The Impact of β-Radiation Crosslinking on Flammability Properties of PA6 Modified by Commercially Available Flame-Retardant Additives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation
2.2. Degree of Crosslinking
2.3. Thermal and Gas Analytics
2.4. Fire Testing
2.5. Char Residue Analysis
2.6. Dynamic Mechanical Analysis (DMA)
3. Results
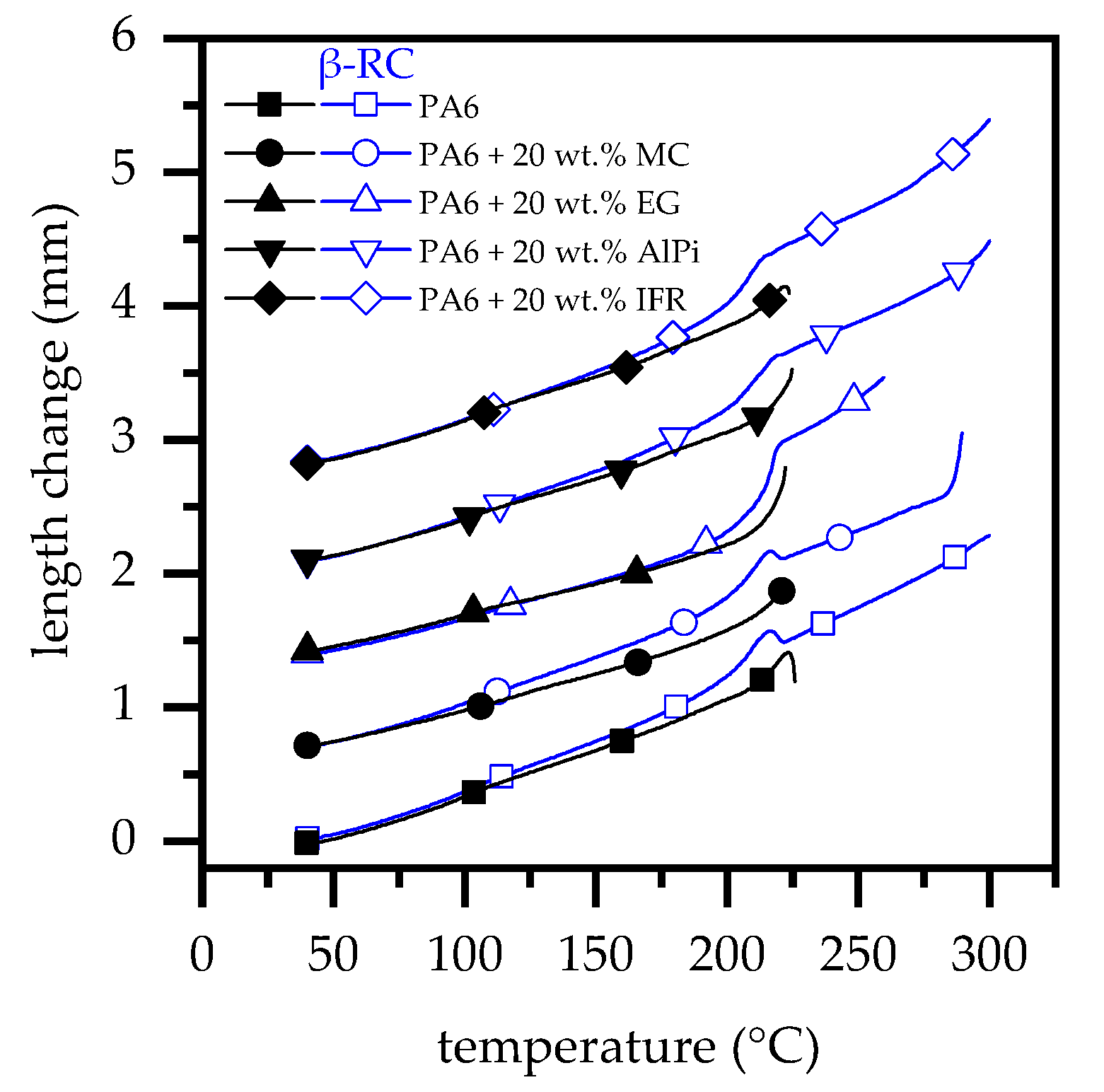
3.1. Degree of Radiation Crosslinking—Cristallization Behavior and Gel Content
3.2. Thermal Decomposition Behavior
3.2.1. Thermal Decomposition Behavior—PA6
3.2.2. Thermal Decomposition Behavior—PA6 Containing MC
3.2.3. Thermal Decomposition Behavior—PA6 Containing EG
3.2.4. Thermal Decomposition Behavior—PA6 Containing AlPi
3.2.5. Thermal Decomposition Behavior—PA6 Containing an IFR
3.2.6. Thermal Decomposition Behavior—Summary
3.3. Dynamic Mechanic Analysis
3.4. Fire Behavior—Cone Calorimeter Testing
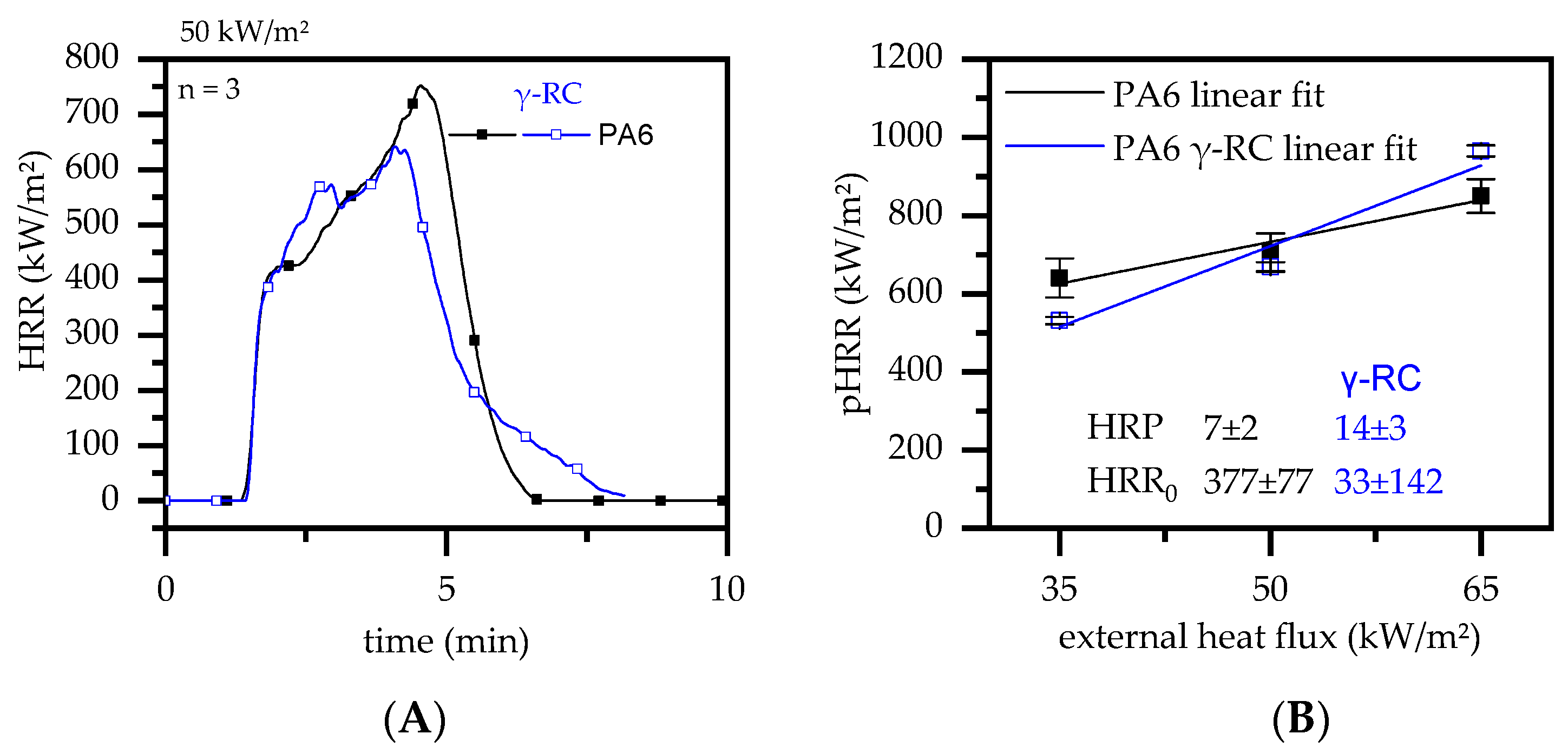
3.4.1. Cone Calorimeter Testing—PA6
3.4.2. Cone Calorimeter Testing—MC
3.4.3. Cone Calorimeter Testing—EG
- (1)
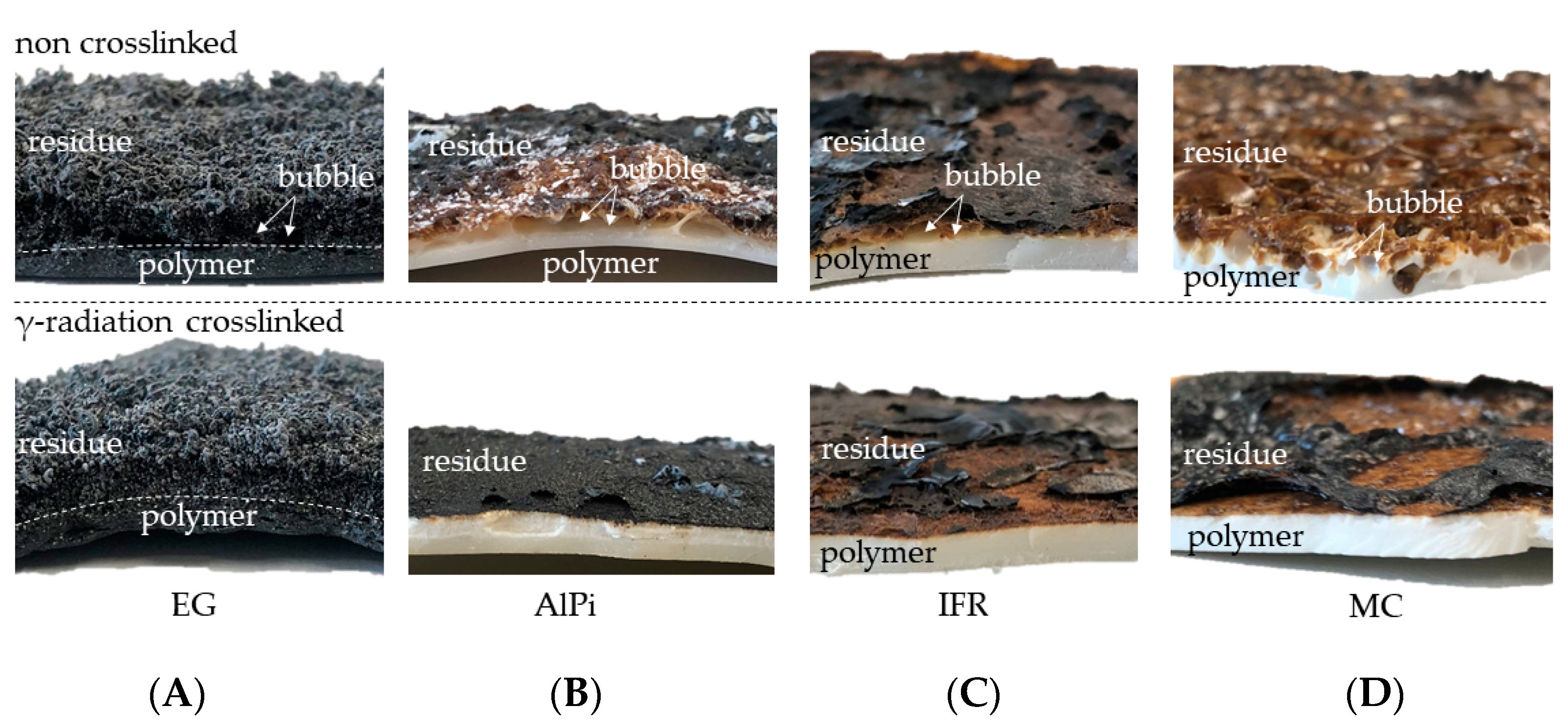
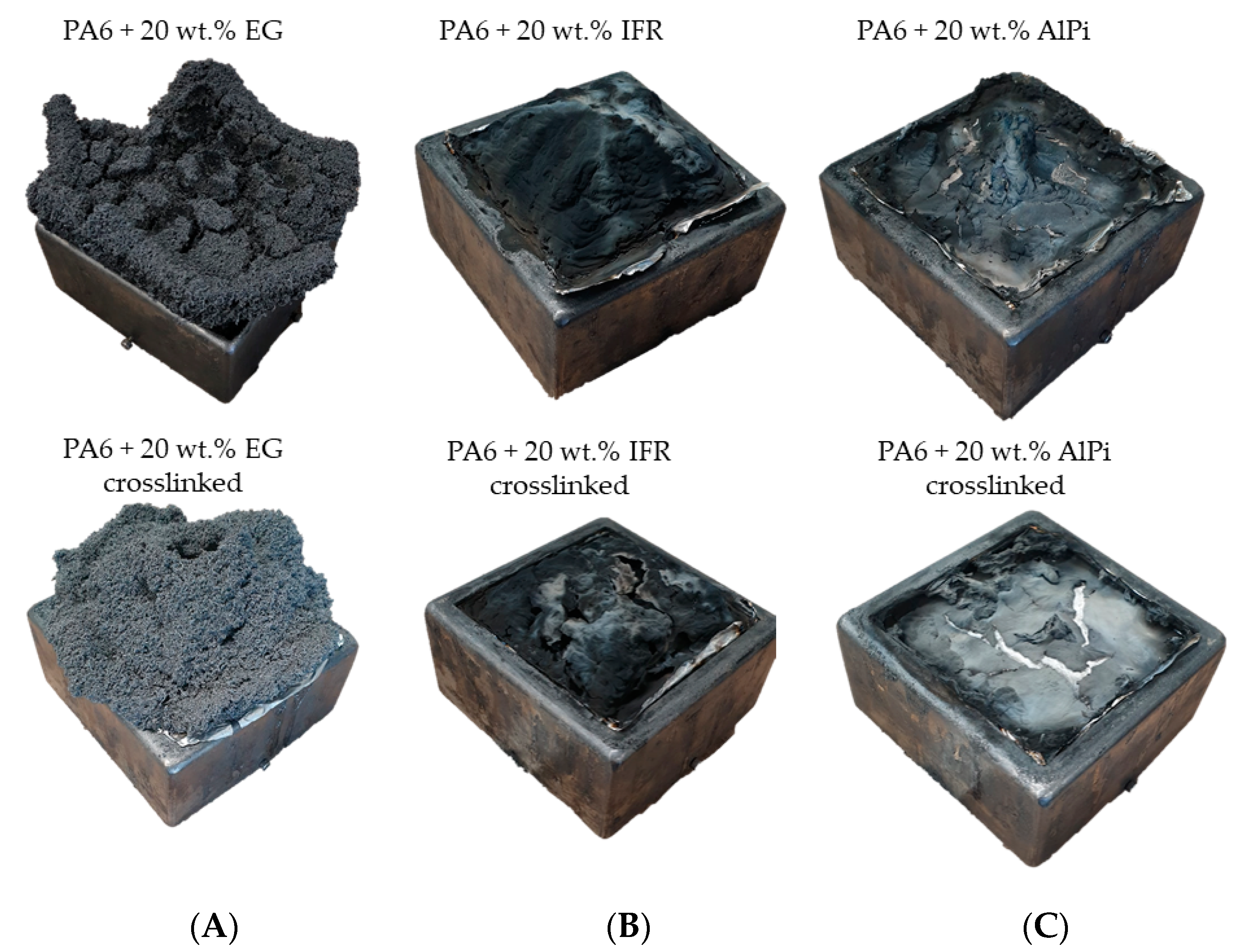
- Warpage and residue disruption: As described earlier for net PA6, β-RC samples tended to warp. Warpage caused the distance between the sample surface and cone heater to shrink, which increased the impact of heat. Additionally, cracks appeared within the growing graphite char residue due to the surface movement and were triggered through internal stress elimination. These cracks locally reduced the insulation performance and led to an increased impact of heat, subsequently causing accelerated pyrolysis gas production. This was particularly evident for formulations containing 15 and 20 wt.% EG.
- (2)
- Mass transportation rate: Even though no shifts in the decomposition onset temperature could be detected, β-RC samples showed a higher physical stability throughout the burning process. This effect was attributed to a lack of low molecular melt pools, bubbling and random geometrical collapses, which constantly change the surface area during the burning process of non-β-RC samples. Particularly in the early burning stages, in which the decomposition zone of β-RC samples containing PA6 and EG occurred rather close to the surface, pyrolysis gas production was more limited. Since the flame retardant effect of EG strongly depends on the formation of residue and as the build up of residue is a somewhat time-consuming process, sufficient fire protection cannot be provided in early burning stages. However, since β-RC seemed to decelerate the mass loss rate development, EG particles were provided with a longer expansion time before ignition. The ignition was thus carried out slightly later.
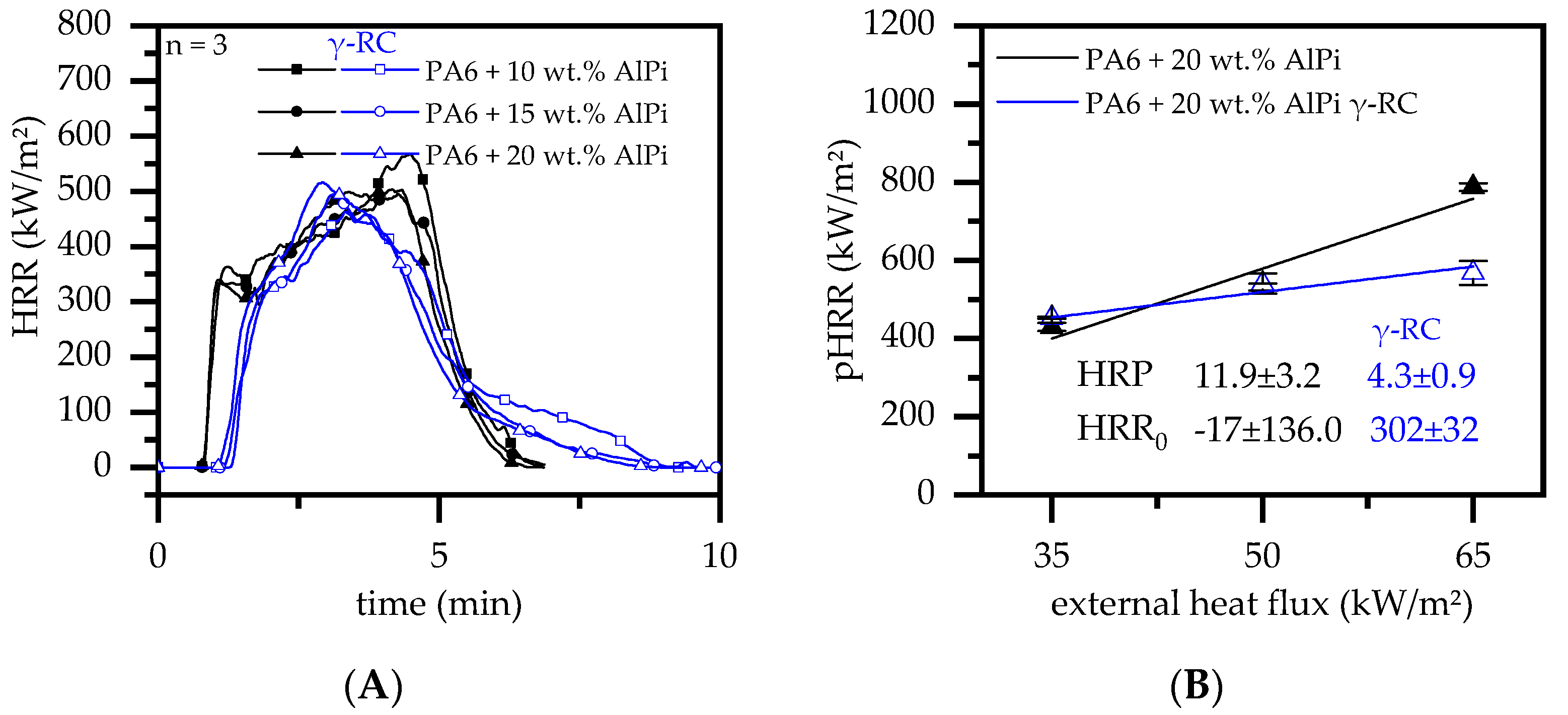
3.4.4. Cone Calorimeter Testing—AlPi
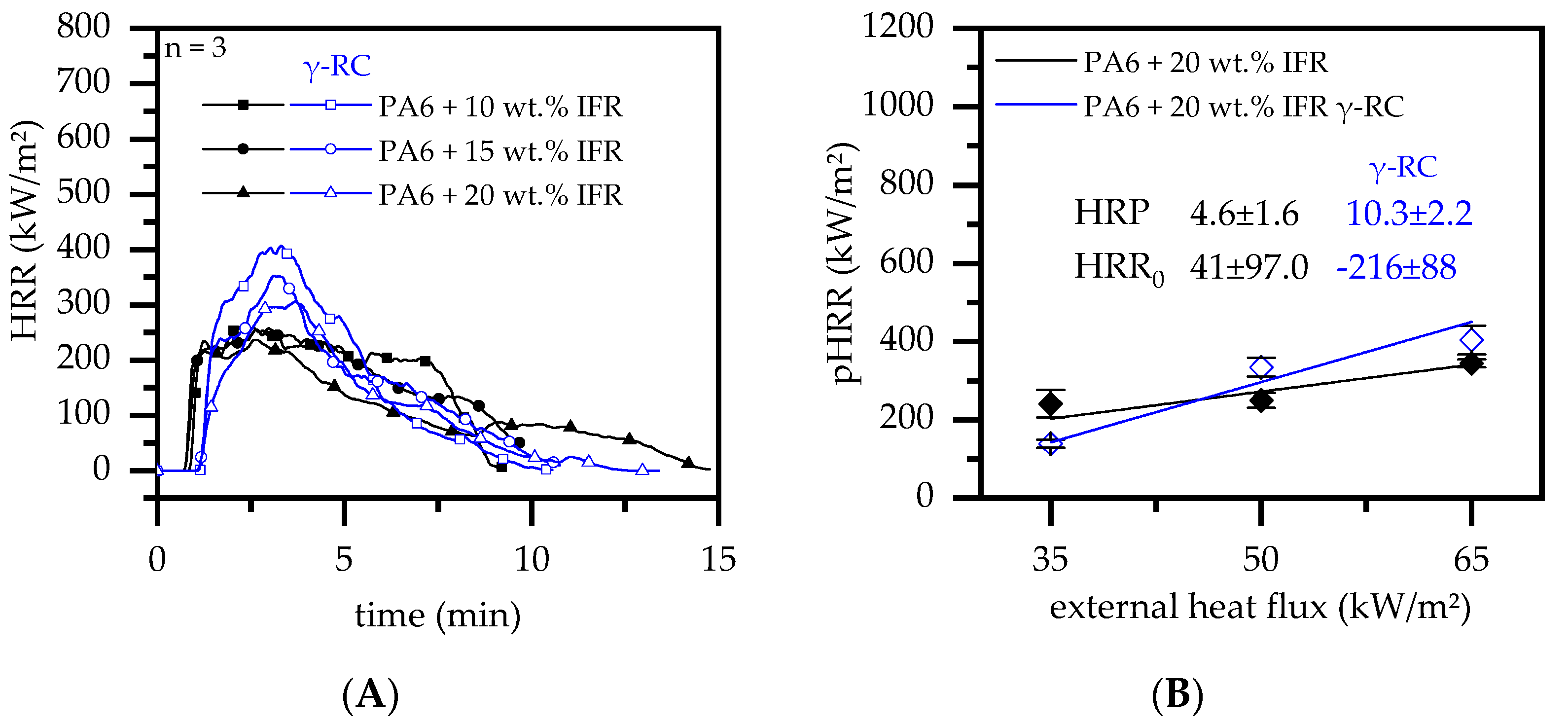
3.4.5. Cone Calorimeter Testing—IFR
3.4.6. Summary—Cone Calorimeter Testing
- (1)
- β-RC samples tended to sufficiently suppress the mass transport of pyrolysis gases that originated from lower polymer layers, which was otherwise evident through the strong bubbling in non-crosslinked samples. The limited mass transport of pyrolysis gases was not only relevant in the early burning stages, but rather reduced the overall heat development over the burning process. This effect can be attributed to the higher structural stability provided by the three-dimensional crosslinked network.
- (2)
- Chemical reactions triggered by flame-retardant additives that react with the matrix polymer and form a char residue predominantly occur between the melting point and the decomposition onset of the polymer. Since the three-dimensional crosslinked network physically stabilizes the polymeric melt until decomposition occurs, the freedom in movement as well as the amount of reactive and unbound polymer fractions is more limited than in non-crosslinked samples.
- (3)
- The ongoing burning process caused warpage for all tested formulations. The physical deformation caused the proximity between the sample surface and cone heater to shrink, which resulted in a higher heat impact and accelerated the burning process. Additionally, when flame inhibition effects provided residual formation of any kind, the residual characteristics tended to deteriorate as a consequence of the physical deformation.
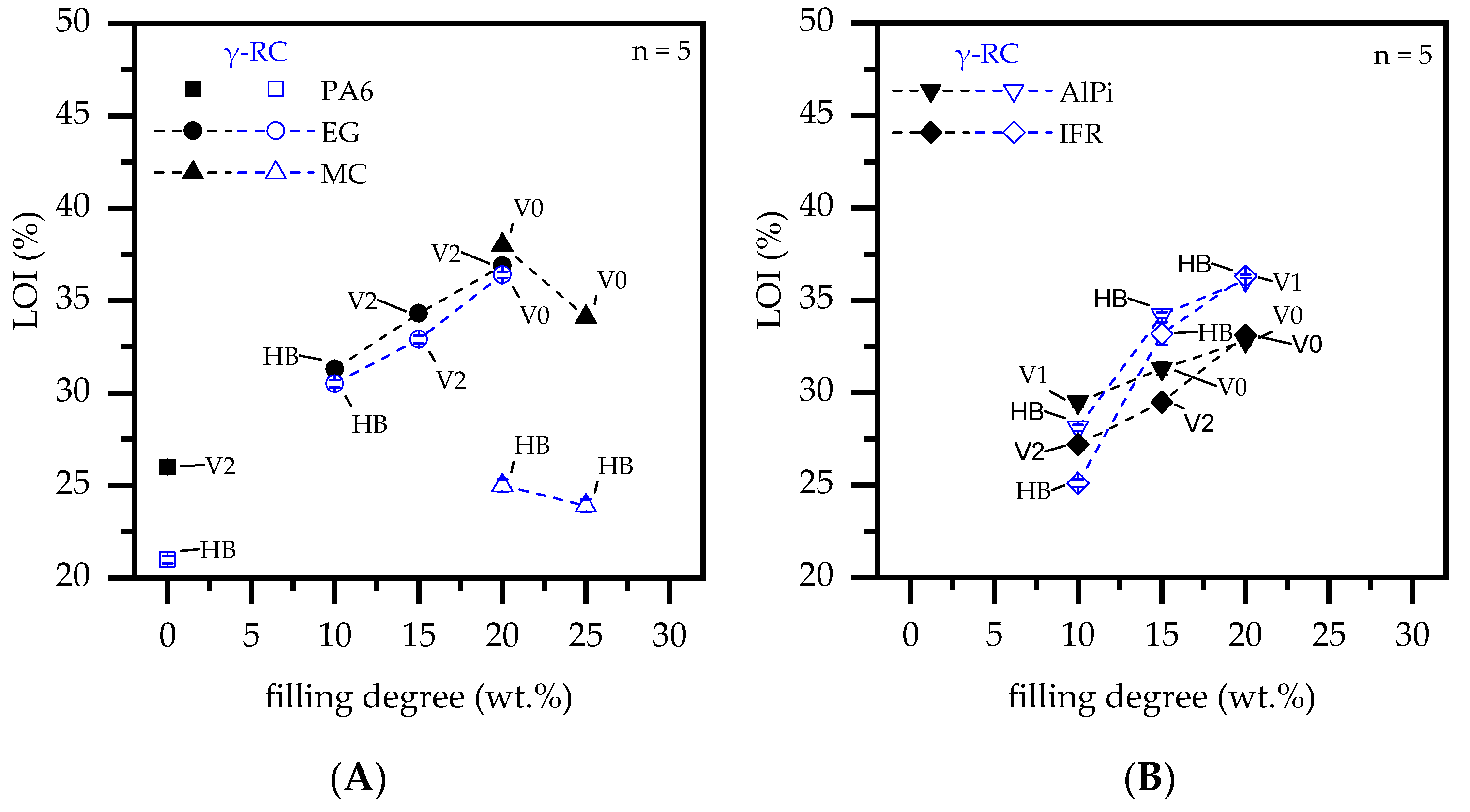
3.5. Fire Testing—LOI and Vertical UL-94
3.6. Residue Analysis—Cone Calorimeter
4. Discussion
- The limited physical mass transport of pyrolysis gasses through the material cross section (e.g., bubbling).
- The limited decomposition depth to near surface areas.
- Anti-dripping
- Physical stability at elevated temperatures; no structural collapse occurred.
- Strong warpage.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bradler, P.R.; Fischer, J.; Wallner, G.M.; Lang, R.W. Characterization of Irradiation Crosslinked Polyamides for Solar Thermal Applications-Basic Thermo-Analytical and Mechanical Properties. Polymers 2018, 10, 969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, E.H.; Kim, S.B.; Choi, Y.-T.; Park, E.-S. Effects of electron beam irradiation on thermal and mechanical properties of nylon 6, nylon 66 and nylon 1212. Res. Rev. Polym. 2016, 7, 1. [Google Scholar]
- Mizera, A.; Manas, M.; Holik, Z.; Manas, D.; Stanek, M.; Cerny, J.; Bednarik, M.; Ovsik, M. Properties of Selected Polymers after Radiation Cross-linking. Int. J. Math. Comput. Simul. 2018, 6, 592–599. [Google Scholar]
- Dadbin, S.; Frounchi, M.; Saeid, M.H.; Gangi, F. Molecular structure and physical properties of E-beam crosslinked low-density polyethylene for wire and cable insulation applications. J. Appl. Polym. Sci. 2002, 86, 1959–1969. [Google Scholar] [CrossRef]
- Manas, D.; Ovsik, M.; Mizera, A.; Manas, M.; Hylova, L.; Bednarik, M.; Stanek, M. The Effect of Irradiation on Mechanical and Thermal Properties of Selected Types of Polymers. Polymers 2018, 10, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bednarik, M.; Manas, D.; Maňas, M.; Mizera, A.; Šenkeřík, V. Mechanical Properties of Irradiated Polyamide under Thermal Stress. DDF 2016, 368, 178–181. [Google Scholar] [CrossRef]
- Rouif, S. Radiation cross-linked plastics: A versatile material solution for packaging, automotive, Electrotechnic and Electronics. Radiat. Phys. Chem. 2004, 71, 527–530. [Google Scholar] [CrossRef]
- Dadbin, S.; Frounchi, M.; Goudarzi, D. Electron beam induced crosslinking of nylon 6 with and without the presence of TAC. Polym. Degrad. Stab. 2005, 89, 436–441. [Google Scholar] [CrossRef]
- Dawes, K.; Glover, L.C.; Vroom, D.A. The Effects of Electron Beam and g-Irradiation on Polymeric Materials. In Physical Properties of Polymers Handbook; Mark, J.E., Ed.; Springer Science + Business Media LLC.: New York, NY, USA, 2007; pp. 867–887. [Google Scholar] [CrossRef]
- Bee, S.-T.; Hassan, A.; Ratnam, C.T.; Tee, T.-T.; Sin, L.T. Investigation of nano-size montmorillonite on electron beam irradiated flame retardant polyethylene and ethylene vinyl acetate blends. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2013, 299, 42–50. [Google Scholar] [CrossRef]
- Liu, H.; Fang, Z.; Peng, M.; Shen, L.; Wang, Y. The effects of irradiation cross-linking on the thermal degradation and flame-retardant properties of the HDPE/EVA/magnesium hydroxide composites. Radiat. Phys. Chem. 2009, 78, 922–926. [Google Scholar] [CrossRef]
- Lee, J.-G.; Jeong, J.-O.; Jeong, S.-I.; Park, J.-S. Radiation-Based Crosslinking Technique for Enhanced Thermal and Mechanical Properties of HDPE/EVA/PU Blends. Polymers 2021, 13, 2832. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Zhang, Z.; Du, Z.; Teng, R.; Wang, Z. A study of the dynamic flammability of radiation cross-linked flame-retardant HDPE/EPDM/silicon-elastomer compound. Radiat. Phys. Chem. 2003, 66, 349–355. [Google Scholar] [CrossRef]
- Jia, S.; Zhang, Z.; Wang, Z.; Zhang, X.; Du, Z. A study of gamma-radiation-crosslinked HDPE/EPDM composites as flame retardants. Polym. Int. 2005, 54, 320–326. [Google Scholar] [CrossRef]
- Herve, C.; Alexus, C.; Carlo, P. Radiation Crosslinking of Halogen-Fre Flame Retardant Polymer. European Patent EP 2305741B1, 3 March 2006. Available online: https://patentimages.storage.googleapis.com/bf/65/0c/58ff40aa706454/EP2305741B1.pdf (accessed on 20 July 2022).
- Balabanovich, A.I.; Levchik, G.F.; Levchik, S.V.; Schnabel, W. Fire retardance in polyamide-6. The effects of red phosphorus and radiation-induced cross-links. Fire Mater. 2001, 25, 179–184. [Google Scholar] [CrossRef]
- Balabanovich, A.I.; Schnabel, W. Fire Retardance in Polyamide-6,6. The Effects of Red Phosphorus and Radiation-Induced Cross-Links. Macromol. Mater. Eng. 2002, 287, 187. [Google Scholar] [CrossRef]
- Balabanovich, A.I.; Zevaco, T.A.; Schnabel, W. Fire Retardance in Poly(butylene terephthalate). The Effects of Red Phosphorus and Radiation-Induced Cross-Links. Macromol. Mater. Eng. 2004, 289, 181–190. [Google Scholar] [CrossRef]
- Elton, S. Reduction of the thermoplastic melt hazard of polyester fabric through the application of a radiation cross-linking technique. Fire Mater. 1998, 22, 19–23. [Google Scholar] [CrossRef]
- Babrauskas, V.; Schnabel, W. Influence of high energy radiation on the thermal stability of PA6. In Fire Retardancy of Polymers. The Use of Intumescence; Bras, M., Camino, G., Bourbigot, S., Delobel, R., Eds.; The Royal Society of Chemistry: Cambridge, UK, 1998; pp. 236–251. [Google Scholar]
- Hamdani, S.; Longuet, C.; Perrin, D.; Lopez-cuesta, J.-M.; Ganachaud, F. Flame retardancy of silicone-based materials. Polym. Degrad. Stab. 2009, 94, 465–495. [Google Scholar] [CrossRef]
- Wang, B.; Tai, Q.; Nie, S.; Zhou, K.; Tang, Q.; Hu, Y.; Song, L. Electron Beam Irradiation Cross Linking of Halogen-Free Flame-Retardant Ethylene Vinyl Acetate (EVA) Copolymer by Silica Gel Microencapsulated Ammonium Polyphosphate and Char-Forming Agent. Ind. Eng. Chem. Res. 2011, 50, 5596–5605. [Google Scholar] [CrossRef]
- Gilman, J.W.; Kashiwagi, T. Chapter 10—Silicon-Based Flame Retardants. In Fire Retardancy of Polymeric Materials; Grand, A.F., Wilkie, C.A., Eds.; Dekker: New York, NY, USA, 2000. [Google Scholar]
- Sonnier, R.; Caro-Bretelle, A.-S.; Dumazert, L.; Longerey, M.; Otazaghine, B. Influence of radiation-crosslinking on flame retarded polymer materials—How crosslinking disrupts the barrier effect. Radiat. Phys. Chem. 2015, 106, 278–288. [Google Scholar] [CrossRef]
- Nyden, M.R.; Forney, G.P.; Brown, J.E. Molecular modeling of polymer flammability: Application to the design of flame-resistant polyethylene. Macromolecules 1992, 25, 1658–1666. [Google Scholar] [CrossRef]
- Nyden, M.R.; Brown, J.E.; Lomakin, S.M. Molecular Dynamics Modeling of Polymer Flammability. MRS Proc. 1992, 278, 47. [Google Scholar] [CrossRef]
- Vyazovkin, S. Evaluation of activation energy of thermally stimulated solid-state reactions under arbitrary variation of temperature. J. Comput. Chem. 1997, 18, 393–402. [Google Scholar] [CrossRef]
- Ozawa, T.; Koto, T. A simple method for estimating activation energy from derivative thermoanalytical curves and its application to thermal shrinkage of polycarbonate. J. Therm. Anal. 1991, 37, 1299–1307. [Google Scholar] [CrossRef]
- Pramanik, N.K.; Haldar, R.S.; Bhardwaj, Y.K.; Sabharwal, S.; Niyogi, U.K.; Khandal, R.K. Radiation processing of Nylon 6 by e-beam for improved properties and performance. Radiat. Phys. Chem. 2009, 78, 199–205. [Google Scholar] [CrossRef]
- Lehrle, R.S.; Parsons, I.W.; Rollinson, M. Thermal degradation mechanisms of nylon 6 deduced from kinetic studies by pyrolysis-g.c. Polym. Degrad. Stab. 2000, 67, 21–33. [Google Scholar] [CrossRef]
- Ohtani, H.; Nagaya, T.; Sugimura, Y.; Tsuge, S. Studies on thermal degradation of aliphatic polyamides by pyrolysis-glass capillary chromatography. J. Anal. Appl. Pyrolysis 1982, 4, 117–131. [Google Scholar] [CrossRef]
- Nagase, Y.; Komatsu, T.; Sumiya, Y.; Ikeda, K.; Sekine, Y. Thermal Degradation of Polyamides. Nippon Kagaku Kaishi 1979, 11, 1560–1568. [Google Scholar] [CrossRef]
- Holland, B.J.; Hay, J.N. Thermal degradation of nylon polymers. Polym. Int. 2000, 49, 943–948. [Google Scholar] [CrossRef]
- Michal, J.; Mitera, J.; Kubat, J. Major pyrolysis and thermoxidative products from certain polyamides. Fire Mater. 1981, 5, 1–5. [Google Scholar] [CrossRef]
- Price, D.; Horrocks, A.R. Polymer Degradation and the Matching of FR Chemistry to Degradation. In Fire Retardancy of Polymeric Materials; Wilkie, C.A., Morgan, A.B., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 15–43. [Google Scholar]
- Levchik, S.V.; Weil, E.D.; Lewin, M. Thermal decomposition of aliphatic nylons. Polym. Int. 1999, 48, 532–557. [Google Scholar] [CrossRef]
- Bhuiyan, A.L. Some aspects of the thermal stability action of the structure in aliphatic polyamides and polyacrylamides. Polymer 1984, 25, 1699–1710. [Google Scholar] [CrossRef]
- Herrera, M.; Matuschek, G.; Kettrup, A. Comparative studies of polymers using TA–MS, macro TA–MS and TA–FTIR. Thermochim. Acta 2000, 361, 69–76. [Google Scholar] [CrossRef]
- Herrera, M.; Wilhelm, M.; Matuschek, G.; Kettrup, A. Thermoanalytical and pyrolysis studies of nitrogen containing polymers. J. Anal. Appl. Pyrolysis 2001, 58–59, 173–188. [Google Scholar] [CrossRef]
- Herrera, M. Dissertation—Untersuchung Flüchtiger Verbindungen bei der Thermischen Zersetzung von Stickstoffhaltigen Polymerwerkstoffen. Ph.D. Thesis, Technische Universität München, Munich, Germany, 2001. [Google Scholar]
- Zimmerman, J. Polyamides. In The Radiation Chemistry of Macromolecules; Dole, M., Ed.; Elsevier Science: Burlington, NJ, USA, 1973; Volume II, pp. 121–135. [Google Scholar]
- Balabanovich, A.I.; Levchik, S.V.; Levchik, G.F.; Schnabel, W.; Wilkie, C.A. Thermal decomposition and combustion of γ-irradiated polyamide 6 containing phosphorus oxynitride or phospham. Polym. Degrad. Stab. 1999, 64, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Bockhorn, H.; Hornung, A.; Hornung, U.; Weichmann, J. Kinetic study on the non-catalysed and catalysed degradation of polyamide 6 with isothermal and dynamic methods. Thermochim. Acta 1999, 337, 97–110. [Google Scholar] [CrossRef]
- Dole, M. (Ed.) The Radiation Chemistry of Macromolecules; Elsevier Science: Burlington, NJ, USA, 1973; Volume II. [Google Scholar]
- Zhu, S.; Shi, M.; Zhu, M. Effects of electron-beam irradiation crosslinking on PA6 fibers. Fibers Polym. 2013, 14, 525–529. [Google Scholar] [CrossRef]
- Isbasar, C.; Hacaloglu, J. Investigation of thermal degradation characteristics of polyamide-6 containing melamine or melamine cyanurate via direct pyrolysis mass spectrometry. J. Anal. Appl. Pyrolysis 2012, 98, 221–230. [Google Scholar] [CrossRef]
- Shimasaki, C.; Watanabe, N.; Fukushima, K.; Rengakuji, S.; Nakamura, Y.; Ono, S.; Yoshimura, T.; Morita, H.; Takakura, M.; Shiroishi, A. Effect of the fire-retardant, melamine, on the combustion and the thermal decomposition of polyamide-6, polypropylene and low-density polyethylene. Polym. Degrad. Stab. 1997, 58, 171–180. [Google Scholar] [CrossRef]
- Doğan, M.; Yılmaz, A.; Bayramlı, E. Effect of boron containing materials on flammability and thermal degradation of polyamide-6 composites containing melamine cyanurate. Polym. Adv. Technol. 2011, 22, 560–566. [Google Scholar] [CrossRef]
- Herrera, M.; Matuschek, G.; Kettrup, A. Main products and kinetics of the thermal degradation of polyamides. Chemosphere 2001, 42, 601–607. [Google Scholar] [CrossRef]
- Tomiak, F.; Rathberger, K.; Schöffel, A.; Drummer, D. Expandable Graphite for Flame Retardant PA6 Applications. Polymers 2021, 13, 2733. [Google Scholar] [CrossRef] [PubMed]
- Tomiak, F.; Schoeffel, A.; Rathberger, K.; Drummer, D. Expandable Graphite, Aluminum Diethylphospinate and Melamine Polyphosphate as Flame Retarding System in Glass Fiber-Reinforced PA6. Polymers 2022, 14, 1263. [Google Scholar] [CrossRef] [PubMed]
- Tomiak, F.; Schoeffel, A.; Rathberger, K.; Drummer, D. A Synergistic Flame Retardant System Based on Expandable Graphite, Aluminum (Diethyl-)Polyphospinate and Melamine Polyphosphate for Polyamide 6. Polymers 2021, 13, 2712. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, H.; Luo, Z.; Yang, B.; Guo, S.; Yu, J. Synergistic effects of expandable graphite with magnesium hydroxide on the flame retardancy and thermal properties of polypropylene. Polym. Eng. Sci. 2007, 47, 1756–1760. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, Y.; Zhang, L.; Wang, H. Synergistic effect of expandable graphite and intumescent flame retardants on the flame retardancy and thermal stability of polypropylene. J. Mater. Sci. 2016, 51, 5857–5871. [Google Scholar] [CrossRef]
- Schartel, B.; Braun, U.; Schwarz, U.; Reinemann, S. Fire retardancy of polypropylene/flax blends. Polymer 2003, 44, 6241–6250. [Google Scholar] [CrossRef]
- Dittrich, B.; Wartig, K.-A.; Hofmann, D.; Mülhaupt, R.; Schartel, B. Carbon black, multiwall carbon nanotubes, expanded graphite and functionalized graphene flame retarded polypropylene nanocomposites. Polym. Adv. Technol. 2013, 24, 916–926. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, M.; Ning, X.; Chen, X.; Sun, Z.; Ma, Y.; Qin, J.; Yu, J.; Zhang, Z.; Yang, L.; et al. The Thermal Stability and Flammability of Expandable Graphite-Filled Polypropylene/Thermoplastic Polyurethane Blends. J. Macromol. Sci. Part B 2014, 53, 756–768. [Google Scholar] [CrossRef]
- Mattausch, H.; Laske, S.; Hohenwarter, D.; Holzer, C. The effect of mineral fillers on the rheological, mechanical and thermal properties of halogen-free flame-retardant polypropylene/expandable graphite compounds. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2015. [Google Scholar] [CrossRef] [Green Version]
- Dittrich, B.; Wartig, K.-A.; Hofmann, D.; Mülhaupt, R.; Schartel, B. The influence of layered, spherical, and tubular carbon nanomaterials’ concentration on the flame retardancy of polypropylene. Polym. Compos. 2015, 36, 1230–1241. [Google Scholar] [CrossRef]
- Sun, Z.; Ma, Y.; Xu, Y.; Chen, X.; Chen, M.; Yu, J.; Hu, S.; Zhang, Z. Effect of the particle size of expandable graphite on the thermal stability, flammability, and mechanical properties of high-density polyethylene/ethylene vinyl-acetate/expandable graphite composites. Polym. Eng. Sci. 2014, 54, 1162–1169. [Google Scholar] [CrossRef]
- Wang, H.; Cao, J.; Luo, F.; Cao, C.; Qian, Q.; Huang, B.; Xiao, L.; Chen, Q. Hugely enhanced flame retardancy and smoke suppression properties of UHMWPE composites with silicone-coated expandable graphite. Polym. Adv. Technol. 2019, 30, 1673–1683. [Google Scholar] [CrossRef]
- Tang, M.; Qi, F.; Chen, M.; Sun, Z.; Xu, Y.; Chen, X.; Zhang, Z.; Shen, R. Synergistic effects of ammonium polyphosphate and red phosphorus with expandable graphite on flammability and thermal properties of HDPE/EVA blends. Polym. Adv. Technol. 2016, 27, 52–60. [Google Scholar] [CrossRef]
- Focke, W.W.; Kruger, H.J.; Mhike, W.; Taute, A.; Roberson, A.; Ofosu, O. Polyethylene flame retarded with expandable graphite and a novel intumescent additive. J. Appl. Polym. Sci. 2014, 131, 13. [Google Scholar] [CrossRef] [Green Version]
- Ge, L.-L.; Duan, H.-J.; Zhang, X.-G.; Chen, C.; Tang, J.-H.; Li, Z.-M. Synergistic effect of ammonium polyphosphate and expandable graphite on flame-retardant properties of acrylonitrile-butadiene-styrene. J. Appl. Polym. Sci. 2012, 126, 1337–1343. [Google Scholar] [CrossRef]
- Li, Z.; Qu, B. Flammability characterization and synergistic effects of expandable graphite with magnesium hydroxide in halogen-free flame-retardant EVA blends. Polym. Degrad. Stab. 2003, 81, 401–408. [Google Scholar] [CrossRef]
- Pang, X.-Y.; Tian, Y.; Shi, X.-Z. Synergism between hydrotalcite and silicate-modified expandable graphite on ethylene vinyl acetate copolymer combustion behavior. J. Appl. Polym. Sci. 2017, 134, 863. [Google Scholar] [CrossRef]
- Alongi, J.; Frache, A.; Gioffredi, E. Fire-retardant poly(ethylene terephthalate) by combination of expandable graphite and layered clays for plastics and textiles. Fire Mater. 2011, 35, 383–396. [Google Scholar] [CrossRef]
- Sover, A.; Marzynkevitsch, S.; Munack, B. Processing Conditions of Expandable Graphite in PP andPA Matrix and their Performance. Mater. Plast. 2018, 55, 507–510. [Google Scholar] [CrossRef]
- Wang, G.; Bai, S. Synergistic effect of expandable graphite and melamine phosphate on flame-retardant polystyrene. J. Appl. Polym. Sci. 2017, 134, 45474. [Google Scholar] [CrossRef]
- Focke, W.W.; Muiambo, H.; Mhike, W.; Kruger, H.J.; Ofosu, O. Flexible PVC flame retarded with expandable graphite. Polym. Degrad. Stab. 2014, 100, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Focke, W.W.; Badenhorst, H.; Mhike, W.; Kruger, H.J.; Lombaard, D. Characterization of commercial expandable graphite fire retardants. Thermochim. Acta 2014, 584, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Fang, K.; Chen, Y.F.; Zhang, S.C.; Sun, H.R.; Wang, G.H.; Sun, X.K. The Effect of Particle Size of Expandable Graphite on the Properties of an Expandable Thermal Insulation Material. KEM 2016, 697, 441–444. [Google Scholar] [CrossRef]
- Camino, G.; Duquesne, S.; Delobel, R.; Eling, B.; Lindsay, C.; Roels, T. Mechanism of Expandable Graphite Fire Retardant Action in Polyurethanes. In Fire and Polymers. Materials and Solutions for Hazard Prevention, Proceedings of the 220th National Meeting of the American Chemical Society, Washington, DC, USA, 20–24 August 2000; Nelson, G.L., Ed.; ACS Symposium Series 797; American Chemical Society: Washington, DC, USA, 2001; pp. 90–109. [Google Scholar] [CrossRef]
- Braun, U.; Bahr, H.; Schartel, B. Fire retardancy effect of aluminium phosphinate and melamine polyphosphate in glass fibre reinforced polyamide 6. e-Polymers 2010, 10, 1. [Google Scholar] [CrossRef]
- Braun, U.; Schartel, B.; Fichera, M.A.; Jäger, C. Flame retardancy mechanisms of aluminium phosphinate in combination with melamine polyphosphate and zinc borate in glass-fibre reinforced polyamide 6,6. Polym. Degrad. Stab. 2007, 92, 1528–1545. [Google Scholar] [CrossRef]
- Seefeldt, H.; Duemichen, E.; Braun, U. Flame retardancy of glass fiber reinforced high temperature polyamide by use of aluminum diethylphosphinate: Thermal and thermo-oxidative effects. Polym. Int. 2013, 62, 1608–1616. [Google Scholar] [CrossRef]
- Li, M.; Zhong, Y.; Wang, Z.; Fischer, A.; Ranft, F.; Drummer, D.; Wu, W. Flame retarding mechanism of Polyamide 6 with phosphorus-nitrogen flame retardant and DOPO derivatives. J. Appl. Polym. Sci. 2016, 133, 6. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C.D.; Pfaendner, R. Polyamide 6 Filled with Melamine Cyanurate and Layered Silicates: Evaluation of Flame Retardancy and Physical Properties. Macromol. Mater. Eng. 2008, 293, 740–751. [Google Scholar] [CrossRef]
- Tomiak, F.; Schneider, K.; Schoeffel, A.; Rathberger, K.; Drummer, D. Expandable Graphite as a Multifunctional Flame-Retarding Additive for Highly Filled Thermal Conductive Polymer Formulations. Polymers 2022, 14, 1613. [Google Scholar] [CrossRef]
- Batistella, M.A.; Sonnier, R.; Otazaghine, B.; Petter, C.O.; Lopez-Cuesta, J.-M. Interactions between kaolinite and phosphinate-based flame retardant in Polyamide 6. Appl. Clay Sci. 2018, 157, 248–256. [Google Scholar] [CrossRef]
- Witkowski, A.; Stec, A.A.; Hull, T.R. Thermal Decomposition of Polymeric Materials. In SFPE Handbook of Fire Protection Engineering; Hurley, M.J., Gottuk, D.T., Hall, J.R., Jr., Harada, K., Kuligowski, E.D., Puchovsky, M., Torero, J.L., Watts, J.M., Jr., Wieczorek, C.J., Eds.; Springer: New York, NY, USA, 2016. [Google Scholar]
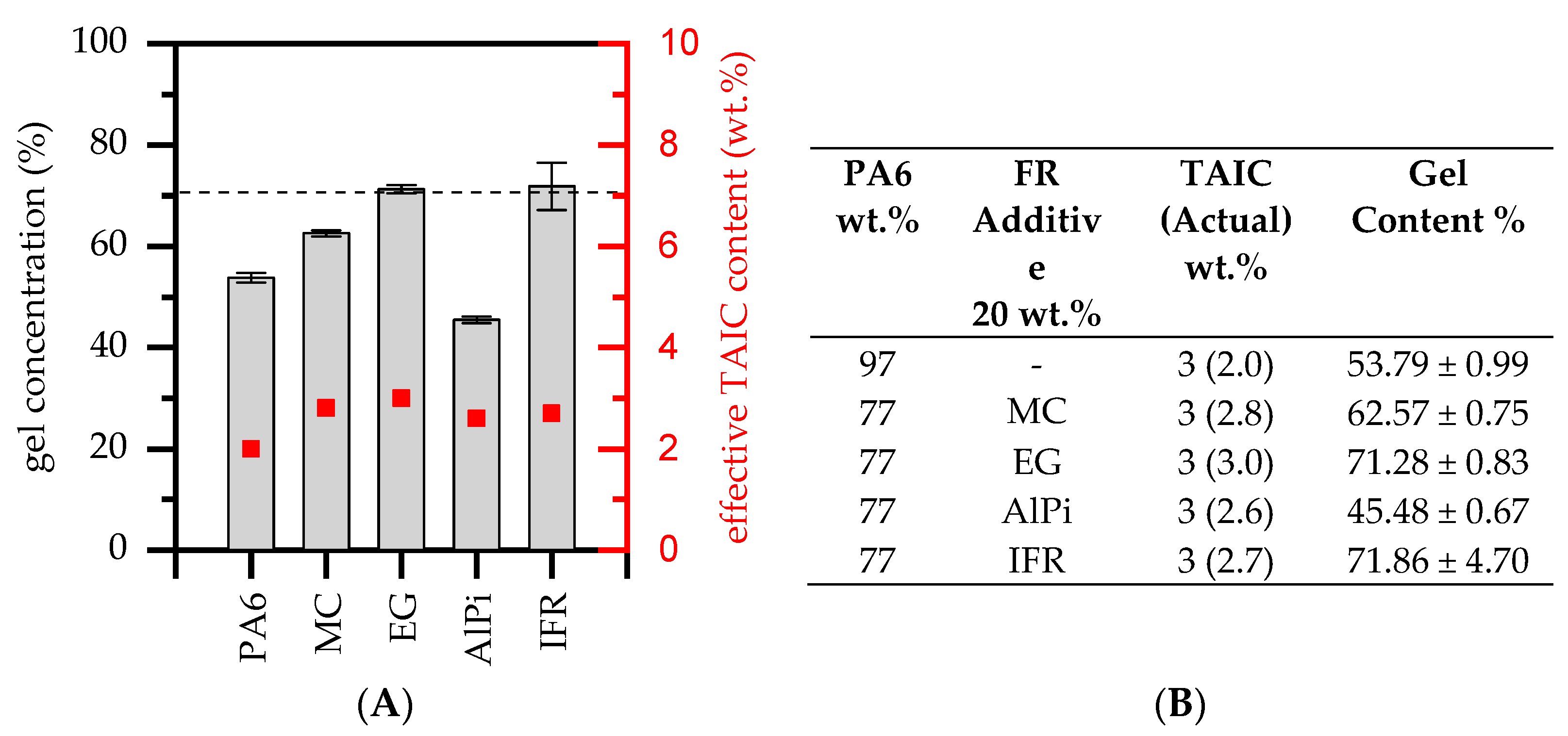
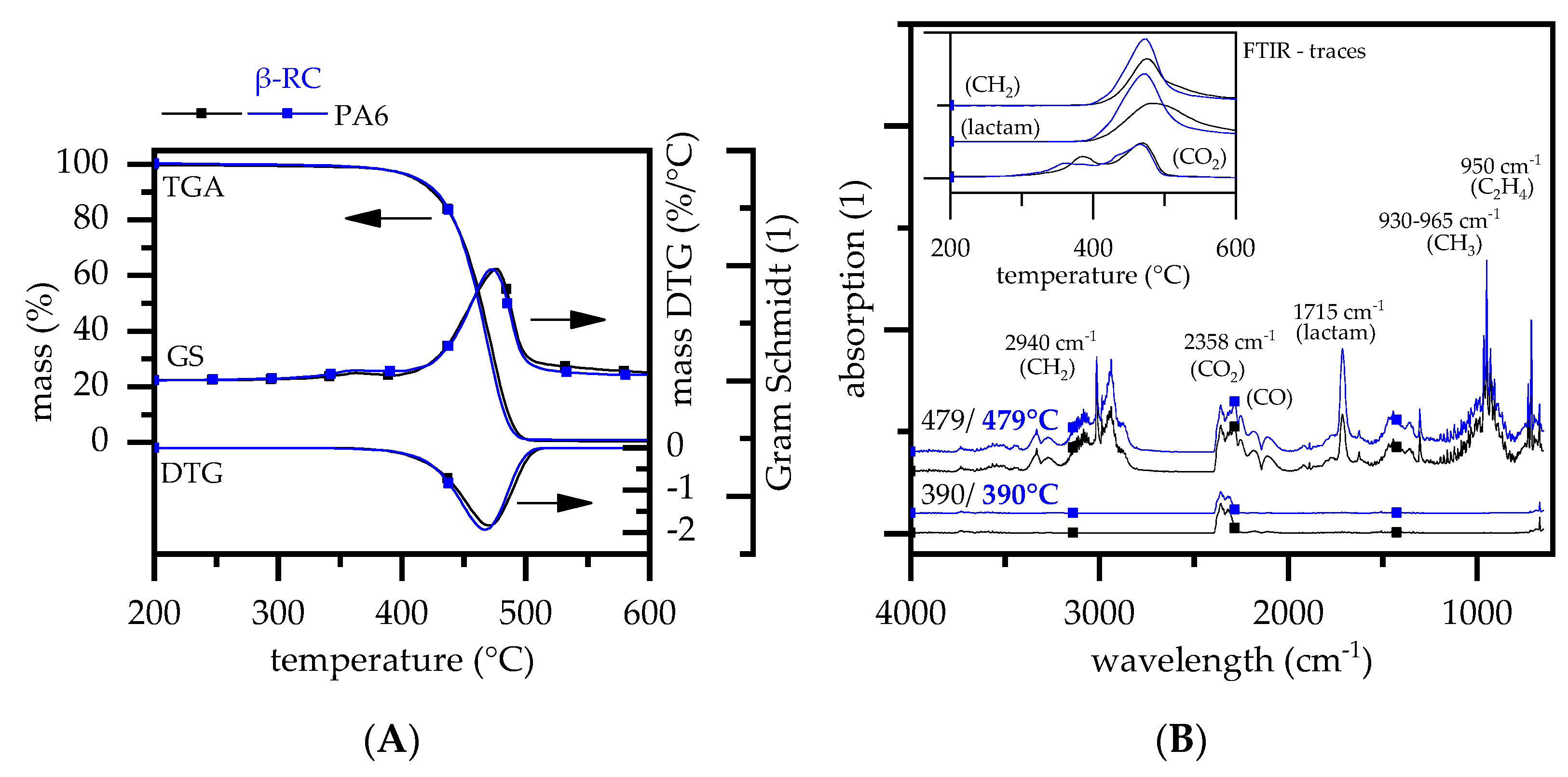

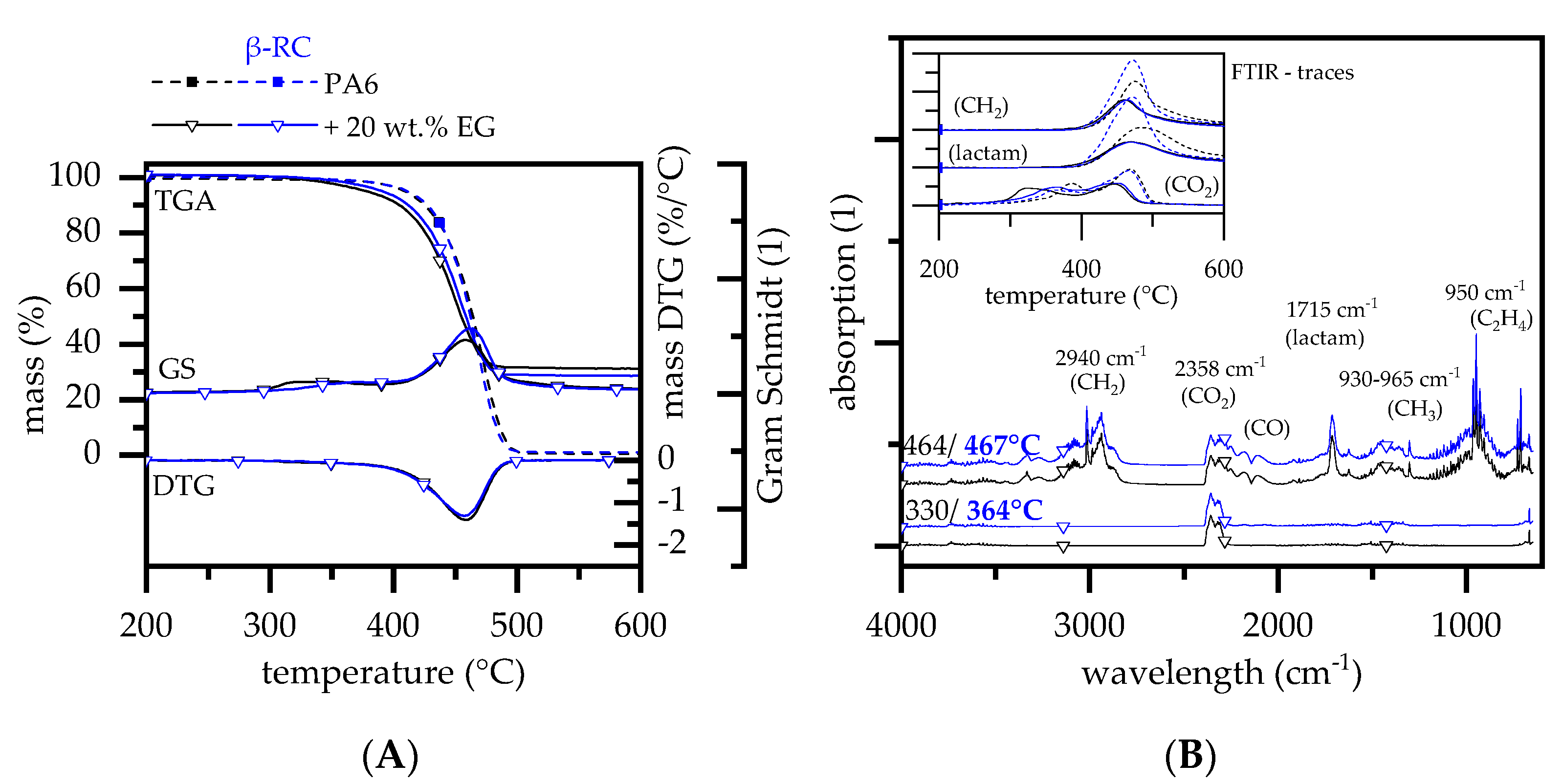



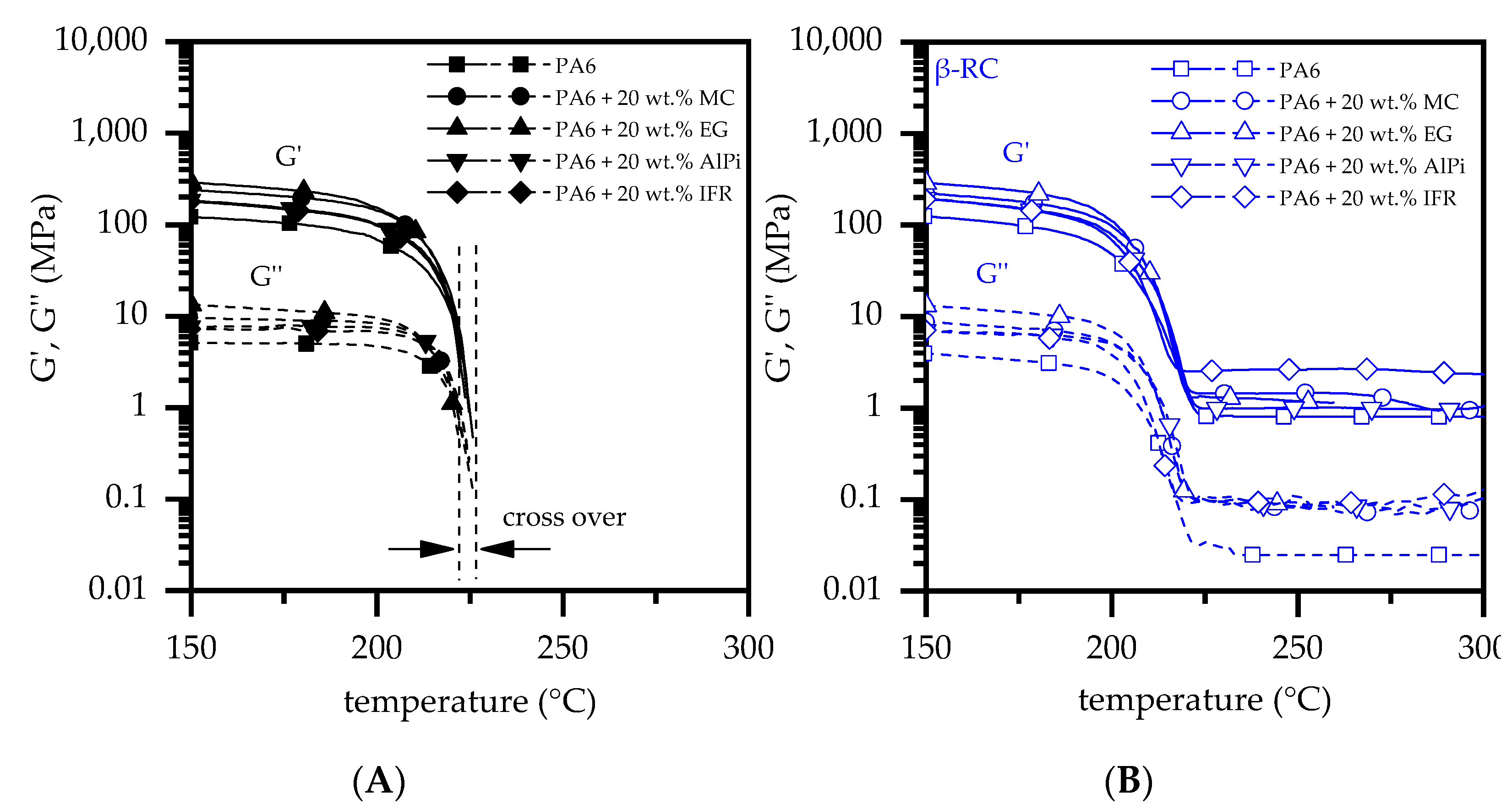



| Materials | Abr. | Filling Degree (wt.%) |
|---|---|---|
| Polyamide 6 (B27E) | PA6 | matrix |
| melamine cyanurat (MC50) | MC | 20, 25 |
| expandable graphite (GHL PX 95 HT) | EG | 10, 15, 20 |
| Aluminum (diethyl-)polyphosphinat (Exolit OP 1230) | AlPi | 10, 15, 20 |
| intumescent combination (Exolit OP 1314) | IFR | 10, 15, 20 |
| Triallyl Isocyanurate TAIC (TAICROS PA6 60); 40% TAIC/60% PA6 | TAIC | 3 |
| PA6 wt.% | MC wt.% | EG wt.% | AlPi wt.% | IFR wt.% | TAIC wt.% (RC) | T99% Onset °C | T97% Onset °C | T90% Onset °C | DTG-Peak °C/min | Residue % | Vyazovkin [27] kJ/mol | Ozawa [28] kJ/mol |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 351 ± 2 | 399 ± 1 | 425 ± 1 | 479 ± 2 | 1.7 ± 0.0 | 195 | 218 | |||||
| 97 | 3 | 366 ± 2 | 400 ± 1 | 428 ± 2 | 468 ± 2 | 0.9 ± 0.0 | 187 | 210 | ||||
| 80 | 20 | 320 ± 1 | 335 ± 2 | 355 ± 2 | 357 ± 2; 466 ± 2 | 1.0 ± 0.0 | 175, 148 | 194, 170 | ||||
| 77 | 20 | 3 | 320 ± 2 | 335 ± 2 | 355 ± 2 | 357 ± 2; 466 ± 2 | 1.1 ± 0.4 | 201, 155 | 221, 177 | |||
| 80 | 20 | 333 ± 2 | 359 ± 2 | 404 ± 1 | 460 ± 2 | 23.4 ± 1.0 | 183 | 206 | ||||
| 77 | 20 | 3 | 350 ± 1 | 376 ± 1 | 412 ± 2 | 460 ± 2 | 26.3 ± 2.0 | 193 | 216 | |||
| 80 | 20 | 357 ± 2 | 390 ± 2 | 419 ± 2 | 447 ± 1 | 3.8 ± 0.3 | 200 | 222 | ||||
| 77 | 20 | 3 | 376 ± 2 | 397 ± 2 | 419 ± 2 | 454 ± 2 | 2.5 ± 0.1 | 212 | 234 | |||
| 80 | 20 | 369 ± 1 | 385 ± 2 | 409 ± 1 | 445 ± 1 | 5.5 ± 0.5 | 207 | 230 | ||||
| 77 | 20 | 3 | 369 ± 2 | 385 ± 2 | 409 ± 2 | 446 ± 2 | 5.7 ± 0.3 | 204 | 227 |
| PA6 wt.% | MC50 wt.% | EG wt.% | Exo 1230 wt.% | Exo 1314 wt.% | TAIC wt.% | tign s | pHRR kW/m2 | THE MJ/m2 | TSP m2 |
|---|---|---|---|---|---|---|---|---|---|
| 100 | 91 ± 4 | 778 ± 45 | 134 ± 3 | 7.4 ± 0.2 | |||||
| 97 | 3 | 91 ± 1 | 669 ± 12 | 128 ± 2 | 8.0 ± 0.1 | ||||
| 80 | 20 | 114 ± 3 | 802 ± 141 | 112 ± 5 | 6.6 ± 0.5 | ||||
| 75 | 25 | 116 ± 6 | 836 ± 67 | 119 ± 4 | 7.2 ± 0.5 | ||||
| 77 | 20 | 3 | 82 ± 9 | 572 ± 27 | 112 ± 7 | 7.1 ± 0.3 | |||
| 72 | 25 | 3 | 88 ± 5 | 549 ± 40 | 119 ± 3 | 6.8 ± 0.8 | |||
| 90 | 10 | 71 ± 1 | 359 ± 23 | 103 ± 2 | 7.5 ± 0.1 | ||||
| 85 | 15 | 72 ± 3 | 167 ± 29 | 26 ± 2 | 2.2 ± 1.3 | ||||
| 80 | 20 | 77 ± 1 | 122 ± 3 | 24 ± 2 | 1.6 ± 0.2 | ||||
| 87 | 10 | 3 | 79 ± 2 | 211 ± 13 | 74 ± 5 | 3.3 ± 0.5 | |||
| 82 | 15 | 3 | 85 ± 3 | 195 ± 20 | 62 ± 3 | 2.5 ± 0.1 | |||
| 77 | 20 | 3 | 90 ± 2 | 191 ± 21 | 48 ± 0 | 1.9 ± 0.3 | |||
| 90 | 10 | 54 ± 4 | 587 ± 29 | 119 ± 1 | 23.5 ± 0.1 | ||||
| 85 | 15 | 54 ± 1 | 531 ± 45 | 111 ± 5 | 26.5 ± 1.2 | ||||
| 80 | 20 | 53 ± 2 | 532 ± 9 | 109 ± 2 | 26.6 ± 1.1 | ||||
| 87 | 10 | 3 | 86 ± 4 | 482 ± 8 | 104 ± 1 | 23.6 ± 0.1 | |||
| 82 | 15 | 3 | 85 ± 8 | 518 ± 26 | 96 ± 1 | 24.8 ± 0.9 | |||
| 77 | 20 | 3 | 77 ± 5 | 540 ± 25 | 99 ± 3 | 26.3 ± 0.6 | |||
| 90 | 10 | 58 ± 2 | 287 ± 8 | 94 ± 7 | 22.8 ± 0.7 | ||||
| 85 | 15 | 50 ± 1 | 276 ± 10 | 98 ± 5 | 24.0 ± 0.6 | ||||
| 80 | 20 | 52 ± 2 | 250 ± 20 | 92 ± 5 | 21.6 ± 1.3 | ||||
| 87 | 10 | 3 | 77 ± 2 | 430 ± 54 | 99 ± 8 | 22.6 ± 0.5 | |||
| 82 | 15 | 3 | 75 ± 3 | 370 ± 20 | 94 ± 2 | 22.9 ± 0.4 | |||
| 77 | 20 | 3 | 77 ± 5 | 335 ± 24 | 84 ± 11 | 22.8 ± 0.7 |
| Material | β-RC | LOI | UL-94 | t1 + t2 | Burn Dripping |
|---|---|---|---|---|---|
| PA6 | no | 26 | V2 | 3 s | yes |
| yes | 21 | HB | - | no | |
| PA6 + 20, 25 wt.% MC | no | 38, 34 | V0, V0 | 3 s, 6 s | no, no |
| yes | 25, 24 | HB, HB | -, - | no, no | |
| PA6 + 10, 15, 20 wt.% EG | no | 31, 34, 37 | HB, V2, V2 | -, 8 s, 8 s | yes, yes, yes |
| yes | 30, 33, 36 | HB, V2, V0 | -, 2 s, 2 s | yes, yes, no | |
| PA6 + 10, 15, 20 wt.% AlPi | no | 30, 31, 33 | V1, V0, V0 | 14 s, 4s, 3 s | no, no, no |
| yes | 28, 34, 36 | HB, HB, HB | -, -, - | no, no, no | |
| PA6 + 10, 15, 20 wt.% IFR | no | 27, 30, 33 | V2, V2, V0 | 11s, 8s, 6 s | yes, yes, no |
| yes | 25, 33, 36 | HB, HB, V1 | -, -, 14 s | yes, yes, no |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomiak, F.; Drummer, D. The Impact of β-Radiation Crosslinking on Flammability Properties of PA6 Modified by Commercially Available Flame-Retardant Additives. Polymers 2022, 14, 3168. https://doi.org/10.3390/polym14153168
Tomiak F, Drummer D. The Impact of β-Radiation Crosslinking on Flammability Properties of PA6 Modified by Commercially Available Flame-Retardant Additives. Polymers. 2022; 14(15):3168. https://doi.org/10.3390/polym14153168
Chicago/Turabian StyleTomiak, Florian, and Dietmar Drummer. 2022. "The Impact of β-Radiation Crosslinking on Flammability Properties of PA6 Modified by Commercially Available Flame-Retardant Additives" Polymers 14, no. 15: 3168. https://doi.org/10.3390/polym14153168
APA StyleTomiak, F., & Drummer, D. (2022). The Impact of β-Radiation Crosslinking on Flammability Properties of PA6 Modified by Commercially Available Flame-Retardant Additives. Polymers, 14(15), 3168. https://doi.org/10.3390/polym14153168







