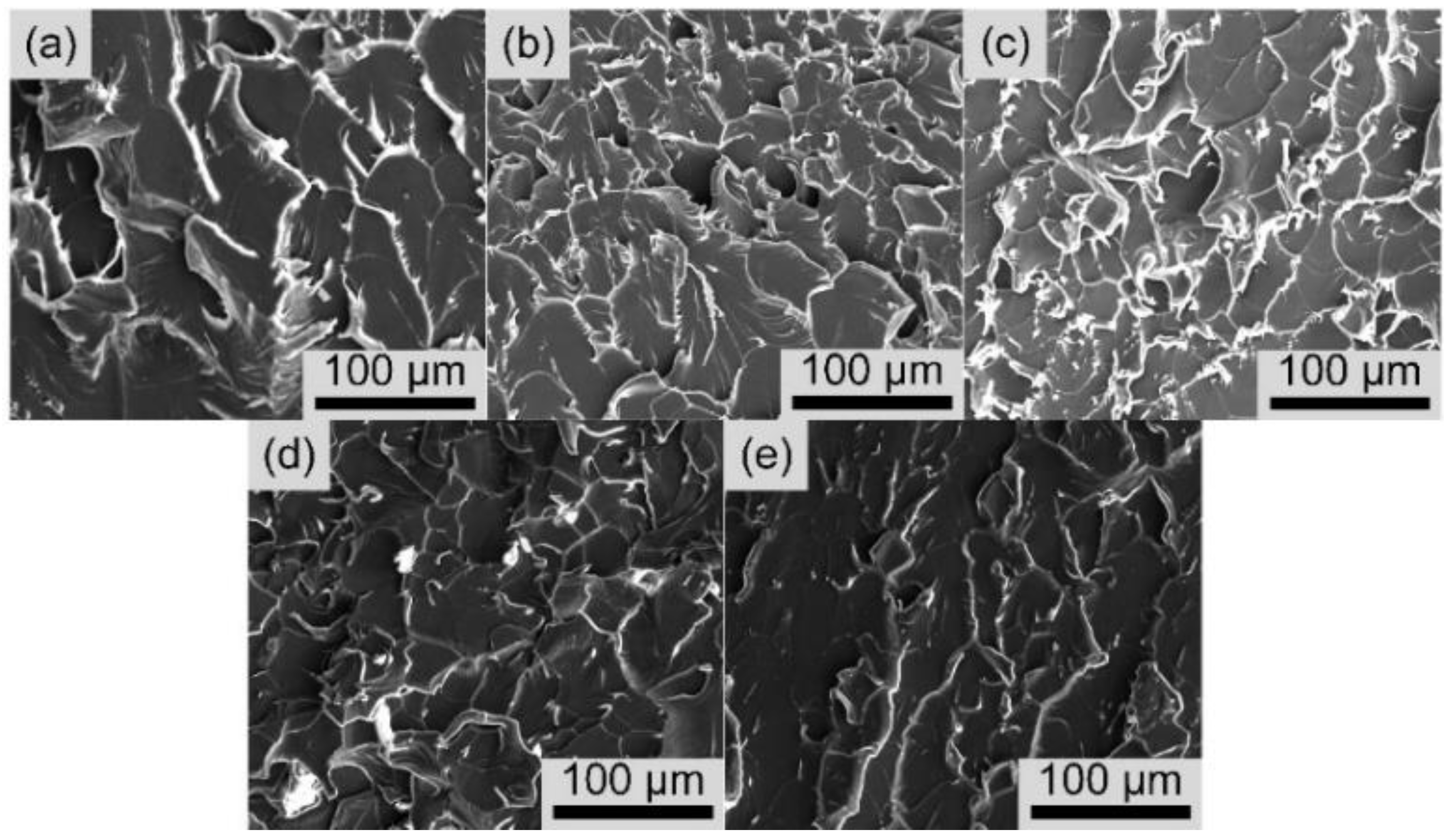
3.6. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.; Tang, Y.; Ye, Y.S.; Xue, Z.; Xue, Y.; Xie, X.; Mai, Y.-W. High-performance epoxy/silica coated silver nanowire composites as underfill material for electronic packaging. Compos. Sci. Technol. 2014, 105, 80–85. [Google Scholar] [CrossRef]
- Domun, N.; Hadavinia, H.; Zhang, T.; Sainsbury, T.; Liaghat, G.H.; Vahid, S. Improving the fracture toughness and the strength of epoxy using nanomaterials—A review of the current status. Nanoscale 2015, 7, 10294–10329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marouf, B.T.; Mai, Y.-W.; Bagheri, R.; Pearson, R.A. Toughening of epoxy nanocomposites: Nano and hybrid effects. Polym. Rev. 2016, 56, 70–112. [Google Scholar] [CrossRef]
- Xie, X.L.; Mai, Y.W.; Zhou, X.P. Dispersion and alignment of carbon nanotubes in polymer matrix: A review. Mater. Sci. Eng. R-Rep. 2005, 49, 89–112. [Google Scholar] [CrossRef]
- Williams, J.G.; Blackman, B.R.K.; Steininger, H.; Zuo, K. Toughening by plastic cavitation around cylindrical particles and fibres. Compos. Sci. Technol. 2014, 103, 119–126. [Google Scholar] [CrossRef]
- Sun, L.; Warren, G.L.; O’Reilly, J.Y.; Everett, W.N.; Lee, S.M.; Davis, D.; Lagoudas, D.; Sue, H.J. Mechanical properties of surface-functionalized SWCNT/epoxy composites. Carbon 2008, 46, 320–328. [Google Scholar] [CrossRef]
- Song, S.H.; Park, K.H.; Kim, B.H.; Choi, Y.W.; Jun, G.H.; Lee, D.J.; Kong, B.-S.; Paik, K.-W.; Jeon, S. Enhanced Thermal Conductivity of EpoxyGraphene Composites by Using Non-Oxidized Graphene Flakes with Non-Covalent Functionalization. Adv. Mater. 2013, 25, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Mostovoy, A.; Yakovlev, A.; Tseluikin, V.; Lopukhova, M. Epoxy Nanocomposites Reinforced with Functionalized Carbon Nanotubes. Polymers 2020, 12, 1816. [Google Scholar] [CrossRef]
- Mostovoy, A.; Shcherbakov, A.; Yakovlev, A.; Arzamastsev, S.; Lopukhova, M. Reinforced Epoxy Composites Modified with Functionalized Graphene Oxide. Polymers 2022, 14, 338. [Google Scholar] [CrossRef] [PubMed]
- Amirbeygi, H.; Khosravi, H.; Tohidlou, E. Reinforcing effects of aminosilane-functionalized graphene on the tribological and mechanical behaviors of epoxy nanocomposites. J. Appl. Polym. Sci. 2019, 136, 47410. [Google Scholar] [CrossRef]
- Bekeshev, A.; Mostovoy, A.; Kadykova, Y.; Akhmetova, M.; Tastanova, L.; Lopukhova, M. Development and Analysis of the Physicochemical and Mechanical Properties of Diorite-Reinforced Epoxy Composites. Polymers 2021, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Islam, M.; Ahmad, I.; Mahmood, N.; Saeed, S.; Javed, H. Thermal and mechanical properties of carbon nanotube/epoxy nanocomposites reinforced with pristine and functionalized multiwalled carbon nanotubes. Polym. Compos. 2015, 36, 1891–1898. [Google Scholar] [CrossRef]
- Zhang, Q.; Bai, G.; Xiao, W.; Sui, G.; Yang, X. Effect of amine functionalized MWCNT-epoxy interfacial interaction on MWCNT dispersion and mechanical properties of epoxy-amine composites. Polym. Compos. 2018, 39, E2552–E2561. [Google Scholar] [CrossRef]
- Yoon, J.T.; Lee, S.C.; Jeong, Y.G. Effects of grafted chain length on mechanical and electrical properties of nanocomposites containing polylactide-grafted carbon nanotubes. Compos. Sci. Technol. 2010, 70, 776–782. [Google Scholar] [CrossRef]
- Zou, W.; Du, Z.-j.; Liu, Y.-x.; Yang, X.; Li, H.-q.; Zhang, C. Functionalization of MWNTs using polyacryloyl chloride and the properties of CNT–epoxy matrix nanocomposites. Compos. Sci. Technol. 2008, 68, 3259–3264. [Google Scholar] [CrossRef]
- Gu, H.; Tadakamalla, S.; Zhang, X.; Huang, Y.; Jiang, Y.; Colorado, H.A.; Luo, Z.; Wei, S.; Guo, Z. Epoxy resin nanosuspensions and reinforced nanocomposites from polyaniline stabilized multi-walled carbon nanotubes. J. Mater. Chem. C 2013, 1, 729–743. [Google Scholar] [CrossRef]
- Katti, P.; Bose, S.; Kumar, S. Tailored interface resulting in improvement in mechanical properties of epoxy composites containing poly (ether ether ketone) grafted multiwall carbon nanotubes. Polymer 2016, 102, 43–53. [Google Scholar] [CrossRef]
- Park, Y.T.; Qian, Y.; Chan, C.; Suh, T.; Nejhad, M.G.; Macosko, C.W.; Stein, A. Epoxy toughening with low graphene loading. Adv. Funct. Mater. 2015, 25, 575–585. [Google Scholar] [CrossRef]
- Gao, J.; Li, J.; Zhao, S.; Benicewicz, B.C.; Hillborg, H.; Schadler, L.S. Effect of graft density and molecular weight on mechanical properties of rubbery block copolymer grafted SiO2 nanoparticle toughened epoxy. Polymer 2013, 54, 3961–3973. [Google Scholar] [CrossRef]
- Liu, J.; Sue, H.-J.; Thompson, Z.J.; Bates, F.S.; Dettloff, M.; Jacob, G.; Verghese, N.; Pham, H. Nanocavitation in Self-Assembled Amphiphilic Block Copolymer-Modified Epoxy. Macromolecules 2008, 41, 7616–7624. [Google Scholar] [CrossRef]
- Li, Q.Q.; Zaiser, M.; Koutsos, V. Carbon nanotube/epoxy resin composites using a block copolymer as a dispersing agent. Phys. Status Solidi A-Appl. Res. 2004, 201, R89–R91. [Google Scholar] [CrossRef]
- Gonzalez-Dominguez, J.M.; Tesa-Serrate, M.A.; Anson-Casaos, A.; Diez-Pascual, A.M.; Gomez-Fatou, M.A.; Martinez, M.T. Wrapping of SWCNTs in Polyethylenoxide-Based Amphiphilic Diblock Copolymers: An Approach to Purification, Debundling, and Integration into the Epoxy Matrix. J. Phys. Chem. C 2012, 116, 7399–7408. [Google Scholar] [CrossRef]
- Ye, Y.S.; Shen, W.C.; Tseng, C.Y.; Rick, J.; Huang, Y.J.; Chang, F.C.; Hwang, B.J. Versatile grafting approaches to star-shaped POSS-containing hybrid polymers using RAFT polymerization and click chemistry. Chem. Commun. 2011, 47, 10656–10658. [Google Scholar] [CrossRef]
- Bahr, J.L.; Mickelson, E.T.; Bronikowski, M.J.; Smalley, R.E.; Tour, J.M. Dissolution of small diameter single-wall carbon nanotubes in organic solvents? Chem. Commun. 2001, 2, 193–194. [Google Scholar] [CrossRef]
- Li, H.M.; Cheng, F.O.; Duft, A.M.; Adronov, A. Functionalization of single-walled carbon nanotubes with well-defined polystyrene by “click” coupling. J. Am. Chem. Soc. 2005, 127, 14518–14524. [Google Scholar] [CrossRef]
- Sinani, V.A.; Gheith, M.K.; Yaroslavov, A.A.; Rakhnyanskaya, A.A.; Sun, K.; Mamedov, A.A.; Wicksted, J.P.; Kotov, N.A. Aqueous dispersions of single-wall and multiwall carbon nanotubes with designed amphiphilic polycations. J. Am. Chem. Soc. 2005, 127, 3463–3472. [Google Scholar] [CrossRef]
- Ye, Y.-S.; Chen, Y.-N.; Wang, J.-S.; Rick, J.; Huang, Y.-J.; Chang, F.-C.; Hwang, B.-J. Versatile Grafting Approaches to Functionalizing Individually Dispersed Graphene Nanosheets Using RAFT Polymerization and Click Chemistry. Chem. Mater. 2012, 24, 2987–2997. [Google Scholar] [CrossRef]
- Ma, P.-C.; Mo, S.-Y.; Tang, B.-Z.; Kim, J.-K. Dispersion, interfacial interaction and re-agglomeration of functionalized carbon nanotubes in epoxy composites. Carbon 2010, 48, 1824–1834. [Google Scholar] [CrossRef]
- Tang, L.-C.; Wan, Y.-J.; Yan, D.; Pei, Y.-B.; Zhao, L.; Li, Y.-B.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. The effect of graphene dispersion on the mechanical properties of graphene/epoxy composites. Carbon 2013, 60, 16–27. [Google Scholar] [CrossRef]
- Feng, Q.-P.; Shen, X.-J.; Yang, J.-P.; Fu, S.-Y.; Mai, Y.-W.; Friedrich, K. Synthesis of epoxy composites with high carbon nanotube loading and effects of tubular and wavy morphology on composite strength and modulus. Polymer 2011, 52, 6037–6045. [Google Scholar] [CrossRef]
- Wang, F.; Drzal, L.T.; Qin, Y.; Huang, Z. Enhancement of fracture toughness, mechanical and thermal properties of rubber/epoxy composites by incorporation of graphene nanoplatelets. Compos. Part A-Appl. Sci. Manuf. 2016, 87, 10–22. [Google Scholar] [CrossRef]
- Hwang, G.L.; Shieh, Y.T.; Hwang, K.C. Efficient Load Transfer to Polymer-Grafted Multiwalled Carbon Nanotubes in Polymer Composites. Adv. Funct. Mater. 2004, 14, 487–491. [Google Scholar] [CrossRef]
- Bagheri, R.; Marouf, B.T.; Pearson, R.A. Rubber-toughened epoxies: A critical review. Polym. Rev. 2009, 49, 201–225. [Google Scholar] [CrossRef]
- Guan, L.-Z.; Wan, Y.-J.; Gong, L.-X.; Yan, D.; Tang, L.-C.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. Toward effective and tunable interphases in graphene oxide/epoxy composites by grafting different chain lengths of polyetheramine onto graphene oxide. J. Mater. Chem. A 2014, 2, 15058. [Google Scholar] [CrossRef]
- Vennerberg, D.; Rueger, Z.; Kessler, M.R. Effect of silane structure on the properties of silanized multiwalled carbon nanotube-epoxy nanocomposites. Polymer 2014, 55, 1854–1865. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Wang, G.-T.; Mai, Y.-W.; Zeng, Y. On fracture toughness of nano-particle modified epoxy. Compos. Part B-Eng. 2011, 42, 2170–2175. [Google Scholar] [CrossRef]
- Li, Z.; Wang, R.; Young, R.J.; Deng, L.; Yang, F.; Hao, L.; Jiao, W.; Liu, W. Control of the functionality of graphene oxide for its in epoxy nanocomposites application. Polymer 2013, 54, 6437–6446. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Winey, K.I. Polymer nanocomposites containing carbon nanotubes. Macromolecules 2006, 39, 5194–5205. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Tang, L.-C.; Yan, D.; Zhao, L.; Li, Y.-B.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. Improved dispersion and interface in the graphene/epoxy composites via a facile surfactant-assisted process. Compos. Sci. Technol. 2013, 82, 60–68. [Google Scholar] [CrossRef]
- Mi, X.; Liang, N.; Xu, H.; Wu, J.; Jiang, Y.; Nie, B.; Zhang, D. Toughness and its mechanisms in epoxy resins. Prog. Mater. Sci. 2022, 130, 100977. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Estaji, S.; Paydayesh, A.; Arjmand, M.; Jafari, S.H.; Nouranian, S.; Khonakdar, H.A. A review of recent progress in improving the fracture toughness of epoxy-based composites using carbonaceous nanofillers. Polym. Compos. 2022, 43, 1871–1886. [Google Scholar] [CrossRef]
- Li, Q.; Dong, L.; Li, L.; Su, X.; Xie, H.; Xiong, C. The effect of the addition of carbon nanotube fluids to a polymeric matrix to produce simultaneous reinforcement and plasticization. Carbon 2012, 50, 2056–2060. [Google Scholar] [CrossRef]
- Wang, K.; Chen, L.; Wu, J.; Toh, M.L.; He, C.; Yee, A.F. Epoxy Nanocomposites with Highly Exfoliated Clay: Mechanical Properties and Fracture Mechanisms. Macromolecules 2005, 38, 788–800. [Google Scholar] [CrossRef]





| The Qualitative Characteristics of E51 | Value |
|---|---|
| Viscosity (25 °C, Pa·s) | 12–15 |
| Epoxy Value (eq/100 g) | 0.51–0.53 |
| Epoxide equivalent weight (g/1 eq) | 191–193 |
| Density at 25 °C (g/cm3) | 1.16 |
| Name | Viscosity (25 °C, mPa·s) | Density at 25 °C (g/cm3) |
|---|---|---|
| MHHPA | 40~50 | 1.16 |
| BDA | 90 | 0.9 |
| Mixture (40:1) | 40–50 | 1.15 |
| The Qualitative Characteristics of MWNTs | Value |
|---|---|
| Internal diameter (nm) | 8−15 |
| External diameter (nm) | 15–20 |
| Length (μm) | 0.5–2 |
| Total amount of admixtures (%) | ≤1 |
| Code | CTA | [M]:[CTA]:[I] | Con (%) | Mn,PHMA a. | Mn, PGMA c. | Mn,PHMA-b-GMA c. | PDI a. |
|---|---|---|---|---|---|---|---|
| H1 | CTA-N3 | 25:1:0.2 | 90 | 4480 | − | − | 1.08 |
| H2 | CTA-N3 | 50:1:0.2 | 94 | 8250 | − | − | 1.09 |
| H3 | CTA-N3 | 90:1:0.2 | 85 | 13,800 | − | − | 1.07 |
| H4 | CTA-N3 | 180:1:0.2 | 70 | 20,800 | − | − | 1.10 |
| B1 | PHMA24-N3 b. | 35:1:0.2 | 88 | 4480 | 6290 | 10,700 | 1.12 |
| B2 | PHMA46-N3 b. | 50:1:0.2 | 85 | 8250 | 6900 | 15,150 | 1.13 |
| B3 | PHMA79-N3 b. | 85:1:0.2 | 49 | 13,800 | 5700 | 19,500 | 1.12 |
| B4 | PHMA120-N3 b. | 95:1:0.2 | 41 | 20,800 | 6500 | 27,300 | 1.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Ye, Y.; Xie, X.; Zhou, X. Effect of MWNT Functionalization with Tunable-Length Block Copolymers on Dispersity of MWNTs and Mechanical Properties of Epoxy/MWNT Composites. Polymers 2022, 14, 3137. https://doi.org/10.3390/polym14153137
Liu J, Ye Y, Xie X, Zhou X. Effect of MWNT Functionalization with Tunable-Length Block Copolymers on Dispersity of MWNTs and Mechanical Properties of Epoxy/MWNT Composites. Polymers. 2022; 14(15):3137. https://doi.org/10.3390/polym14153137
Chicago/Turabian StyleLiu, Jingwei, Yunsheng Ye, Xiaolin Xie, and Xingping Zhou. 2022. "Effect of MWNT Functionalization with Tunable-Length Block Copolymers on Dispersity of MWNTs and Mechanical Properties of Epoxy/MWNT Composites" Polymers 14, no. 15: 3137. https://doi.org/10.3390/polym14153137






