Characterization and Application in Packaging Grease of Gelatin–Sodium Alginate Edible Films Cross-Linked by Pullulan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
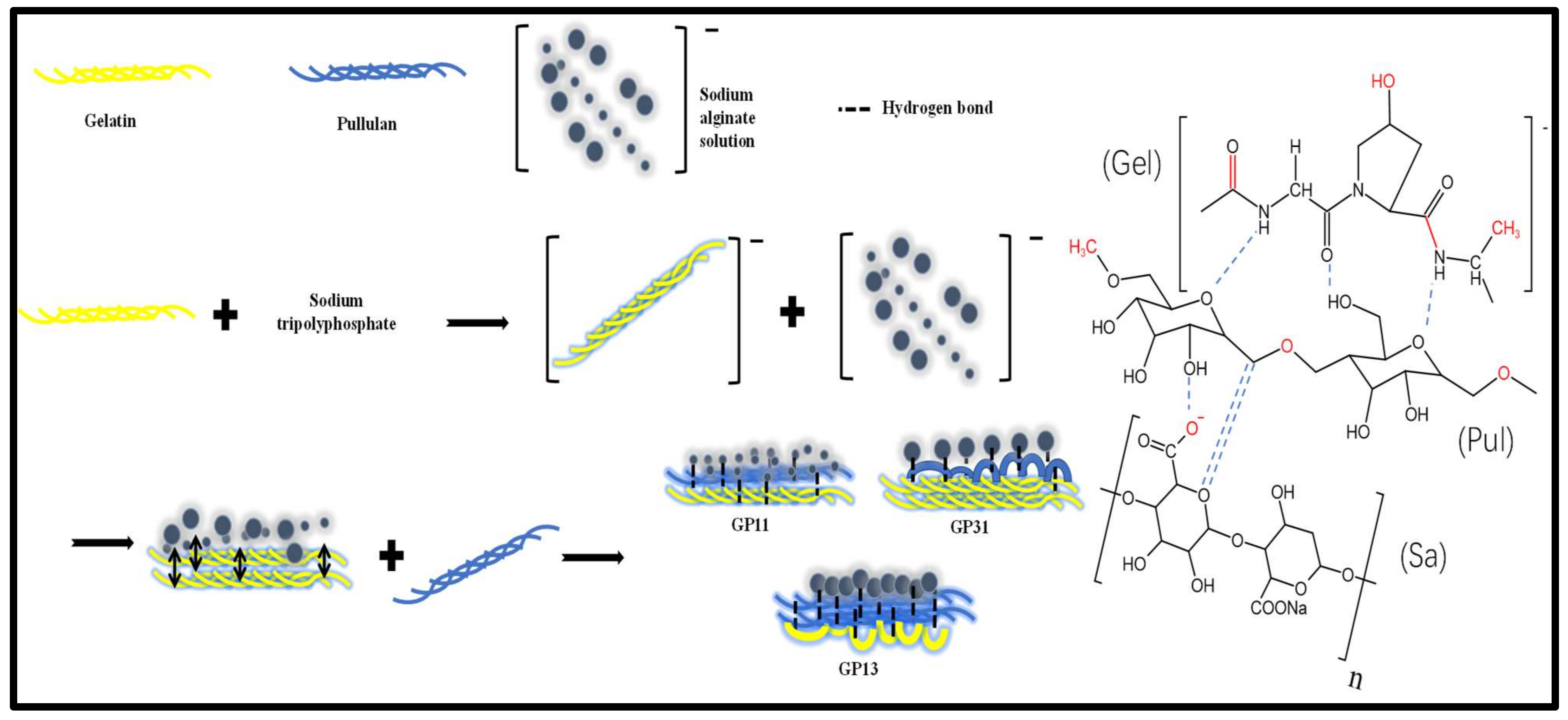
2.2. Preparation of Edible Films
2.3. Characterization
2.3.1. Thickness
2.3.2. Mechanical Property
2.3.3. Oxygen Transmission Rate (OTR)
2.3.4. Water Solubility (WS)
2.3.5. SEM
2.3.6. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
2.3.7. Thermal Stability
2.3.8. Transparency and Color
2.3.9. DPPH Antioxidant Analysis
2.4. Application of Films in Fish Liver Oil Storage
2.4.1. Preparation and Bagging of Fish Liver Oil
2.4.2. Determination of Malondialdehyde (MDA) in Liver Oil
2.4.3. Determination of Peroxide Value (PV), Anisidine Value (An.V) and Total Oxidation Value (TOV) in Cod Liver Oil
2.5. Statistical Analysis
3. Results and Discussion
3.1. FTIR Analysis
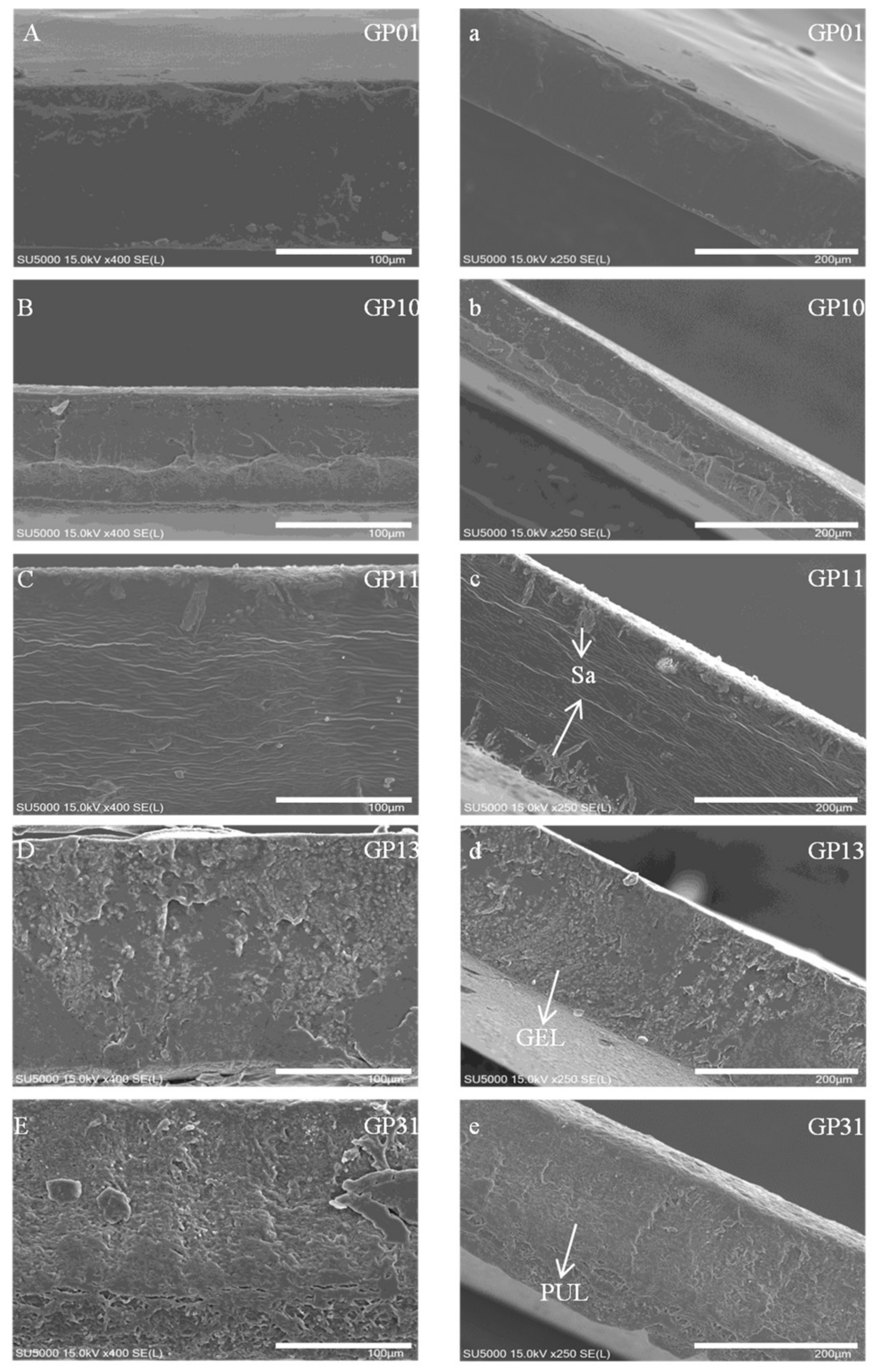
3.2. Microstructure
3.3. Thickness
3.4. Oxygen Permeability
3.5. Solubility
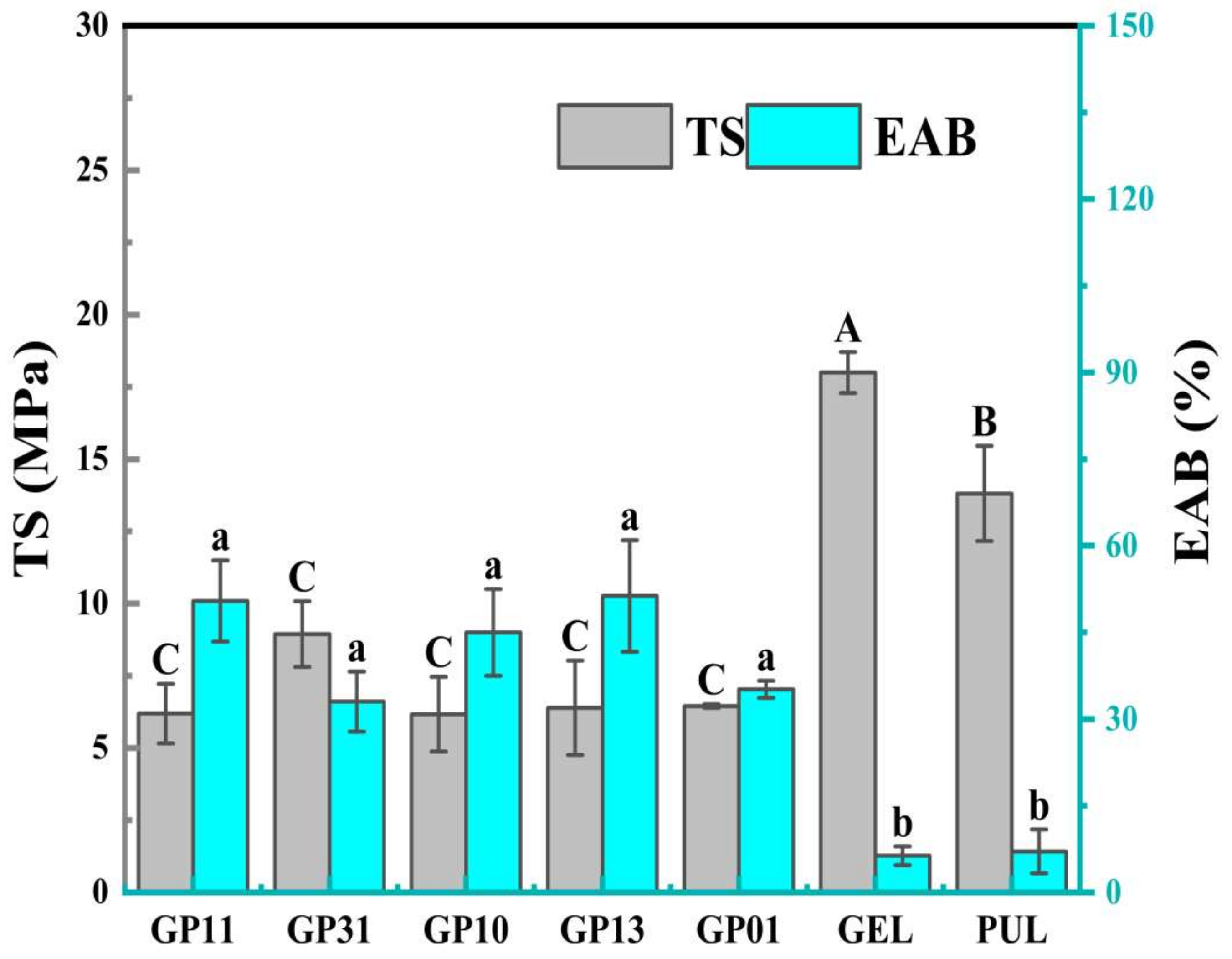
3.6. Mechanical Properties
3.7. Thermodynamic Properties
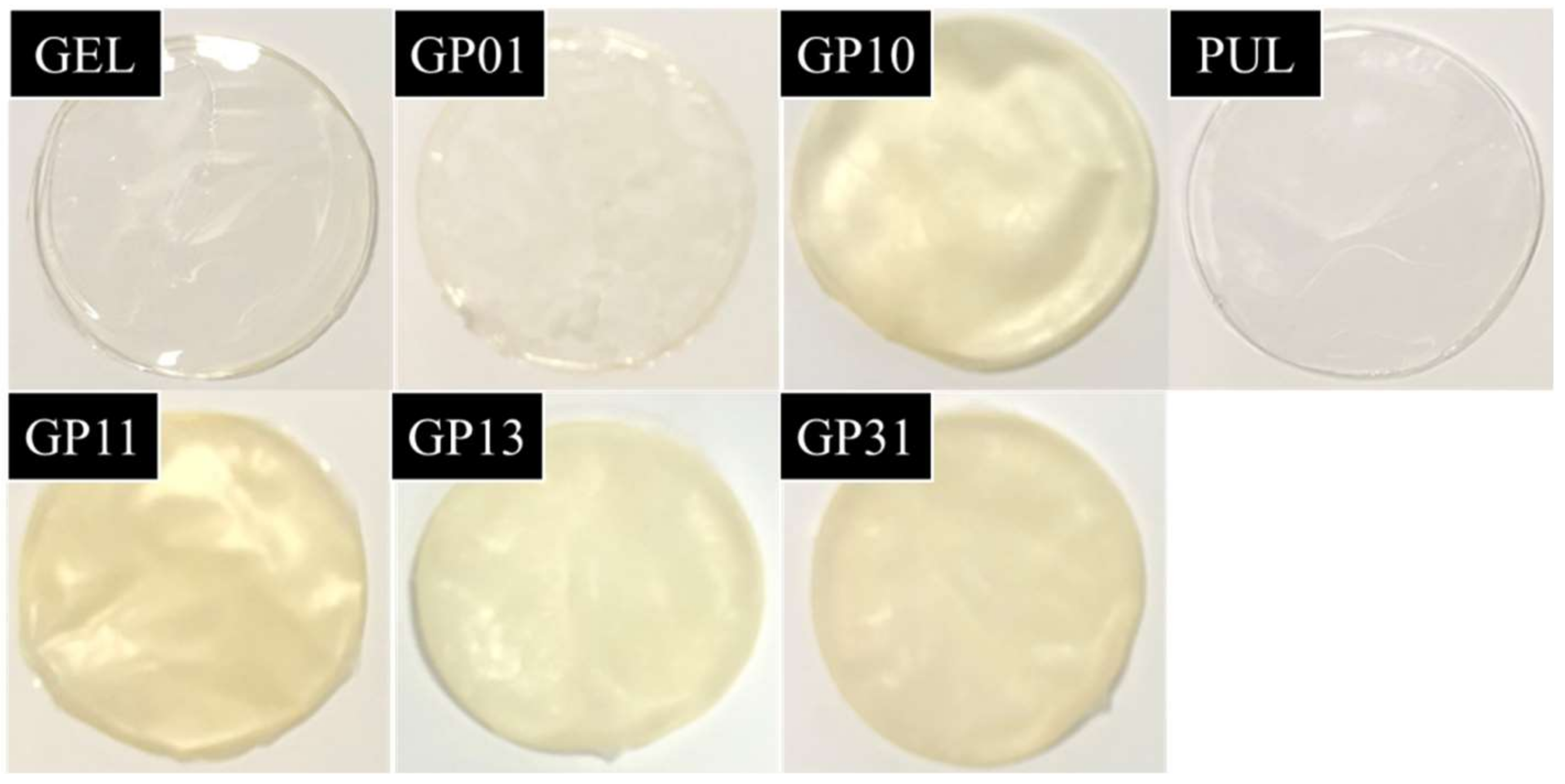
3.8. Color and Light Transmission
3.9. Films Antioxidant Activity
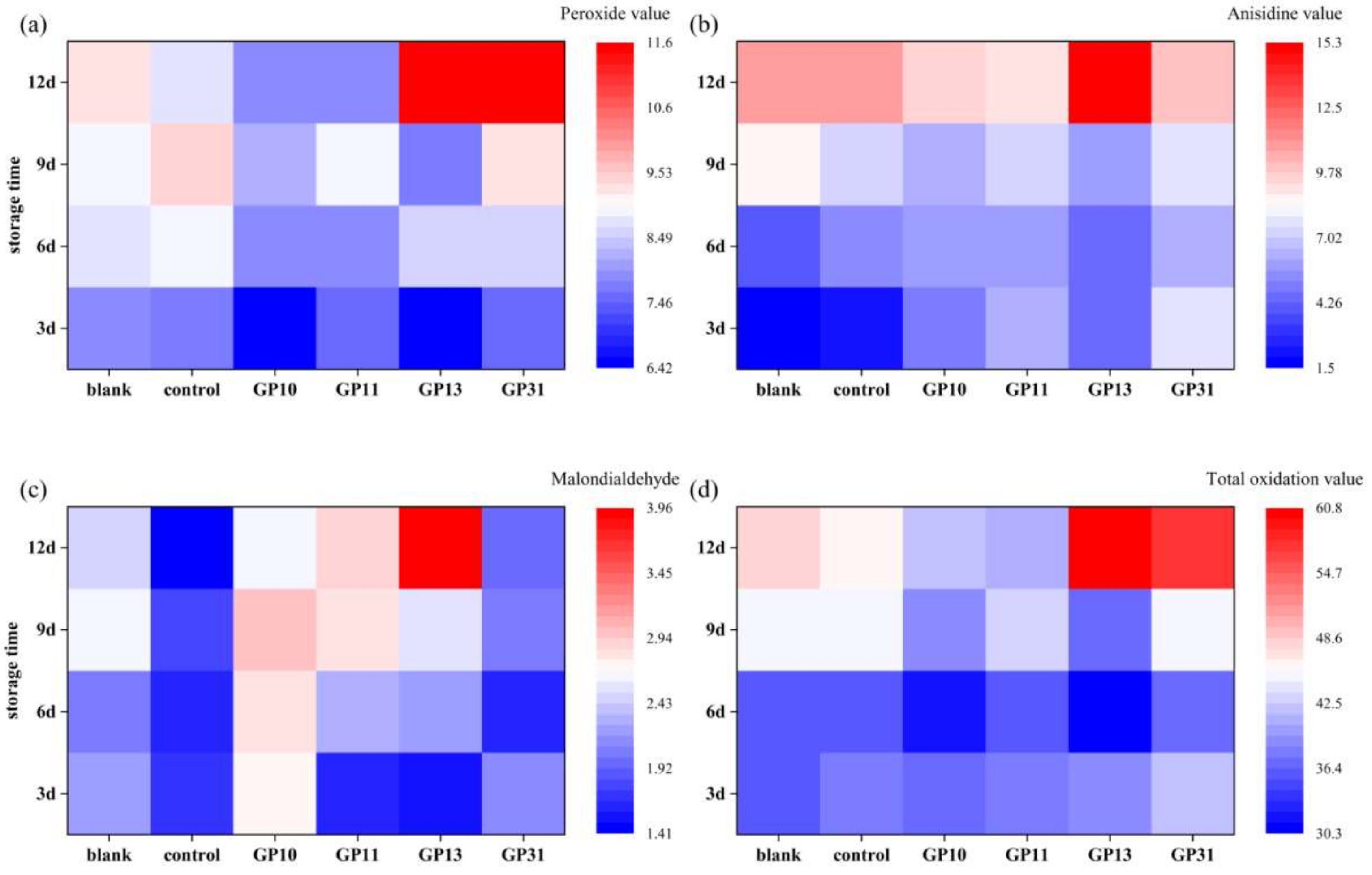
3.10. Protection against Oxidation of Oils and Fats
3.10.1. Changes in Primary Oxidation Products of Liver Oil
3.10.2. Changes in Secondary Oxidation Products of Liver Oil
3.11. Correlation Analysis between Resistance to Grease Oxidation and Key Properties of Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Han, L.; Wang, D.; Sun, Y.; Huang, J.; Shahidi, F. Stability and stabilization of omega-3 oils: A review. Trends Food Sci. Technol. 2021, 118, 17–35. [Google Scholar] [CrossRef]
- Otoni, C.G.; Avena-Bustillos, R.J.; Azeredo, H.M.C.; Lorevice, M.V.; Moura, M.R.; Mattoso, L.H.C.; McHugh, T.H. Recent Advances on Edible Films Based on Fruits and Vegetables—A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Johnson, D.R.; Tian, F.; Roman, M.J.; Decker, E.A.; Goddard, J.M. Development of Iron-Chelating Poly(ethylene terephthalate) Packaging for Inhibiting Lipid Oxidation in Oil-in-Water Emulsions. J. Agric. Food Chem. 2015, 63, 5055–5060. [Google Scholar] [CrossRef]
- Oenel, A.; Fekete, A.; Krischke, M.; Faul, S.C.; Gresser, G.; Havaux, M.; Mueller, M.J.; Berger, S. Enzymatic and Non-enzymatic Mechanisms Contribute to Lipid Oxidation during Seed Aging. Plant Cell Physiol. 2017, 58, 925–933. [Google Scholar] [CrossRef]
- Zhang, M.; Tao, N.; Li, L.; Xu, C.; Deng, S.; Wang, Y. Non-migrating active antibacterial packaging and its application in grass carp fillets. Food Packag. Shelf Life. 2022, 31. [Google Scholar] [CrossRef]
- Sutthasupa, S.; Padungkit, C.; Suriyong, S. Colorimetric ammonia (NH3) sensor based on an alginate-methylcellulose blend hydrogel and the potential opportunity for the development of a minced pork spoilage indicator. Food Chem. 2021, 362, 130151. [Google Scholar] [CrossRef]
- Dierings de Souza, E.J.; Kringel, D.H.; Guerra Dias, A.R.; da Rosa Zavareze, E. Polysaccharides as wall material for the encapsulation of essential oils by electrospun technique. Carbohydr. Polym. 2021, 265, 118068. [Google Scholar] [CrossRef]
- Hamed, S.F.; Hashim, A.F.; Abdel Hamid, H.A.; Abd-Elsalam, K.A.; Golonka, I.; Musial, W.; El-Sherbiny, I.M. Edible alginate/chitosan-based nanocomposite microspheres as delivery vehicles of omega-3 rich oils. Carbohydr. Polym. 2020, 239, 116201. [Google Scholar] [CrossRef]
- Jiang, J.; Dong, Q.; Gao, H.; Han, Y.; Li, L. Enhanced mechanical and antioxidant properties of biodegradable poly (lactic) acid-poly(3-hydroxybutyrate-co-4-hydroxybutyrate) film utilizing α-tocopherol for peach storage. Packag. Technol. Sci. 2020, 34, 187–199. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Kouhi, M.; Prabhakaran, M.P.; Ramakrishna, S. Edible polymers: An insight into its application in food, biomedicine and cosmetics. Trends Food Sci. Technol. 2020, 103, 248–263. [Google Scholar] [CrossRef]
- Umaraw, P.; Munekata, P.E.S.; Verma, A.K.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Gounga, M.E.; Xu, S.Y.; Wang, Z.; Yang, W.G. Effect of whey protein isolate-pullulan edible coatings on the quality and shelf life of freshly roasted and freeze-dried Chinese chestnut. J. Food Sci. 2008, 73, E155–E161. [Google Scholar] [CrossRef]
- Zibaei, R.; Hasanvand, S.; Hashami, Z.; Roshandel, Z.; Rouhi, M.; Guimaraes, J.T.; Mortazavian, A.M.; Sarlak, Z.; Mohammadi, R. Applications of emerging botanical hydrocolloids for edible films: A review. Carbohydr. Polym. 2021, 256, 117554. [Google Scholar] [CrossRef]
- Cao, Y.; Mezzenga, R. Design principles of food gels. Nat. Food. 2020, 1, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Cui, C.; Ji, N.; Wang, Y.; Xiong, L.; Sun, Q. Bioactive and intelligent starch-based films: A review. Trends Food Sci. Technol. 2021, 116, 854–869. [Google Scholar] [CrossRef]
- Chen, Z.; Zong, L.; Chen, C.; Xie, J. Development and characterization of PVA-Starch active films incorporated with β-cyclodextrin inclusion complex embedding lemongrass (Cymbopogon citratus) oil. Food Packag. Shelf Life 2020, 26. [Google Scholar] [CrossRef]
- Etxabide, A.; Kilmartin, P.A.; Maté, J.I.; Gómez-Estaca, J. Characterization of glucose-crosslinked gelatin films reinforced with chitin nanowhiskers for active packaging development. LWT 2022, 154. [Google Scholar] [CrossRef]
- Maryam Adilah, Z.A.; Jamilah, B.; Nur Hanani, Z.A. Functional and antioxidant properties of protein-based films incorporated with mango kernel extract for active packaging. Food Hydrocoll. 2018, 74, 207–218. [Google Scholar] [CrossRef]
- Yin, Y.; Li, Z.; Sun, Y.; Yao, K. A preliminary study on chitosan/gelatin polyelectrolyte complex formation. J. Mater. Sci. 2005, 40, 4649–4652. [Google Scholar] [CrossRef]
- Soltanzadeh, M.; Peighambardoust, S.H.; Ghanbarzadeh, B.; Amjadi, S.; Mohammadi, M.; Lorenzo, J.M.; Hamishehkar, H. Active gelatin/cress seed gum-based films reinforced with chitosan nanoparticles encapsulating pomegranate peel extract: Preparation and characterization. Food Hydrocoll. 2022, 129. [Google Scholar] [CrossRef]
- Thongsrikhem, N.; Taokaew, S.; Sriariyanun, M.; Kirdponpattara, S. Antibacterial activity in gelatin-bacterial cellulose composite film by thermally crosslinking with cinnamaldehyde towards food packaging application. Food Packag. Shelf Life 2022, 31. [Google Scholar] [CrossRef]
- He, B.; Wang, S.; Lan, P.; Wang, W.; Zhu, J. Topography and physical properties of carboxymethyl cellulose films assembled with calcium and gelatin at different temperature and humidity. Food Chem. 2022, 382, 132391. [Google Scholar] [CrossRef]
- Pan, J.; Li, Y.; Chen, K.; Zhang, Y.; Zhang, H. Enhanced physical and antimicrobial properties of alginate/chitosan composite aerogels based on electrostatic interactions and noncovalent crosslinking. Carbohydr. Polym. 2021, 266, 118102. [Google Scholar] [CrossRef]
- Xiao, Q.; Tong, Q.; Lim, L.-T. Pullulan-sodium alginate based edible films: Rheological properties of film forming solutions. Carbohydr. Polym. 2012, 87, 1689–1695. [Google Scholar] [CrossRef]
- Han, K.; Liu, Y.; Liu, Y.; Huang, X.; Sheng, L. Characterization and film-forming mechanism of egg white/pullulan blend film. Food Chem. 2020, 315, 126201. [Google Scholar] [CrossRef]
- Krasniewska, K.; Gniewosz, M.; Kosakowska, O.; Cis, A. Preservation of Brussels Sprouts by Pullulan Coating Containing Oregano Essential Oil. J. Food Prot. 2016, 79, 493–500. [Google Scholar] [CrossRef]
- Chu, Y.; Gao, C.; Liu, X.; Zhang, N.; Xu, T.; Feng, X.; Yang, Y.; Shen, X.; Tang, X. Improvement of storage quality of strawberries by pullulan coatings incorporated with cinnamon essential oil nanoemulsion. LWT 2020, 122. [Google Scholar] [CrossRef]
- Da Nóbrega Santos, E.; Cesar de Albuquerque Sousa, T.; Cassiano de Santana Neto, D.; Brandão Grisi, C.V.; Cardoso da Silva Ferreira, V.; Pereira da Silva, F.A. Edible active film based on gelatin and Malpighia emarginata waste extract to inhibit lipid and protein oxidation in beef patties. LWT 2022, 154. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, H.; Li, M.; Lu, Y.; Du, Y.; Tong, C.; Pang, J.; Wu, C. Preparation and characterization of multifunctional konjac glucomannan/carboxymethyl chitosan biocomposite films incorporated with epigallocatechin gallate. Food Hydrocoll. 2020, 105. [Google Scholar] [CrossRef]
- Jin, H.; Li, P.; Jin, Y.; Sheng, L. Effect of sodium tripolyphosphate on the interaction and aggregation behavior of ovalbumin-lysozyme complex. Food Chem. 2021, 352, 129457. [Google Scholar] [CrossRef]
- Zhang, A.Q.; He, J.L.; Wang, Y.; Zhang, X.; Piao, Z.-H.; Xue, Y.-T.; Zhang, T.-H. Whey protein isolate modified with sodium tripolyphosphate gel: A novel pH-sensitive system for controlled release of Lactobacillus plantarum. Food Hydrocoll. 2021, 120, 106924. [Google Scholar] [CrossRef]
- Szerman, N.; Ferrari, R.; Sancho, A.M.; Vaudagna, S. Response surface methodology study on the effects of sodium chloride and sodium tripolyphosphate concentrations, pressure level and holding time on beef patties properties. LWT — Food Sci. Technol. 2019, 109, 93–100. [Google Scholar] [CrossRef]
- Wang, Y.R.; Zhang, B.; Fan, J.L.; Yang, Q.; Chen, H.Q. Effects of sodium tripolyphosphate modification on the structural, functional, and rheological properties of rice glutelin. Food Chem. 2019, 281, 18–27. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Zhai, J.; Gu, L.; Su, Y.; Chang, C.; Yang, Y. Improving gelling properties of diluted whole hen eggs with sodium chloride and sodium tripolyphosphate: Study on intermolecular forces, water state and microstructure. Food Chem. 2021, 358, 129823. [Google Scholar] [CrossRef]
- Chen, J.; Ren, Y.; Zhang, K.; Xiong, Y.L.; Wang, Q.; Shang, K.; Zhang, D. Site-specific incorporation of sodium tripolyphosphate into myofibrillar protein from mantis shrimp (Oratosquilla oratoria) promotes protein crosslinking and gel network formation. Food Chem. 2020, 312, 126113. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Yi, Y.; Solangi, I.; Zan, L.; Zhu, J. Effects of sodium hexametaphosphate, sodium tripolyphosphate and sodium pyrophosphate on the ultrastructure of beef myofibrillar proteins investigated with atomic force microscopy. Food Chem. 2021, 338, 128146. [Google Scholar] [CrossRef]
- Zhou, W.; He, Y.; Liu, F.; Liao, L.; Huang, X.; Li, R.; Zou, Y.; Zhou, L.; Zou, L.; Liu, Y.; et al. Carboxymethyl chitosan-pullulan edible films enriched with galangal essential oil: Characterization and application in mango preservation. Carbohydr. Polym. 2021, 256, 117579. [Google Scholar] [CrossRef]
- Chen, C.; Zong, L.; Wang, J.; Xie, J. Microfibrillated cellulose reinforced starch/polyvinyl alcohol antimicrobial active films with controlled release behavior of cinnamaldehyde. Carbohydr. Polym. 2021, 272, 118448. [Google Scholar] [CrossRef]
- Jiang, J.; Gong, L.; Dong, Q.; Kang, Y.; Osako, K.; Li, L. Characterization of PLA-P3,4HB active film incorporated with essential oil: Application in peach preservation. Food Chem. 2020, 313, 126134. [Google Scholar] [CrossRef]
- Ghosh, M.; Upadhyay, R.; Mahato, D.K.; Mishra, H.N. Kinetics of lipid oxidation in omega fatty acids rich blends of sunflower and sesame oils using Rancimat. Food Chem. 2019, 272, 471–477. [Google Scholar] [CrossRef]
- GB5009.181-2016; National Standard for Food Safety: Determination of Malondialdehyde in Foods. National Standards of People's Republic of China: China, 2016.
- GB/T 24304-2009; Animal and vegetable fats and oils—Determination of anisidine value. National Standards of People's Republic of China: China, 2009.
- Li, Y.; Dong, Q.; Chen, J.; Li, L. Effects of coaxial electrospun eugenol loaded core-sheath PVP/shellac fibrous films on postharvest quality and shelf life of strawberries. Postharvest Biol. Technol. 2020, 159. [Google Scholar] [CrossRef]
- Ni, Y.; Sun, J.; Wang, J. Enhanced antimicrobial activity of konjac glucomannan nanocomposite films for food packaging. Carbohydr. Polym. 2021, 267, 118215. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, D.; Ma, Y.; Zhao, X. Effect of gelatin addition on properties of pullulan films. J. Food Sci. 2013, 78, C805–C810. [Google Scholar] [CrossRef]
- Chang, S.C.; Hsu, B.Y.; Chen, B.H. Structural characterization of polysaccharides from Zizyphus jujuba and evaluation of antioxidant activity. Int. J. Biol. Macromol. 2010, 47, 445–453. [Google Scholar] [CrossRef]




| Component (%) | GP01 | GP13 | GP11 | GP31 | GP10 | GEL | PUL |
|---|---|---|---|---|---|---|---|
| Gelatin | 0 | 19.44 | 38.89 | 58.33 | 77.77 | 100 | 0 |
| Pullulan | 77.77 | 58.33 | 38.89 | 19.44 | 0 | 0 | 100 |
| Sodium alginate | 5.56 | 5.56 | 5.56 | 5.56 | 5.56 | 0 | 0 |
| Sodium tripolyphosphate | 11.11 | 11.11 | 11.11 | 11.11 | 11.11 | 0 | 0 |
| Glycerol | 5.56 | 5.56 | 5.56 | 5.56 | 5.56 | 0 | 0 |
| Film Groups | Thickness (mm) | OTR (cm3/(m2⋅24 h⋅0.1 MPa)) | WS (g/s) |
|---|---|---|---|
| GP11 | 0.201 ± 0.018 a | 47.42 ± 13.73 ab | 0.10 ± 0.03 ab |
| GP31 | 0.179 ± 0.008 b | 54.47 ± 8.80 a | 0.11 ± 0.01 a |
| GP10 | 0.149 ± 0.006 c | 57.69 ± 10.31 a | 0.07 ± 0.01 ab |
| GP13 | 0.145 ± 0.004 c | 49.06 ± 5.26 ab | 0.10 ± 0.02 ab |
| GP01 | 0.138 ± 0.016 c | - | 0.07 ± 0.02 b |
| GEL | 0.177 ± 0.009 b | 35.86 ± 3.37 b | 0.07 ± 0.01 b |
| PUL | 0.143 ± 0.009 c | 19.09 ± 3.45 c | 0.09 ± 0.01 ab |
| L* | a* | b* | ΔE | VLT (%) | UVR (%) | IRR (%) | |
|---|---|---|---|---|---|---|---|
| GP11 | 90.92 ± 0.52 ab | −2.16 ± 0.06 c | 11.53 ± 0.56 b | 13.03 ± 0.50 b | 27.07 ± 4.21 c | 94.13 ± 3.50 a | 57.67 ± 4.41 ab |
| GP31 | 89.07 ± 2.79 b | −2.56 ± 0.41 d | 15.33 ± 3.36 a | 17.26 ± 4.07 a | 42.63 ± 14.68 bc | 75.17 ± 27.89 a | 69.23 ± 18.08 a |
| GP10 | 92.78 ± 0.07 a | −2.09 ± 0.09 c | 10.33 ± 0.60 b | 11.42 ± 0.60 bc | 56.77 ± 4.01 b | 72.17 ± 10.96 a | 28.93 ± 3.72 cd |
| GP13 | 93.26 ± 0.15 a | −1.21 ± 0.10 b | 6.45 ± 0.50 c | 7.56 ± 0.51 d | 66.57 ± 9.94 ab | 71.9 ± 11.23 a | 20.93 ± 6.79 de |
| GP01 | 91.74 ± 1.71 ab | −1.14 ± 0.20 b | 6.96 ± 1.16 c | 8.53 ± 1.64 cd | 45.5 ± 32.09 bc | 69.17 ± 23.69 a | 44.53 ± 20.74 bc |
| GEL | 93.08 ± 0.19 a | −1.44 ± 0.07 b | 7.87 ± 0.47 c | 8.96 ± 0.43 cd | 90.7 ± 0.95 a | 30.6 ± 2.44 b | 8.63 ± 0.81 e |
| PUL | 92.8 ± 2.56 a | −0.57 ± 0.10 a | 3.87 ± 0.17 d | 5.69 ± 1.00 d | 91.93 ± 0.06 a | 10.57 ± 0.21 b | 8.23 ± 0.55 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Fan, M.; Deng, S.; Tao, N. Characterization and Application in Packaging Grease of Gelatin–Sodium Alginate Edible Films Cross-Linked by Pullulan. Polymers 2022, 14, 3199. https://doi.org/10.3390/polym14153199
Li S, Fan M, Deng S, Tao N. Characterization and Application in Packaging Grease of Gelatin–Sodium Alginate Edible Films Cross-Linked by Pullulan. Polymers. 2022; 14(15):3199. https://doi.org/10.3390/polym14153199
Chicago/Turabian StyleLi, Shuo, Min Fan, Shanggui Deng, and Ningping Tao. 2022. "Characterization and Application in Packaging Grease of Gelatin–Sodium Alginate Edible Films Cross-Linked by Pullulan" Polymers 14, no. 15: 3199. https://doi.org/10.3390/polym14153199
APA StyleLi, S., Fan, M., Deng, S., & Tao, N. (2022). Characterization and Application in Packaging Grease of Gelatin–Sodium Alginate Edible Films Cross-Linked by Pullulan. Polymers, 14(15), 3199. https://doi.org/10.3390/polym14153199






