Development of Adsorptive Membranes for Selective Removal of Contaminants in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Graphitic Carbon Nitride
2.3. Synthesis of Porous Arsenate-Imprinted and Non-Imprinted 30:70 Methacrylic Acid: Methacrylamide Polymer
2.4. Synthesis of Porous Ammonia-Imprinted and Non-Imprinted 70:30 Methacrylic Acid: Methacrylamide Polymer
2.5. Synthesis of Porous Arsenate-Imprinted and Non-Imprinted Polymer with Graphitic Carbon Nitride
2.6. Synthesis of Porous Ammonia-Imprinted and Non-Imprinted Polymer with Graphitic Carbon Nitride
2.7. Adsorptive Membrane Synthesis
2.8. Adsorption Column Removal Experiments
2.9. Adsorptive Membrane Removal Experiments
2.10. Analytical Measurements
3. Results
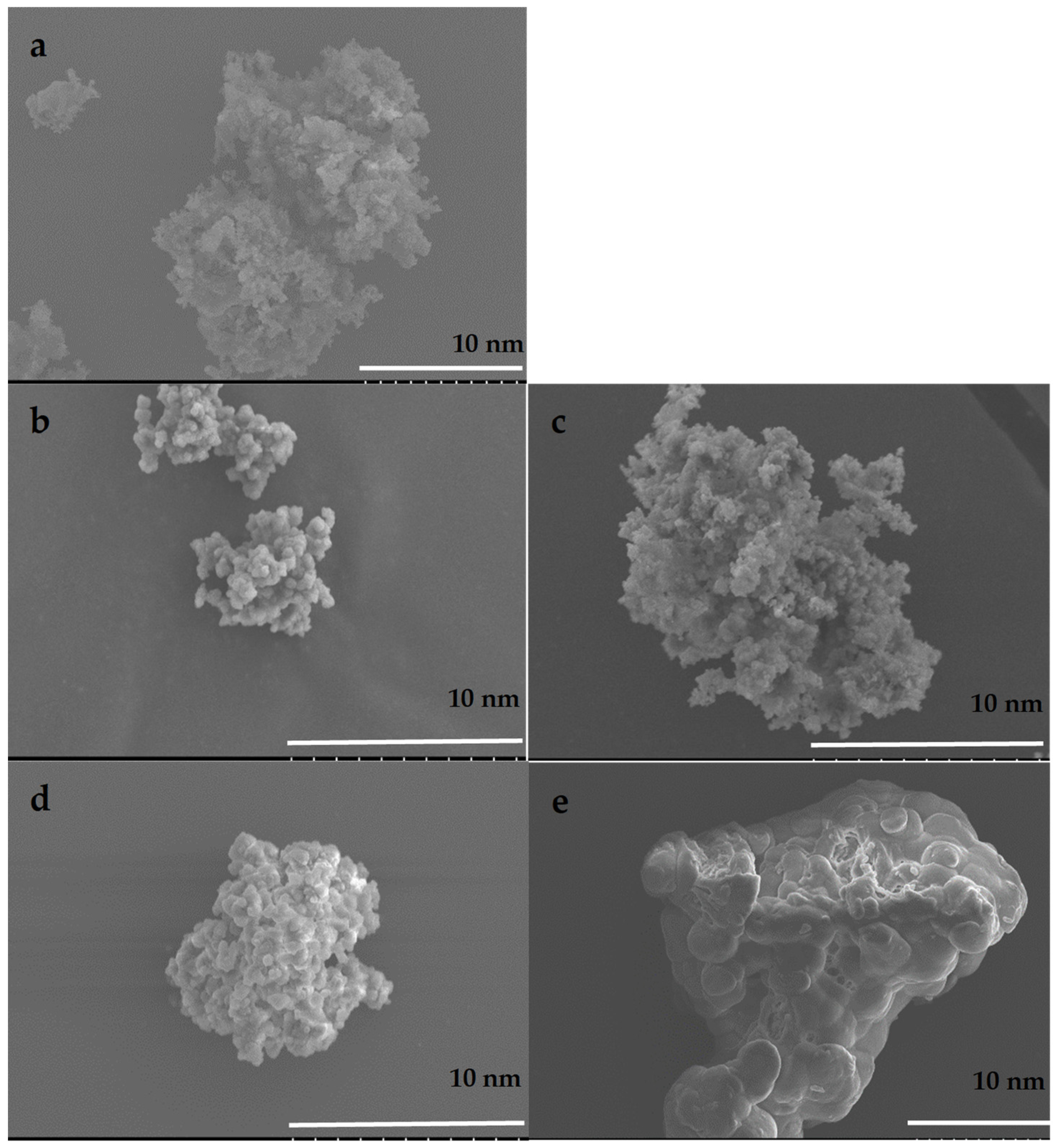
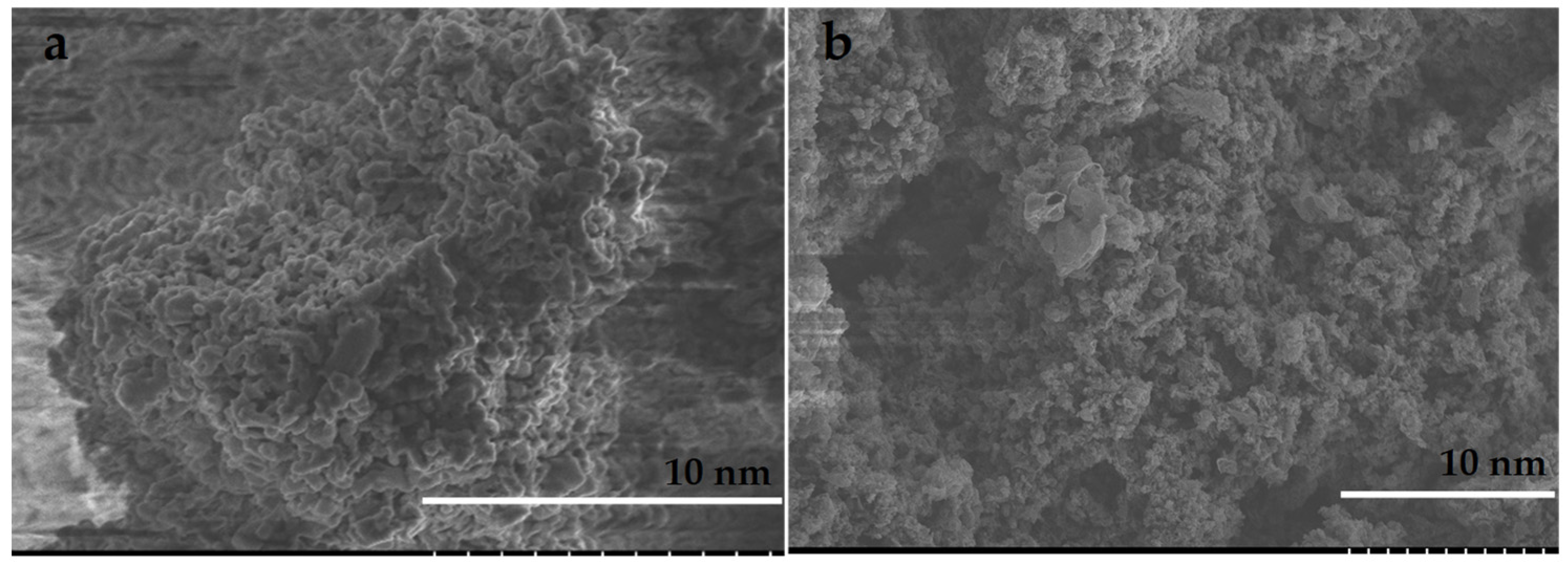
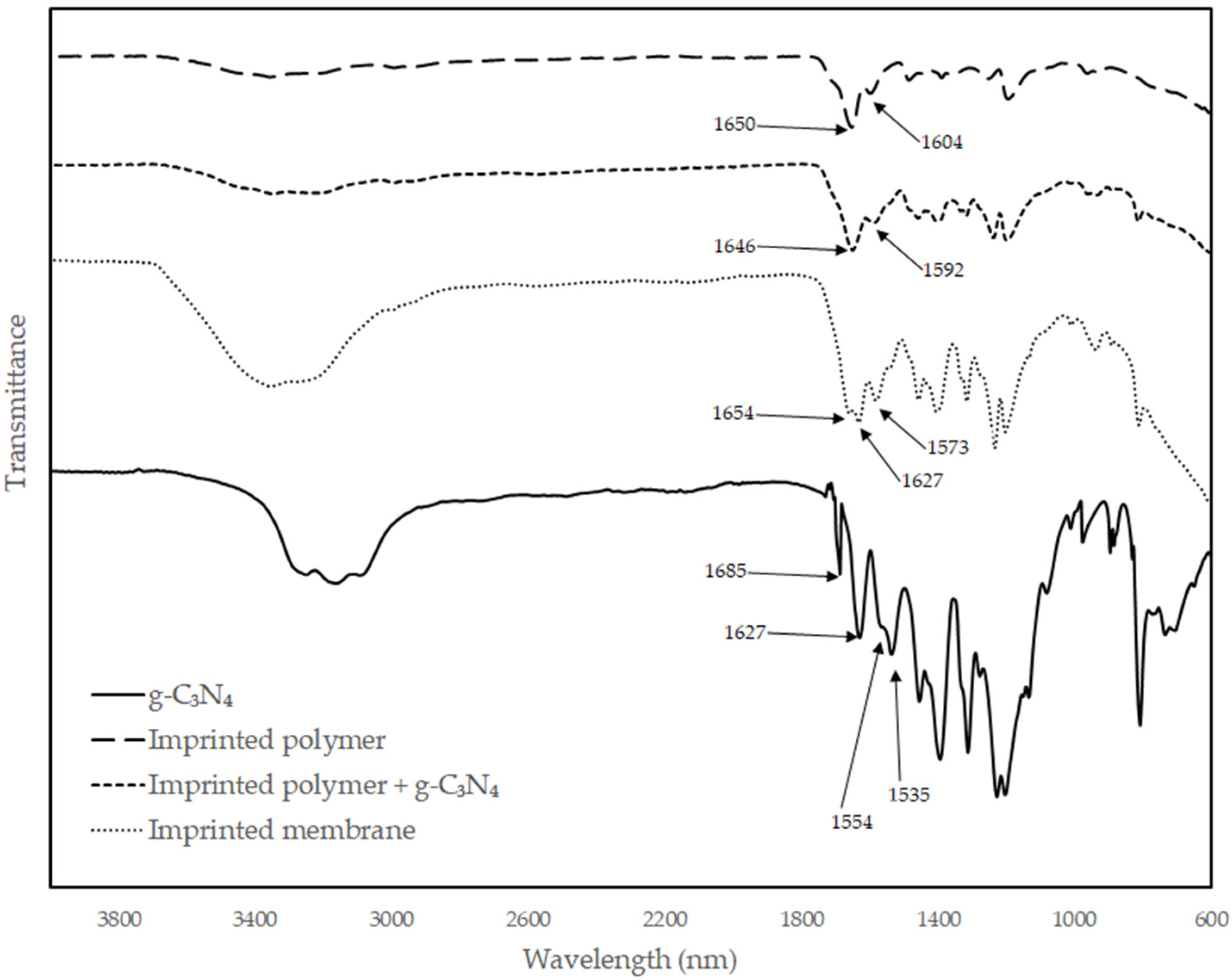
3.1. Materials Synthesis and Characterization
3.2. Removal of Arsenate in Adsorption Columns
3.3. Removal of Ammonia in Adsorption Columns
3.4. Comparison to Conventional Adsorbents
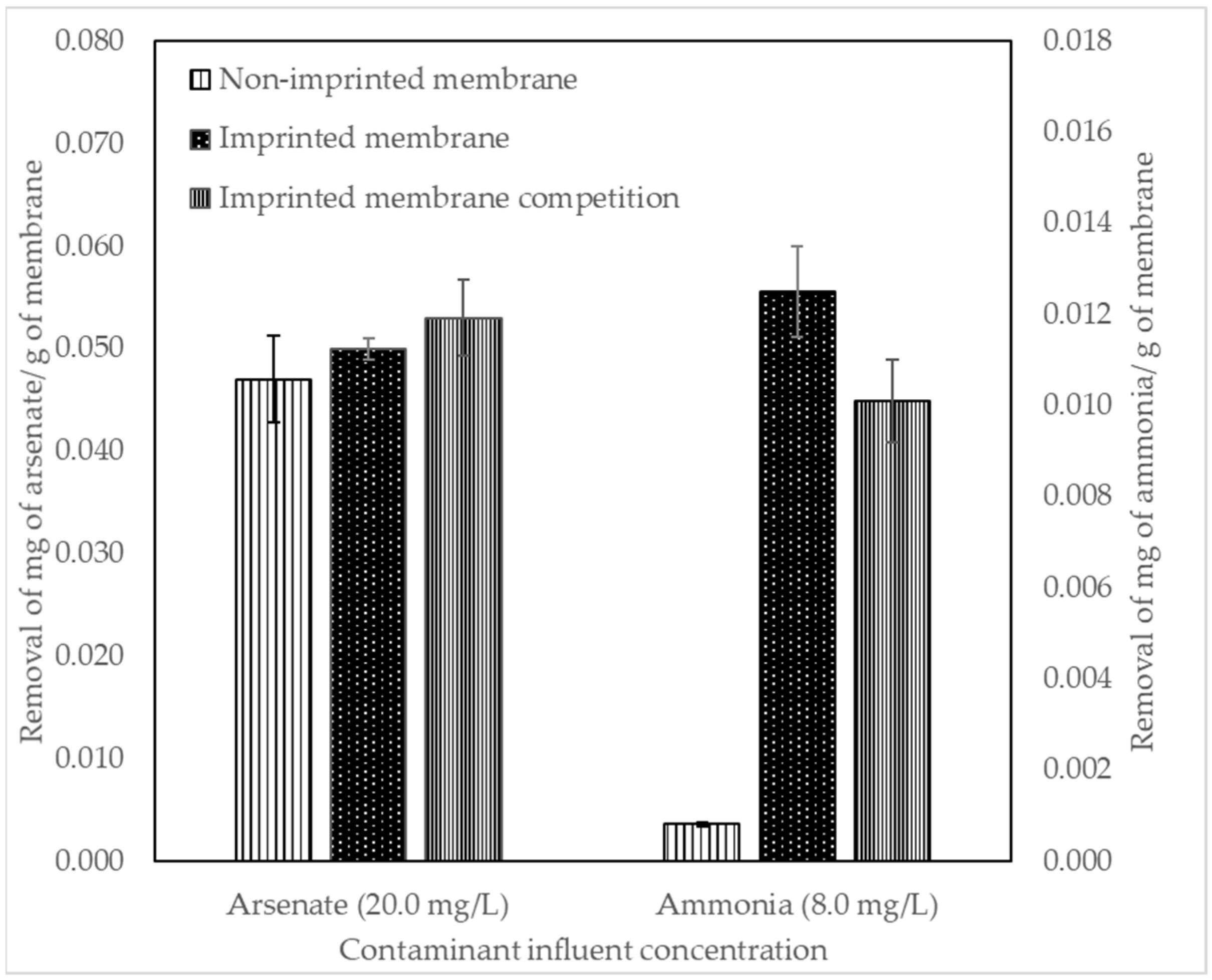
3.5. Initial Membrane Removal Results
4. Discussion
4.1. Materials Synthesis and Characterization
4.2. Removal of Arsenate in Adsorption Columns
4.3. Removal of Ammonia in Adsorption Columns
4.4. Comparison to Conventional Adsorbents
4.5. Initial Membrane Removal Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- John, Y.; David, V.E.; Mmereki, D. A Comparative Study on Removal of Hazardous Anions from Water by Adsorption: A Review. Int. J. Chem. Eng. 2018, 2018, 3975948. [Google Scholar] [CrossRef] [Green Version]
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Rahaman, S.; Rahman, M.; Mise, N.; Sikder, T.; Ichihara, G.; Uddin, K.; Kurasaki, M.; Ichihara, S. Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ. Pollut. 2021, 289, 117940. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.; Ali, I. Arsenic: Occurrence, toxicity and speciation techniques. Water Res. 2000, 34, 4304–4312. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S. A review on sources, identification and treatment strategies for the removal of toxic Arsenic from water system. J. Hazard. Mater. 2021, 418, 126299. [Google Scholar] [CrossRef] [PubMed]
- Alexander, T.C.; Gulledge, E. Arsenic Occurrence, Ecotoxicity and its Potential Remediation. J. Bioremediat. Biodegrad. 2016, 7, e174. [Google Scholar] [CrossRef]
- Alka, S.; Shahir, S.; Ibrahim, N.; Ndejiko, M.J.; Vo, D.-V.N.; Manan, F.A. Arsenic removal technologies and future trends: A mini review. J. Clean. Prod. 2021, 278, 123805. [Google Scholar] [CrossRef]
- DU, Q.; Liu, S.; Cao, Z.; Wang, Y. Ammonia removal from aqueous solution using natural Chinese clinoptilolite. Sep. Purif. Technol. 2005, 44, 229–234. [Google Scholar] [CrossRef]
- Adam, M.R.; Othman, M.H.D.; Abu Samah, R.; Puteh, M.H.; Ismail, A.; Mustafa, A.; Rahman, M.A.; Jaafar, J. Current trends and future prospects of ammonia removal in wastewater: A comprehensive review on adsorptive membrane development. Sep. Purif. Technol. 2019, 213, 114–132. [Google Scholar] [CrossRef]
- Huang, L.; Li, L.; Dong, W.; Liu, Y.; Hou, H. Removal of Ammonia by OH Radical in Aqueous Phase. Environ. Sci. Technol. 2008, 42, 8070–8075. [Google Scholar] [CrossRef]
- Tanne, N.; Xu, R.; Zhou, M.; Zhang, P.; Wang, X.; Wen, X. Influence of pore size and membrane surface properties on arsenic removal by nanofiltration membranes. Front. Environ. Sci. Eng. 2019, 13, 19. [Google Scholar] [CrossRef]
- Shih, M.-C. An overview of arsenic removal by pressure-drivenmembrane processes. Desalination 2005, 172, 85–97. [Google Scholar] [CrossRef]
- Roper, D.; Lightfoot, E.N. Separation of biomolecules using adsorptive membranes. J. Chromatogr. A 1995, 702, 3–26. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Comparison of Polypropylene and Ceramic Microfiltration Membranes Applied for Separation of 1,3-PD Fermentation Broths and Saccharomyces cerevisiae Yeast Suspensions. Membranes 2021, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Alresheedi, M.T.; Barbeau, B.; Basu, O.D. Comparisons of NOM fouling and cleaning of ceramic and polymeric membranes during water treatment. Sep. Purif. Technol. 2019, 209, 452–460. [Google Scholar] [CrossRef]
- Parlapiano, M.; Akyol, Ç.; Foglia, A.; Pisani, M.; Astolfi, P.; Eusebi, A.L.; Fatone, F. Selective removal of contaminants of emerging concern (CECs) from urban water cycle via Molecularly Imprinted Polymers (MIPs): Potential of upscaling and enabling reclaimed water reuse. J. Environ. Chem. Eng. 2021, 9, 105051. [Google Scholar] [CrossRef]
- Ashraf, S.; Cluley, A.; Mercado, C.; Mueller, A. Imprinted polymers for the removal of heavy metal ions from water. Water Sci. Technol. 2011, 64, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Ding, L.; Ren, W.; Hu, H.; Huang, Y.; Shao, P.; Yang, L.; Shi, H.; Ren, Z.; Han, K.; et al. High exposure effect of the adsorption site significantly enhanced the adsorption capacity and removal rate: A case of adsorption of hexavalent chromium by quaternary ammonium polymers (QAPs). J. Hazard. Mater. 2021, 416, 125829. [Google Scholar] [CrossRef]
- Chaipuang, A.; Phungpanya, C.; Thongpoon, C.; Watla-Iad, K.; Inkaew, P.; Machan, T.; Suwantong, O. Effect of ethylene diamine tetra-acetic acid and functional monomers on the structure and adsorption properties of copper (II) ion-imprinted polymers. Polym. Adv. Technol. 2021, 32, 3000–3007. [Google Scholar] [CrossRef]
- Liu, J.; Chen, M.; Cui, H. Synthesis of Ion-imprinted materials with amidoxime groups for enhanced UO22+ adsorption. Inorganica Chim. Acta 2021, 517, 120196. [Google Scholar] [CrossRef]
- Gornik, T.; Shinde, S.; Lamovsek, L.; Koblar, M.; Heath, E.; Sellergren, B.; Kosjek, T. Molecularly Imprinted Polymers for the Removal of Antidepressants from Contaminated Wastewater. Polymers 2021, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.; Ashraf, S.; Mueller, A. The binding of metal ions to molecularly-imprinted polymers. Water Sci. Technol. 2017, 75, 1643–1650. [Google Scholar] [CrossRef]
- Randhawa, M.; Gartner, I.; Becker, C.; Student, J.; Chai, M.; Mueller, A. Imprinted polymers for water purification. J. Appl. Polym. Sci. 2007, 106, 3321–3326. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Kyzas, G.Z.; Katsoyiannis, I.A. Arsenic(III) and Arsenic(V) Removal from Water Sources by Molecularly Imprinted Polymers (MIPs): A Mini Review of Recent Developments. Sustainability 2022, 14, 5222. [Google Scholar] [CrossRef]
- Mann, S.; Johnson, T.; Medendorp, E.; Ocomen, R.; DeHart, L.; Bauer, A.; Li, B.; Tecklenburg, M.; Mueller, A. Pressure-Stable Imprinted Polymers for Waste Water Remediation. Polymers 2018, 10, 704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Gao, B.; Yue, Q.; Wang, Z. Graphitic carbon nitride (g-C3N4)-based membranes for advanced separation. J. Mater. Chem. A 2020, 8, 19133–19155. [Google Scholar] [CrossRef]
- Li, R.; Ren, Y.; Zhao, P.; Wang, J.; Liu, J.; Zhang, Y. Graphitic carbon nitride (g-C3N4) nanosheets functionalized composite membrane with self-cleaning and antibacterial performance. J. Hazard. Mater. 2019, 365, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, G.; Chen, S.; Fan, X.; Quan, X.; Yu, H. Integration of membrane filtration and photoelectrocatalysis on g-C3N4/CNTs/Al2O3 membrane with visible-light response for enhanced water treatment. J. Membr. Sci. 2017, 541, 153–161. [Google Scholar] [CrossRef]
- Lan, H.; Wang, F.; Lan, M.; An, X.; Liu, H.; Qu, J. Hydrogen-Bond-Mediated Self-Assembly of Carbon-Nitride-Based Photo-Fenton-like Membranes for Wastewater Treatment. Environ. Sci. Technol. 2019, 53, 6981–6988. [Google Scholar] [CrossRef]
- Shamim, M.; Perveen, M.; Nazir, S.; Hussnain, M.; Mehmood, R.; Khan, M.I.; Iqbal, J. DFT study of therapeutic potential of graphitic carbon nitride (g-C3N4) as a new drug delivery system for carboplatin to treat cancer. J. Mol. Liq. 2021, 331, 115607. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Dong, F. Graphitic carbon nitride based nanocomposites: A review. Nanoscale 2015, 7, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lyu, C.; Wu, S.; Ben, Y.; Li, X.; Ge, Z.; Zou, H.; Tian, D.; Yu, Y.; Ding, K. Electrophoresis-Deposited Mesoporous Graphitic Carbon Nitride Surfaces with Efficient Bactericidal Properties. ACS Appl. Bio Mater. 2020, 3, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
- Epsztein, R.; Duchanois, R.M.; Ritt, C.L.; Noy, A.; Elimelech, M. Towards single-species selectivity of membranes with subnanometre pores. Nat. Nanotechnol. 2020, 15, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, M.; Liu, F.; Ding, S.; Wang, X.; Du, G.; Liu, J.; Apel, P.; Kluth, P.; Trautmann, C.; et al. Ultrafast ion sieving using nanoporous polymeric membranes. Nat. Commun. 2018, 9, 569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arumugham, T.; Kaleekkal, N.J.; Gopal, S.; Nambikkattu, J.; Rambabu, K.; Aboulella, A.M.; Wickramasinghe, S.R.; Banat, F. Recent developments in porous ceramic membranes for wastewater treatment and desalination: A review. J. Environ. Manag. 2021, 293, 112925. [Google Scholar] [CrossRef] [PubMed]
- Jagirani, M.S.; Balouch, A.; Mahesar, S.A.; Kumar, A.; Abdullah; Mustafai, F.A.; Bhanger, M.I. Preparation of novel arsenic-imprinted polymer for the selective extraction and enhanced adsorption of toxic As3+ ions from the aqueous environment. Polym. Bull. 2019, 77, 5261–5279. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Chakraborty, S.; Tow, E.W.; Plumlee, M.H.; Bellona, C.; Loutatidou, S.; Karimi, L.; Mikelonis, A.M.; Achilli, A.; Ghassemi, A.; et al. A review of polymeric membranes and processes for potable water reuse. Prog. Polym. Sci. 2018, 81, 209–237. [Google Scholar] [CrossRef]
- Asif, M.B.; Zhang, Z. Ceramic membrane technology for water and wastewater treatment: A critical review of performance, full-scale applications, membrane fouling and prospects. Chem. Eng. J. 2021, 418, 129481. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, D.; Yu, P.; Sun, R.; Javed, H.; Wu, G.; Alvarez, P.J.J. Selective Adsorption and Photocatalytic Degradation of Extracellular Antibiotic Resistance Genes by Molecularly-Imprinted Graphitic Carbon Nitride. Environ. Sci. Technol. 2020, 54, 4621–4630. [Google Scholar] [CrossRef]
- Vinardell, S.; Astals, S.; Peces, M.; Cardete, M.A.; Fernández, I.; Mata-Alvarez, J.; Dosta, J. Advances in anaerobic membrane bioreactor technology for municipal wastewater treatment: A 2020 updated review. Renew. Sustain. Energy Rev. 2020, 130, 109936. [Google Scholar] [CrossRef]
- Ogunbiyi, O.O.; Miles, N.J.; Hilal, N. The effects of performance and cleaning cycles of new tubular ceramic microfiltration membrane fouled with a model yeast suspension. Desalination 2008, 220, 273–289. [Google Scholar] [CrossRef]
- Cao, Q.; Kumru, B.; Antonietti, M.; Schmidt, B.V.K.J. Graphitic carbon nitride and polymers: A mutual combination for advanced properties. Mater. Horizons 2019, 7, 762–786. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Peng, R.; Zhang, H.; Cui, Q.; Niu, P.; Li, L. Graphitic carbon nitride colloid as one photoinitiator for two-step polymerization. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129615. [Google Scholar] [CrossRef]
- Kiskan, B.; Zhang, J.; Wang, X.; Antonietti, M.; Yagci, Y. Mesoporous Graphitic Carbon Nitride as a Heterogeneous Visible Light Photoinitiator for Radical Polymerization. ACS Macro Lett. 2012, 1, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; An, T.; Chen, Z.; Wang, Z.; Zhou, H.; Fan, T.; Zhang, D.; Antonietti, M. Carbon nitride nanosheets as visible light photocatalytic initiators and crosslinkers for hydrogels with thermoresponsive turbidity. J. Mater. Chem. A 2017, 5, 8933–8938. [Google Scholar] [CrossRef] [Green Version]
- Morohashi, S.; Takeda, K.; Yamamoto, T.; Hoshino, K.; Sasakura, T. Adsorption Properties of Trivalent Metal Ions onto Hydrolyzed Polyacrylamide Gel. J. Chem. Eng. Jpn. 1996, 29, 1060–1063. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zhu, S.; Pelton, R.H.; Spafford, M. Flocculation of dilute titanium dioxide suspensions by graft cationic polyelectrolytes. Colloid Polym. Sci. 1999, 277, 108–114. [Google Scholar] [CrossRef]
- Wang, L.; Wang, K.; He, T.; Zhao, Y.; Song, H.; Wang, H. Graphitic Carbon Nitride-Based Photocatalytic Materials: Preparation Strategy and Application. ACS Sustain. Chem. Eng. 2020, 8, 16048–16085. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Yang, H.; Jin, D.; Qu, J.; Yuan, X.; Zhang, Y.-N.; Huo, M.; Peijnenburg, W.J. Rapid water purification using modified graphitic carbon nitride and visible light. Appl. Catal. B Environ. 2021, 285, 119864. [Google Scholar] [CrossRef]
- Barrejón, M.; Prato, M. Carbon Nanotube Membranes in Water Treatment Applications. Adv. Mater. Interfaces 2021, 9, 2101260. [Google Scholar] [CrossRef]
- Abu Samah, N.; Rosli, N.A.M.; Manap, A.H.A.; Aziz, Y.F.A.; Yusoff, M.M. Synthesis & characterization of ion imprinted polymer for arsenic removal from water: A value addition to the groundwater resources. Chem. Eng. J. 2020, 394, 124900. [Google Scholar] [CrossRef]
- Gao, B.; Du, J.; Zhang, Y. Preparation of Arsenate Anion Surface-Imprinted Material IIP-PDMC/SiO2 and Study on Its Ion Recognition Property. Ind. Eng. Chem. Res. 2013, 52, 7651–7659. [Google Scholar] [CrossRef]
- Barrio, J.; Barzilai, S.; Karjule, N.; Amo-Ochoa, P.; Zamora, F.; Shalom, M. Synergistic Doping and Surface Decoration of Carbon Nitride Macrostructures by Single Crystal Design. ACS Appl. Energy Mater. 2021, 4, 1868–1875. [Google Scholar] [CrossRef]
- Zhu, L.; You, L.; Wang, Y.; Shi, Z. The application of graphitic carbon nitride for the adsorption of Pb2+ ion from aqueous solution. Mater. Res. Express 2017, 4, 075606. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Pang, H.; Wang, X.; Yu, S.; Wng, X. Study on the Removal of Water Pollutants by Graphite Phase Carbon Nitride Materials. Prog. Chem. 2019, 31, 831–846. [Google Scholar]
- Han, Z.; Xu, Y.; Wang, H.; Tian, H.; Qiu, B.; Sun, D. Synthesis of ammonia molecularly imprinted adsorbents and ammonia adsorption separation during sludge aerobic composting. Bioresour. Technol. 2020, 300, 122670. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Xu, Y.; Tian, H.; Liang, J.; Sun, D. Enhanced ammonia adsorption and separation by a molecularly imprinted polymer after acid hydrolysis of its ester crosslinker. J. Hazard. Mater. 2021, 412, 125145. [Google Scholar] [CrossRef]
- Xi, Y.; Shi, H.; Liu, R.; Yin, X.; Yang, L.; Huang, M.; Luo, X. Insights into ion imprinted membrane with a delayed permeation mechanism for enhancing Cd2+ selective separation. J. Hazard. Mater. 2021, 416, 125772. [Google Scholar] [CrossRef]




| Polymeric Membranes | Ceramic Membranes | g-C3N4 Membranes | |
|---|---|---|---|
| Permeability | High permeability | High permeability [14] | High permeability [26] |
| Selectivity | Low selectivity [33,34] | Some selectivity [35] | High selectivity [24,36] |
| Mechanical strength | Good mechanical strength [37] | Prone to breakage [14] | Good mechanical strength [26] |
| Pore size control | Broader pore size distribution, smaller pore size [37] | Narrow pore size distribution, higher porosity, larger pore size [14,37] | Narrow pore size distribution, higher porosity [27,38,39] |
| Fouling | Susceptible to fouling [15] | Lower fouling [14,35] | Antibacterial and antifouling properties [26,27] |
| Cleaning | Susceptible to cleaning agents | Resistant to cleaning agents [14] | Self-cleaning properties [26,27] |
| Integration with other processes | Bioreactors [40]. Susceptible to oxidation reagents [39] | Advanced Oxidation Processes and bioreactors [39] | Advanced Oxidation Processes and adsorption [39] |
| Thermal stability | Poor [37] | Up to 500 °C [14,41] | Good [26] |
| Cost of production | Low cost [35] | High cost [14] | Expected low cost |
| Adsorbent | Average Mass of Arsenate Removed/Mass of Adsorbent (mg/g) | Standard Deviation |
|---|---|---|
| Non-imprinted polymer | 1.416 | 0.011 |
| Imprinted polymer | 1.412 | 0.100 |
| Imprinted polymer competition | 1.406 | 0.066 |
| g-C3N4 | 1.583 | 0.011 |
| Non-imprinted polymer + g-C3N4 | 1.469 | 0.180 |
| Imprinted polymer + g-C3N4 | 1.505 | 0.078 |
| Imprinted polymer + g-C3N4 competition | 1.857 | 0.040 |
| Adsorbent | Average Mass of Ammonia Removed/Mass of Adsorbent (mg/g) | Standard Deviation |
|---|---|---|
| Non-imprinted polymer | 0.070 | 0.012 |
| Imprinted polymer | 0.217 | 0.012 |
| Imprinted polymer competition | 0.068 | 0.003 |
| g-C3N4 | 0.221 | 0.026 |
| Non-imprinted polymer + g-C3N4 | 0.200 | 0.013 |
| Imprinted polymer + g-C3N4 | 0.271 | 0.004 |
| Imprinted polymer + g-C3N4 competition | 0.057 | 0.013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirisenage, P.M.; Zulqarnain, S.M.; Myers, J.L.; Fahlman, B.D.; Mueller, A.; Marquez, I. Development of Adsorptive Membranes for Selective Removal of Contaminants in Water. Polymers 2022, 14, 3146. https://doi.org/10.3390/polym14153146
Kirisenage PM, Zulqarnain SM, Myers JL, Fahlman BD, Mueller A, Marquez I. Development of Adsorptive Membranes for Selective Removal of Contaminants in Water. Polymers. 2022; 14(15):3146. https://doi.org/10.3390/polym14153146
Chicago/Turabian StyleKirisenage, Priyalatha M., Syed M. Zulqarnain, Jordan L. Myers, Bradley D. Fahlman, Anja Mueller, and Itzel Marquez. 2022. "Development of Adsorptive Membranes for Selective Removal of Contaminants in Water" Polymers 14, no. 15: 3146. https://doi.org/10.3390/polym14153146






