Electrocrystallization of Calcium Oxalate on Electrospun PCL Fibers Loaded with Phytic Acid as a Template
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of the Electrospun PCL Fibers
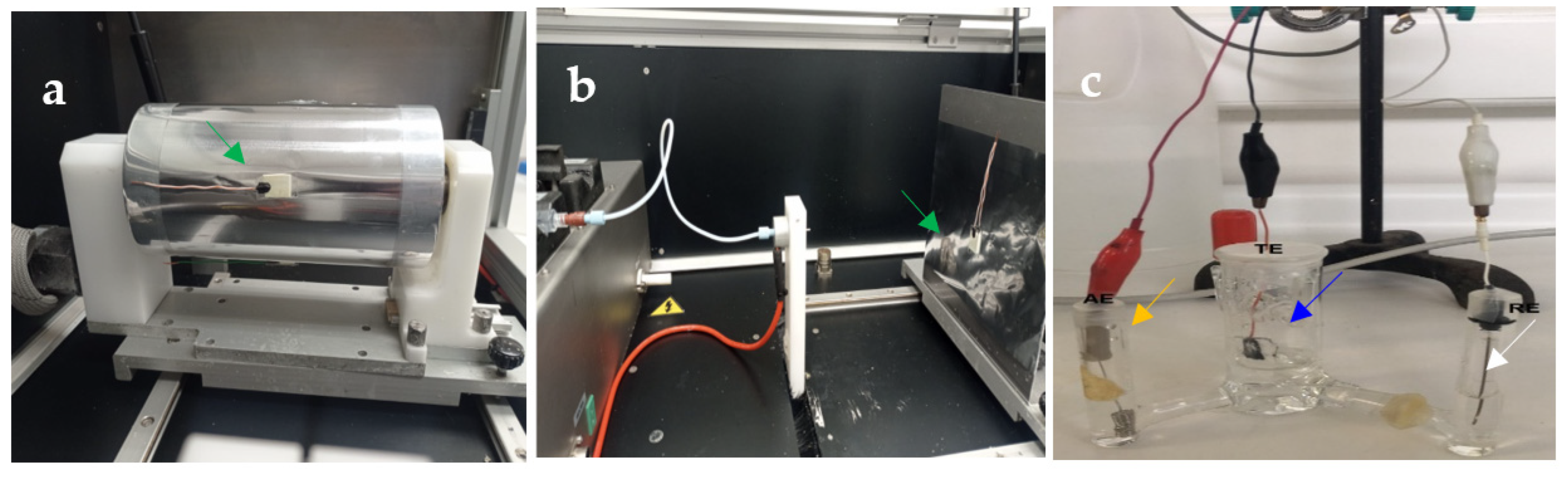
2.3. Electrocrystallization of CaOx
2.4. Characterization of PCL-ESM and CaOx Crystals
2.5. Statistical Analysis
3. Results
3.1. Chronopotentiometry
3.2. Optical Microscopy
3.3. Scanning Electron Microscopy
3.4. X-ray Diffraction (XRD)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lowenstam, H.A.; Weiner, S. On Biomineralization; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Estroff, L.A. Introduction: Biomineralization. Chem. Rev. 2008, 108, 4329–4331. [Google Scholar] [CrossRef] [PubMed]
- Simkiss, K.; Wilbur, K.M. Biomineralization: Cell Biology and Mineral Deposition; Academic Press: San Diego, CA, USA, 1989; p. 337. [Google Scholar]
- Mann, S. Biomineralization Principles and Concepts in Bioinorganic Materials Chemistry; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Weiner, S.; Dove, P.M. An overview of biomineralization processes and the problem of the vital effect. Rev. Mineral. Geochem. 2003, 54, 1–29. [Google Scholar] [CrossRef]
- Aizenberg, J.; Weaver, J.C.; Thanawala, M.S.; Sundar, V.C.; Morse, D.E.; Fratzl, P. Skeleton of Euplectella sp.: Structural Hierarchy from the Nanoscale to the Macroscale. Science 2005, 309, 275–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumper, M.; Brunner, E. Learning from Diatoms: Nature’s Tools for the Production of Nanostructured Silica. Adv. Funct. Mater. 2006, 16, 17–26. [Google Scholar] [CrossRef]
- Arias, J.L.; Fernández, M.S. Biomimetic processes through the study of mineralized shells. Mater. Charac. 2003, 50, 189–195. [Google Scholar] [CrossRef]
- Wasson, J.A.; Ward, M.A. Pathological biomineralziation of kidney stones. Elements 2007, 3, 415–421. [Google Scholar] [CrossRef]
- Daudon, M.; Bazin, D.; Letavernier, E. Randall’s plaque as the origin of calcium oxalate kidney stones. Urolithiasis 2015, 43, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Zhi-Liang, J.; Mei-Xiu, Z.; Lin-Xiu, L. Catalytic spectrophotometric methods for the determination of oxalic acid. Anal. Chim. Acta 1996, 320, 139–143. [Google Scholar] [CrossRef]
- Sezgintürk, M.K.; Dincakaya, E. A novel amperometric biosensor based on spinach (Spinacia oleracea) tissue homogenate for urinary oxalate determination. Talanta 2003, 59, 545–551. [Google Scholar] [CrossRef]
- Neira-Carrillo, A.; Vásquez-Quitral, P.; Sánchez, M.; Vargas-Fernández, A.; Silva, J.F. Control of calcium oxalate morphology through electrocrystallization as an electrochemical approach for preventing pathological disease. Ionics 2015, 21, 3141–3149. [Google Scholar] [CrossRef]
- Ruiz-Agudo, E.; Burgos-Cara, A.; Ruiz-Agudo, C.; Ibañez-Velasco, A.; Cölfen, H.; Rodriguez-Navarro, C. A non-classical view on calcium oxalate precipitation and the role of citrate. Nat. Commun. 2017, 8, 768. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.Y.; Ouyang, J.M.; Xu, M. Synthesis, characterization, and cytotoxicity assay of calcium oxalate dihydrate crystals in various shapes. CrystEngComm 2016, 18, 5463–5473. [Google Scholar] [CrossRef]
- Yu, Z.Q.; Chew, J.W.; Chow, P.S.; Tan, R.B.H. Recent advances in crystallization control: An industrial perspective. Chem. Eng. Res. Des. 2007, 85, 893–905. [Google Scholar] [CrossRef]
- Turki Jalil, A.; Hussein Demin Al-Khafaji, A.; Karevskiy, A.; Hussain Dilfy, S.; Hanan, Z.K. Polymerase chain reaction technique for molecular detection of HPV16 infections among women with cervical cancer in Dhi-Qar Province. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Jasim, S.A.; Hadi, J.M.; Opulencia, M.J.C.; Karim, Y.S.; Mahdi, A.B.; Kadhim, M.M.; Bokov, D.O.; Jalil, A.T.; Mustafa, Y.F.; Falih, K.T. MXene/metal and polymer nanocomposites: Preparation, properties, and applications. J. Alloys Compd. 2022, 917, 165404. [Google Scholar] [CrossRef]
- Obaid Saleh, R.; Bokov, D.O.; Fenjan, M.N.; Abdelbasset, W.K.; Altimari, U.S.; Turki Jalil, A.; Thangavelu, L.; Suksatan, W.; Cao, Y. Application of aluminum nitride nanotubes as a promising nanocarriers for anticancer drug 5-aminosalicylic acid in drug delivery system. J. Mol. Liq. 2022, 352, 118676. [Google Scholar] [CrossRef]
- Jalil, A.T.; Al. Qurabiy, H.E.; Hussain Dilfy, S.; Oudah Meza, S.; Aravindhan, S.; Kadhim, M.M.; Aljeboree, A.M. CuO/ZrO2 nanocomposites: Facile synthesis, characterization and photocatalytic degradation of tetracycline antibiotic. J. Nanostruct. 2021, 11, 333–346. [Google Scholar]
- Li, S.; Tang, W.; Li, M.; Wang, L.; Yang, Y.; Gong, J. Understanding the role of citric acid on the crystallization pathways of calcium oxalate hydrates. Cryst. Growth Des. 2019, 19, 3139–3147. [Google Scholar] [CrossRef]
- Gangu, K.K.; Maddila, S.; Maddila, S.N.; Jonnalagadda, S.B. Novel iron doped calcium oxalates as promising heterogeneous catalysts for one-pot multi-component synthesis of pyranopyrazoles. RSC Adv. 2017, 7, 423–432. [Google Scholar] [CrossRef] [Green Version]
- Modlin, M. Renal stone; a study of 520 patients with special reference to the pattern of recurrence. S. Afr. Med. J. 1957, 31, 824–828. [Google Scholar]
- Modlin, M. Urinary phosphorylated inositols and renal stone. Lancet 1980, 2, 1113. [Google Scholar] [CrossRef]
- Saw, N.K.; Chow, K.; Rao, P.N.; Kavanagh, J.P. Effects of inositol hexaphosphate (phytate) on calcium binding, calcium oxalate crystallization and in vitro stone growth. J. Urol. 2007, 177, 2366–2370. [Google Scholar] [CrossRef]
- Weis, A. Mineralization in organic matrix frameworks. Rev. Miner. Geochem. 2003, 54, 249–289. [Google Scholar]
- Butto, N.; Cotrina Vera, N.; Díaz-Soler, F.; Yazdani-Pedram, M.; Neira-Carrillo, A. Effect of chitosan electrospun fiber mesh as template on the crystallization of calcium oxalate. Crystals 2020, 10, 453. [Google Scholar] [CrossRef]
- Xiao, Y.; Shen, M.; Shi, X. Design of functional electrospun nanofibers for cancer cell capture applications. J. Mater. Chem. B 2018, 6, 1420–1432. [Google Scholar] [CrossRef]
- Dinamani, M.; Kamath, P.V.; Seshadri, R. Deposition of oriented SrSO4 coatings by electrogeneration of acid. Solid State Sci. 2003, 5, 805–810. [Google Scholar] [CrossRef]
- Ketrane, R.; Saidani, B.; Gil, O.; Leleyter, L.; Baraud, F. Efficiency of five scale inhibitors on calcium carbonate precipitation from hard water: Effect of temperature and concentration. Desalination 2009, 249, 1397–1404. [Google Scholar] [CrossRef]





| Factor | n | Median | Grouping | ||
|---|---|---|---|---|---|
| PA-A 1.5 mg/L | 99 | 20.64 | A | ||
| CN-A | 108 | 7.933 | B | ||
| PA-A 1 mg/L | 208 | 5.598 | C | ||
| Factor | n | Median | Grouping | ||
|---|---|---|---|---|---|
| CN-R | 38 | 15.70 | A | ||
| PA-R 1.5 mg/L | 161 | 9.578 | B | ||
| PA-R 1 mg/L | 109 | 4.445 | C | ||
| Factor | n | Median | Grouping | ||
|---|---|---|---|---|---|
| PA-A 1.5 mg/L | 99 | 20.64 | A | ||
| CN-A | 108 | 7.933 | B | ||
| PA-A 1 mg/L | 208 | 5.598 | B | ||
| Factor | n | Median | Grouping | ||
|---|---|---|---|---|---|
| CN-R | 38 | 15.70 | A | ||
| PA-R 1.5 mg/L | 161 | 9.578 | B | ||
| PA-R 1 mg/L | 109 | 4.445 | C | ||
| Factor | n | Median | Grouping | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PA-A 1.5 mg/L | 99 | 20.64 | A | ||||||
| CN-R | 38 | 15.70 | A | B | |||||
| BC | 172 | 12.965 | B | ||||||
| PA-R 1.5 mg/L | 161 | 9.578 | |||||||
| CN-A | 108 | 7.933 | C | D | E | ||||
| PA-A 1 mg/L | 208 | 5.598 | E | F | |||||
| PA-R 1 mg/L | 109 | 4.445 | F | G | |||||
| Total Number of Crystals (N) | Average Crystal Size (n) | |
|---|---|---|
| CN-R | 1° | 6° |
| PA-A 1.5 mg/L | 2° | 7° |
| PA-R 1 mg/L | 3° | 1° |
| CN-A | 4° | 3° |
| PA-R 1.5 mg/L | 5° | 4° |
| BC | 6° | 5° |
| PA-A 1 mg/L | 7° | 2° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arce, T.Z.; Yazdani-Pedram, M.; Neira-Carrillo, A. Electrocrystallization of Calcium Oxalate on Electrospun PCL Fibers Loaded with Phytic Acid as a Template. Polymers 2022, 14, 3190. https://doi.org/10.3390/polym14153190
Arce TZ, Yazdani-Pedram M, Neira-Carrillo A. Electrocrystallization of Calcium Oxalate on Electrospun PCL Fibers Loaded with Phytic Acid as a Template. Polymers. 2022; 14(15):3190. https://doi.org/10.3390/polym14153190
Chicago/Turabian StyleArce, Tatiana Zegers, Mehrdad Yazdani-Pedram, and Andrónico Neira-Carrillo. 2022. "Electrocrystallization of Calcium Oxalate on Electrospun PCL Fibers Loaded with Phytic Acid as a Template" Polymers 14, no. 15: 3190. https://doi.org/10.3390/polym14153190







