A High-Energy-Density Magnesium-Air Battery with Nanostructured Polymeric Electrodes
Abstract
:1. Introduction
2. Experimental Details
2.1. Manufacturing of Electrode for Cathodic Compartment
2.2. Manufacturing of the Electrode for Anodic Compartment
2.3. Assembling of Cathodic Electrodes
2.4. Assembling of Anodic Electrodes
2.5. MAB Battery Electrochemical Testing in an Advanced Configuration
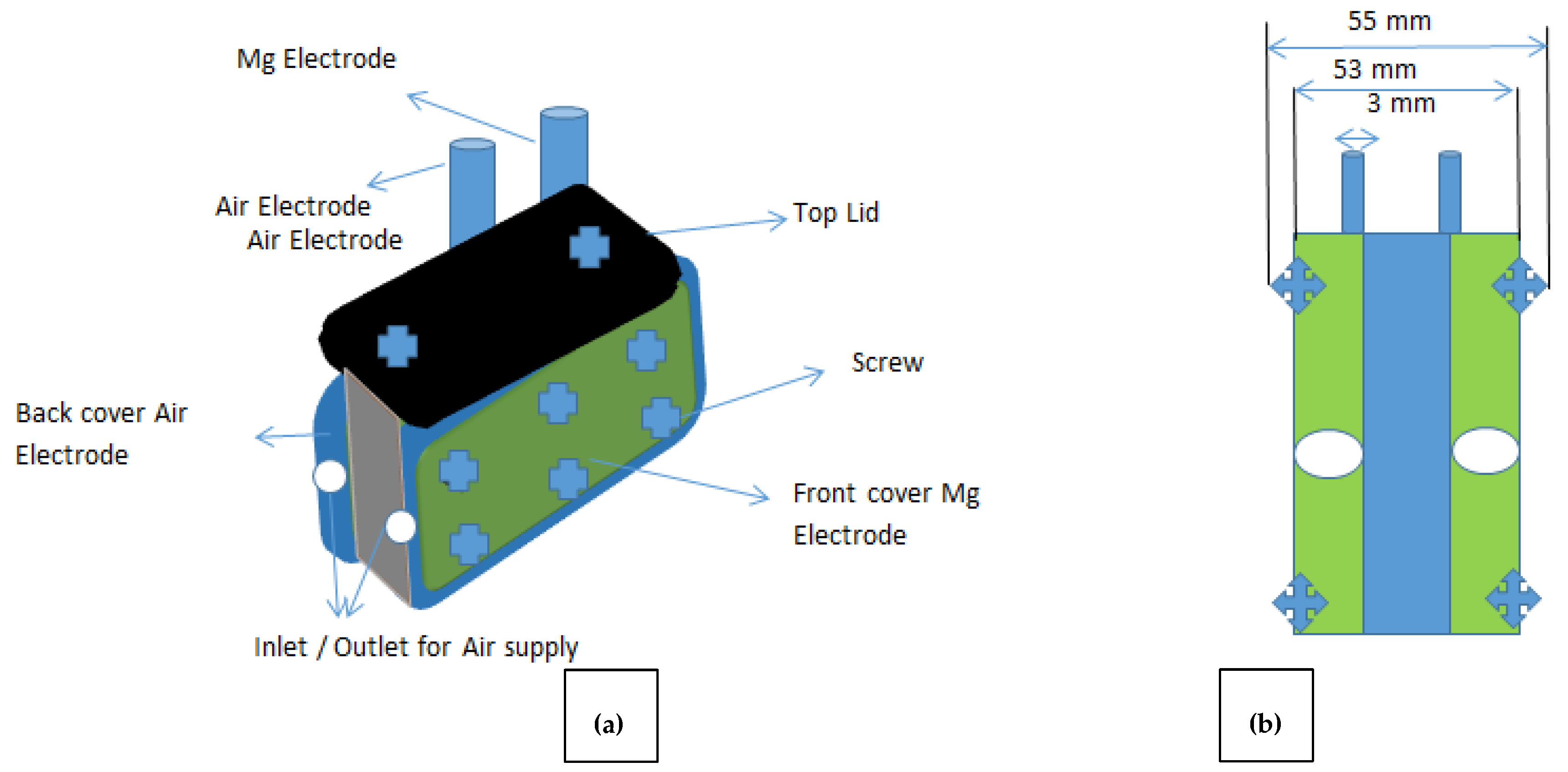
2.6. Blueprint and Assembling of MAB
3. Results and Discussion
3.1. Anodic Electrode Characterization
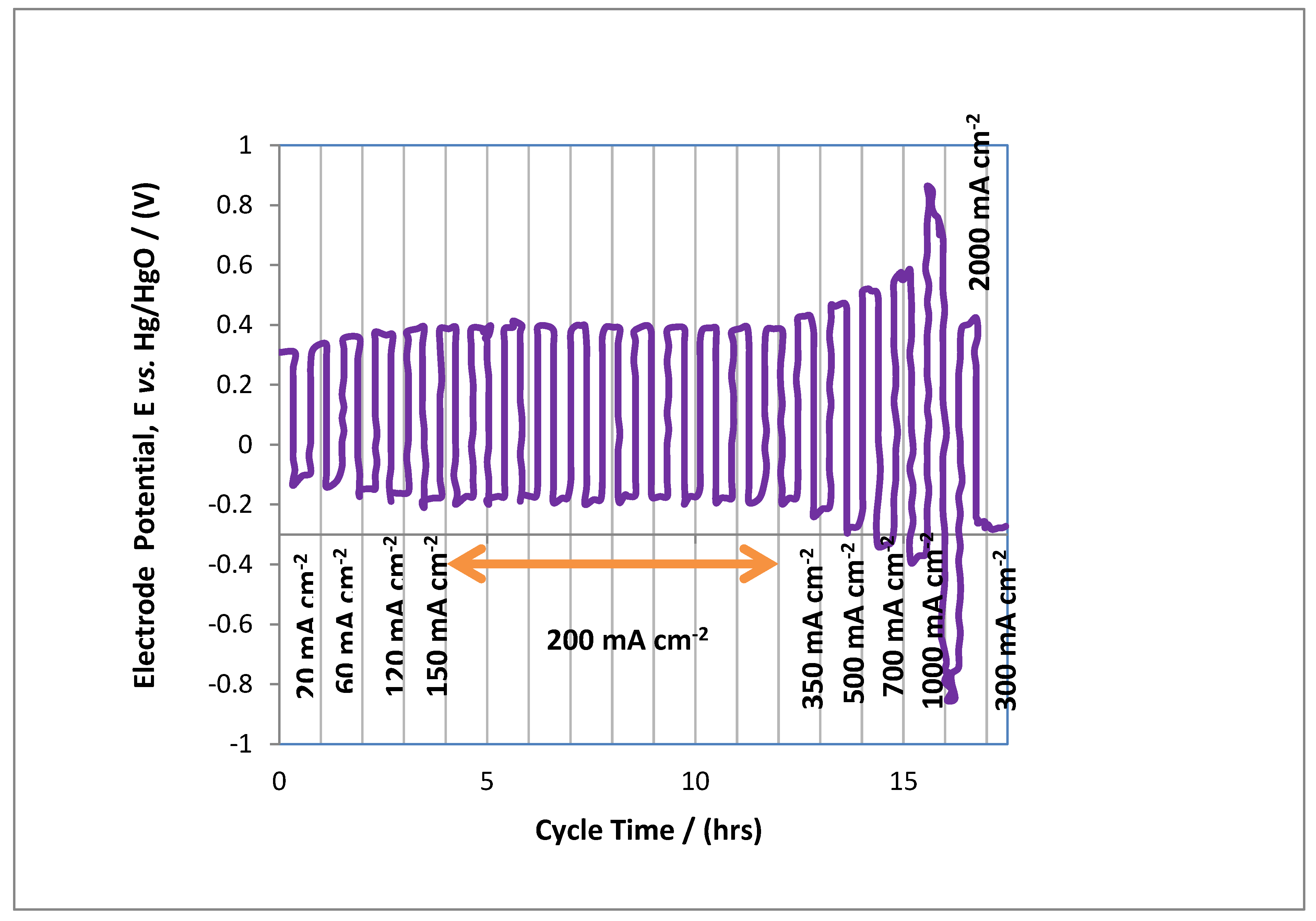
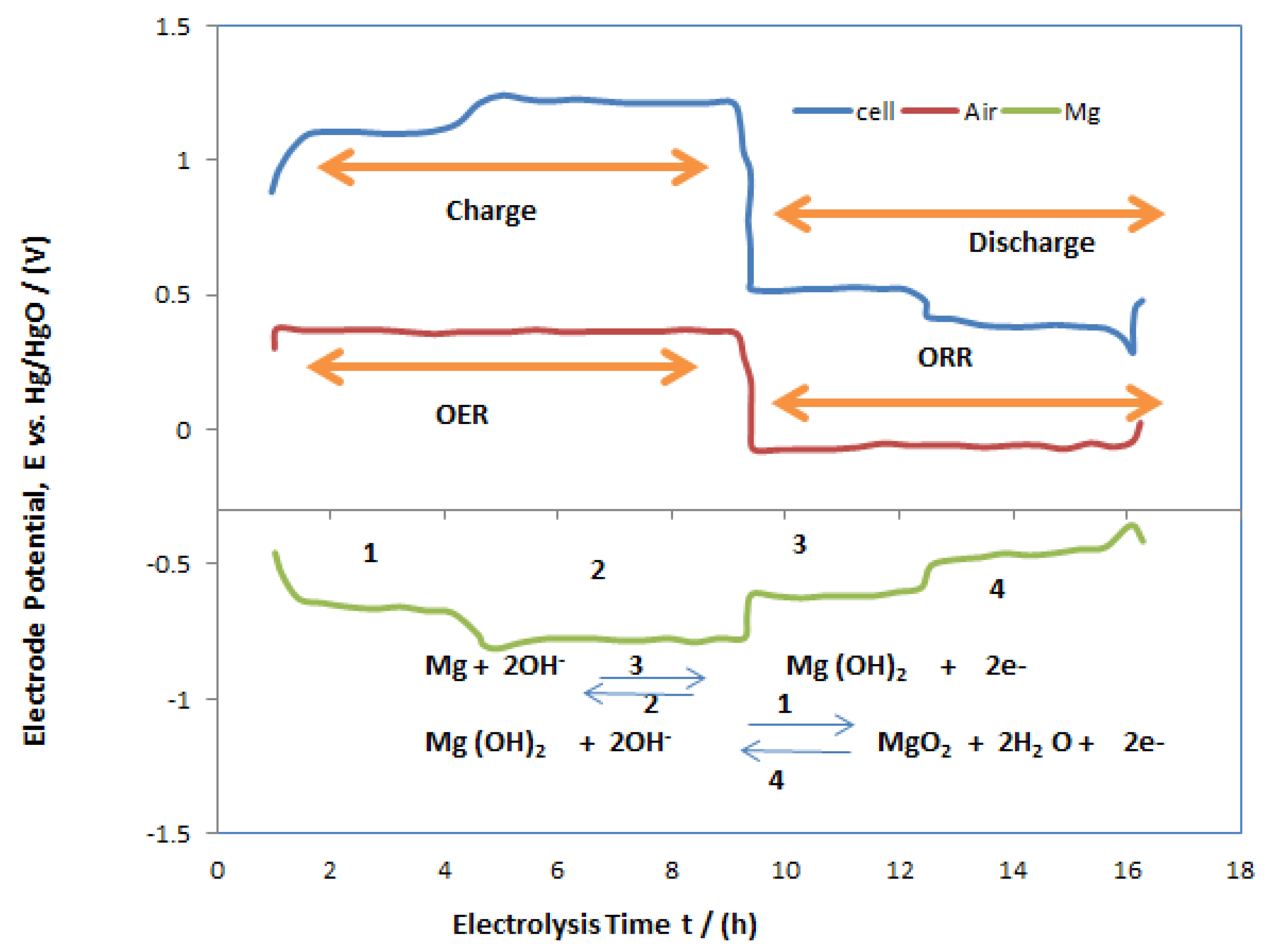
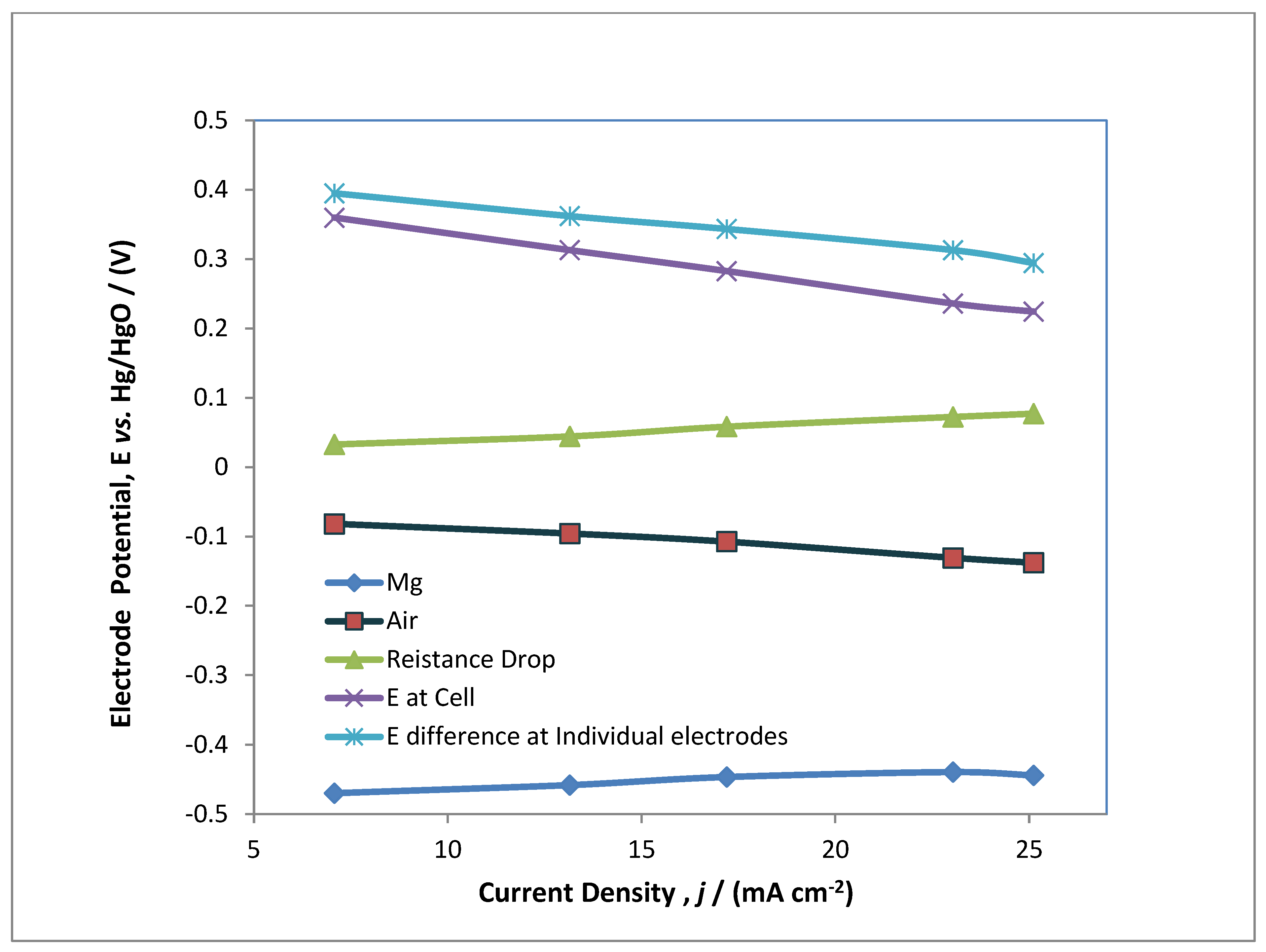
3.2. Performance Characterisation of MAB
3.3. Cathodic Performance of Air Electrodes in MAB
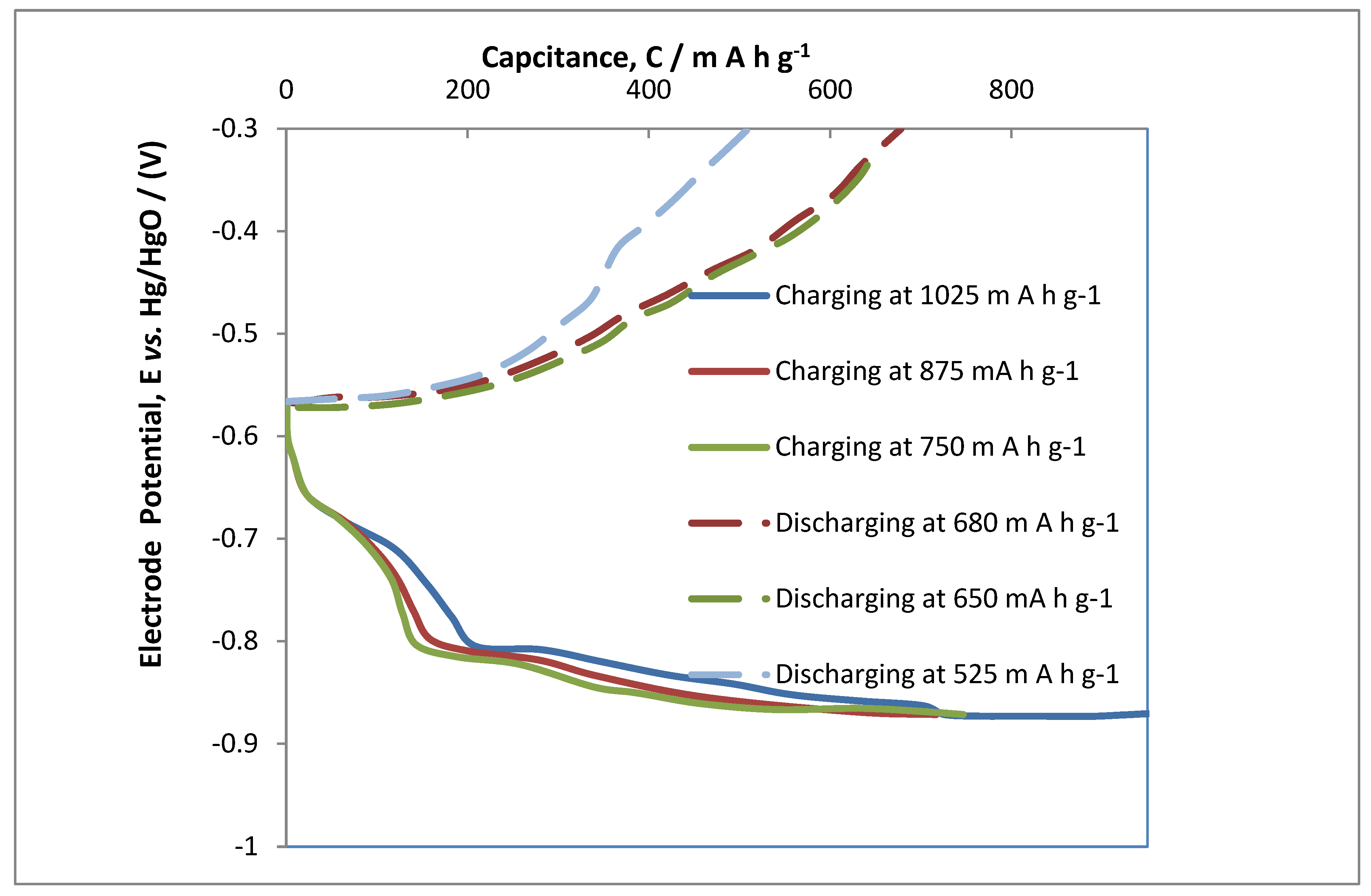
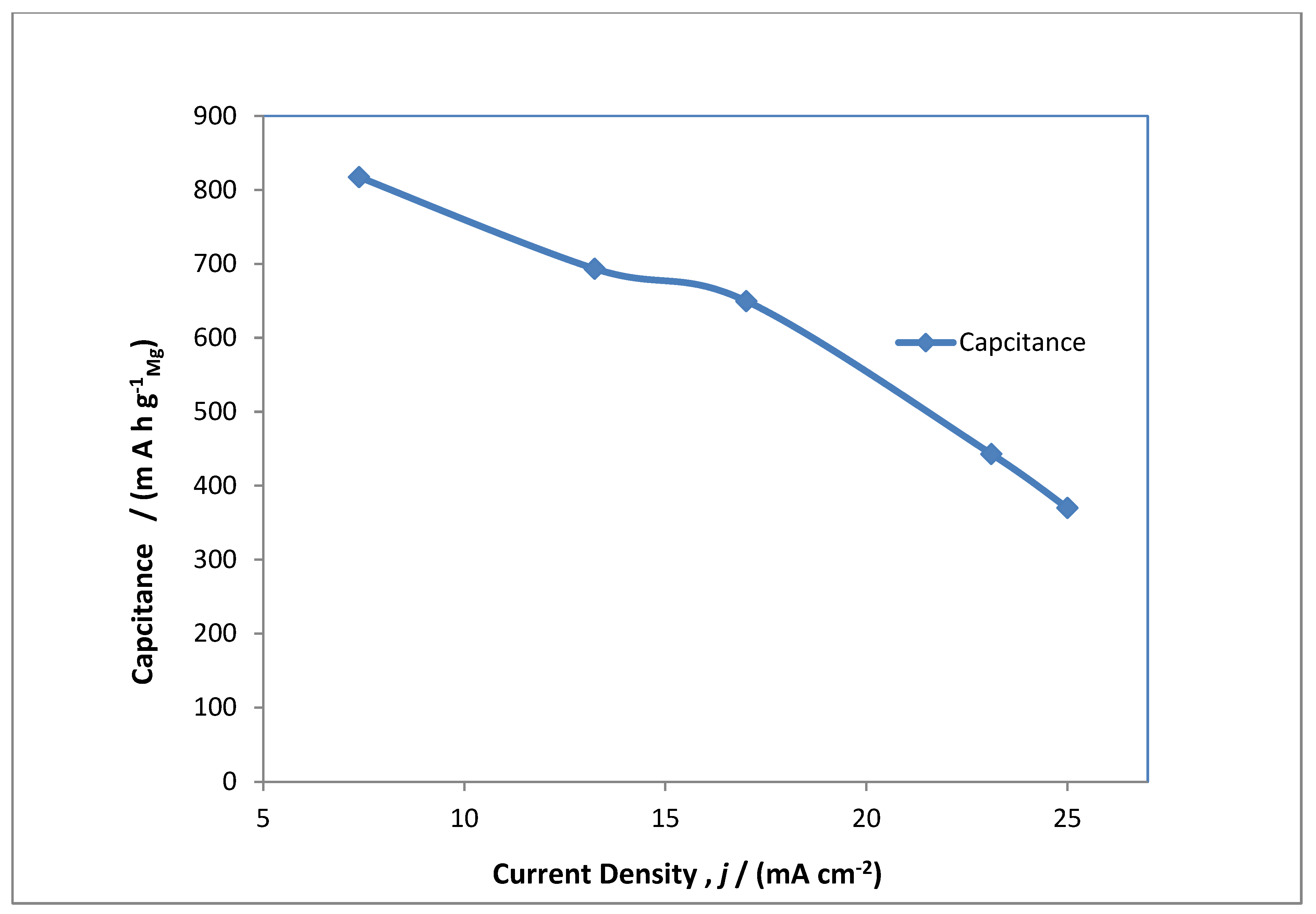
3.4. Performance Capacity Charge/Discharge of MAB
3.5. Electrode Characterisation after Cycling of MAB
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.-G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Sun, Y. Lithium ion conducting membranes for lithium-air batteries. Nano Energy 2013, 2, 801–816. [Google Scholar] [CrossRef]
- Zaidi, S.Z.J.; Nazir, M.H.; Raza, M.; Hassan, S. A High Energy Density Li-ion Battery with Lithium Titanium Oxide Anode. Int. J. Electrochem. Sci. 2022, 17, 2. [Google Scholar]
- Shang, W.; Yu, W.; Tan, P.; Chen, B.; Wu, Z.; Xu, H.; Ni, M. Achieving high energy density and efficiency through integration: Progress in hybrid zinc batteries. J. Mater. Chem. A 2019, 7, 15564–15574. [Google Scholar] [CrossRef]
- Rahman, M.A.; Wang, X.; Wen, C. High Energy Density Metal-Air Batteries: A Review. J. Electrochem. Soc. 2013, 160, A1759–A1771. [Google Scholar] [CrossRef]
- Cui, B.-F.; Han, X.-P.; Hu, W.-B. Micronanostructured Design of Dendrite-Free Zinc Anodes and Their Applications in Aqueous Zinc-Based Rechargeable Batteries. Small Struct. 2021, 2, 2000128. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, T.; Duan, Y.E.; Dai, X.; Tan, Q.; Chen, Y.; Liu, Y. N,O-codoped carbon spheres with uniform mesoporous entangled Co3O4 nanoparticles as a highly efficient electrocatalyst for oxygen reduction in a Zn-air battery. J. Colloid Interface Sci. 2021, 604, 746–756. [Google Scholar] [CrossRef]
- Liu, L.; Guo, H.; Fu, L.; Chou, S.; Thiele, S.; Wu, Y.; Wang, J. Critical Advances in Ambient Air Operation of Nonaqueous Rechargeable Li–Air Batteries. Small 2021, 17, e1903854. [Google Scholar] [CrossRef] [Green Version]
- Huy, V.P.H.; Hieu, L.T.; Hur, J. Zn Metal Anodes for Zn-Ion Batteries in Mild Aqueous Electrolytes: Challenges and Strategies. Nanomaterials 2021, 11, 2746. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, J. Metal–air batteries: From oxygen reduction electrochemistry to cathode catalysts. Chem. Soc. Rev. 2012, 41, 2172–2192. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kim, S.T.; Cao, R.; Choi, N.-S.; Liu, M.; Lee, K.T.; Cho, J. Metal-Air Batteries with High Energy Density: Li-Air versus Zn-Air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Hang, B.T.; Watanabe, T.; Egashira, M.; Watanabe, I.; Okada, S.; Yamaki, J.-I. The effect of additives on the electrochemical properties of Fe/C composite for Fe/air battery anode. J. Power Sources 2006, 155, 461–469. [Google Scholar] [CrossRef]
- Hang, B.T.; Eashira, M.; Watanabe, I.; Okada, S.; Yamaki, J.-I.; Yoon, S.-H.; Mochida, I. The effect of carbon species on the properties of Fe/C composite for metal–air battery anode. J. Power Sources 2005, 143, 256–264. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, X.; Jiang, Y.; Li, J.; Ding, J.; Hu, W.; Zhong, C. Recent advances and challenges in divalent and multivalent metal electrodes for metal–air batteries. J. Mater. Chem. A 2019, 7, 18183–18208. [Google Scholar] [CrossRef]
- Li, C.; Sun, Y.; Gebert, F.; Chou, S. Current Progress on Rechargeable Magnesium–Air Battery. Adv. Energy Mater. 2017, 7, 1700869. [Google Scholar] [CrossRef]
- Reddy, T.B. Lithium primary batteries. In Linden’s Handbook of Batteries; McGraw-Hill Education: New York, NY, USA, 2011; pp. 14.1–14.90. [Google Scholar]
- Zhang, Y.-L.; Goh, K.; Zhao, L.; Sui, X.-L.; Gong, X.-F.; Cai, J.-J.; Zhou, Q.-Y.; Zhang, H.-D.; Li, L.; Kong, F.-R.; et al. Advanced non-noble materials in bifunctional catalysts for ORR and OER toward aqueous metal–air batteries. Nanoscale 2020, 12, 21534–21559. [Google Scholar] [CrossRef]
- Liu, Q.; Pan, Z.; Wang, E.; An, L.; Sun, G. Aqueous metal-air batteries: Fundamentals and applications. Energy Storage Mater. 2020, 27, 478–505. [Google Scholar] [CrossRef]
- Jörissen, L. Bifunctional oxygen/air electrodes. J. Power Sources 2006, 155, 23–32. [Google Scholar] [CrossRef]
- Ma, T.Y.; Dai, S.; Jaroniec, M.; Qiao, S.Z. Metal–organic framework derived hybrid Co3O4-carbon porous nanowire arrays as reversible oxygen evolution electrodes. J. Am. Chem. Soc. 2014, 136, 13925–13931. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.-L.; Xu, D.; Xu, J.-J.; Zhang, X.-B. Oxygen electrocatalysts in metal–air batteries: From aqueous to nonaqueous electrolytes. Chem. Soc. Rev. 2014, 43, 7746–7786. [Google Scholar] [CrossRef]
- Tahir, M.; Pan, L.; Idrees, F.; Zhang, X.; Wang, L.; Zou, J.-J.; Wang, Z.L. Electrocatalytic oxygen evolution reaction for energy conversion and storage: A comprehensive review. Nano Energy 2017, 37, 136–157. [Google Scholar] [CrossRef]
- Avasarala, B.; Moore, R.; Haldar, P. Surface oxidation of carbon supports due to potential cycling under PEM fuel cell conditions. Electrochim. Acta 2010, 55, 4765–4771. [Google Scholar] [CrossRef]
- Golovin, M.N.; Kuznetsov, I.; Atijosan, I.; Tinker, L.A.; Pedicini, C.S. Influence of Carbon Structure and Physical Properties on The Corrosion Behavior in Carbon Based Air Electrodes for Zinc Air Batteries. MRS Proc. 1997, 496. [Google Scholar] [CrossRef]
- Alegre, C.; Stassi, A.; Modica, E.; Vecchio, C.L.; Aricò, A.S.; Baglio, V. Investigation of the activity and stability of Pd-based catalysts towards the oxygen reduction (ORR) and evolution reactions (OER) in iron–air batteries. RSC Adv. 2015, 5, 25424–25427. [Google Scholar] [CrossRef]
- Khan, Z.; Vagin, M.; Crispin, X. Can hybrid Na–air batteries outperform nonaqueous Na–O2 batteries? Adv. Sci. 2020, 7, 1902866. [Google Scholar] [CrossRef] [Green Version]
- Orikasa, Y.; Masese, T.; Koyama, Y.; Mori, T.; Hattori, M.; Yamamoto, K.; Okado, T.; Huang, Z.-D.; Minato, T.; Tassel, C.; et al. High energy density rechargeable magnesium battery using earth-abundant and non-toxic elements. Sci. Rep. 2014, 4, srep05622. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Chen, Y.; Tang, Z.; Liu, Z.; Wang, C. Palladium nanoparticles supported by metal-organic frameworks derived FeNi3Cx nanorods as efficient oxygen reversible catalysts for rechargeable Zn-Air batteries. Electrochim. Acta 2019, 307, 403–413. [Google Scholar] [CrossRef]
- Yang, W.; Yang, S.; Guo, J.; Sun, G.; Xin, Q. Comparison of CNF and XC-72 carbon supported palladium electrocatalysts for magnesium air fuel cell. Carbon 2007, 45, 397–401. [Google Scholar] [CrossRef]
- Wu, N.; Wang, W.; Wei, Y.; Li, T. Studies on the Effect of Nano-Sized MgO in Magnesium-Ion Conducting Gel Polymer Electrolyte for Rechargeable Magnesium Batteries. Energies 2017, 10, 1215. [Google Scholar] [CrossRef]
- Randrianantoandro, N.; Mercier, A.; Hervieu, M.; Greneche, J.-M. Direct phase transformation from hematite to maghemite during high energy ball milling. Mater. Lett. 2001, 47, 150–158. [Google Scholar] [CrossRef]
- Ma, J.; Qin, C.; Li, Y.; Ren, F.; Liu, Y.; Wang, G. Properties of reduced graphene oxide for Mg-air battery. J. Power Sources 2019, 430, 244–251. [Google Scholar] [CrossRef]
- Zuo, Y.; Yu, Y.; Shi, H.; Wang, J.; Zuo, C.; Dong, X. Inhibition of Hydrogen Evolution by a Bifunctional Membrane between Anode and Electrolyte of Aluminum–Air Battery. Membranes 2022, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- The Electrochemical Society. Metal/Air and Metal/Water Batteries; Dudney, N., Ed.; The Electrochemical Society: New Jersey, NJ, USA, 2010. [Google Scholar]
- Jin, C.; Lu, F.; Cao, X.; Yang, Z.; Yang, R. Facile synthesis and excellent electrochemical properties of NiCo2O4 spinel nanowire arrays as a bifunctional catalyst for the oxygen reduction and evolution reaction. J. Mater. Chem. A 2013, 1, 12170–12177. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, A.F.; Abahussain, A.A.M.; Nazir, M.H.; Zaidi, S.Z.J. A High-Energy-Density Magnesium-Air Battery with Nanostructured Polymeric Electrodes. Polymers 2022, 14, 3187. https://doi.org/10.3390/polym14153187
Alharbi AF, Abahussain AAM, Nazir MH, Zaidi SZJ. A High-Energy-Density Magnesium-Air Battery with Nanostructured Polymeric Electrodes. Polymers. 2022; 14(15):3187. https://doi.org/10.3390/polym14153187
Chicago/Turabian StyleAlharbi, Abdulrahman Faraj, Abdulaziz Abdulkarim Mansour Abahussain, Mian Hammad Nazir, and Syed Zohaib Javaid Zaidi. 2022. "A High-Energy-Density Magnesium-Air Battery with Nanostructured Polymeric Electrodes" Polymers 14, no. 15: 3187. https://doi.org/10.3390/polym14153187




