The Study of Chemical and Thermal Influences of the Environment on the Degradation of Mechanical Properties of Carbon Composite with Epoxy Resin
Abstract
:1. Introduction
2. Experimental Material and Methods
2.1. Experimental Material
2.2. Exposure Conditions
2.3. Methods
2.3.1. Macroscopic Observation of the Surface Changes
2.3.2. Tensile Test
2.3.3. Three-Point Bending Test
2.3.4. Dynamic Mechanical Analysis
3. Results
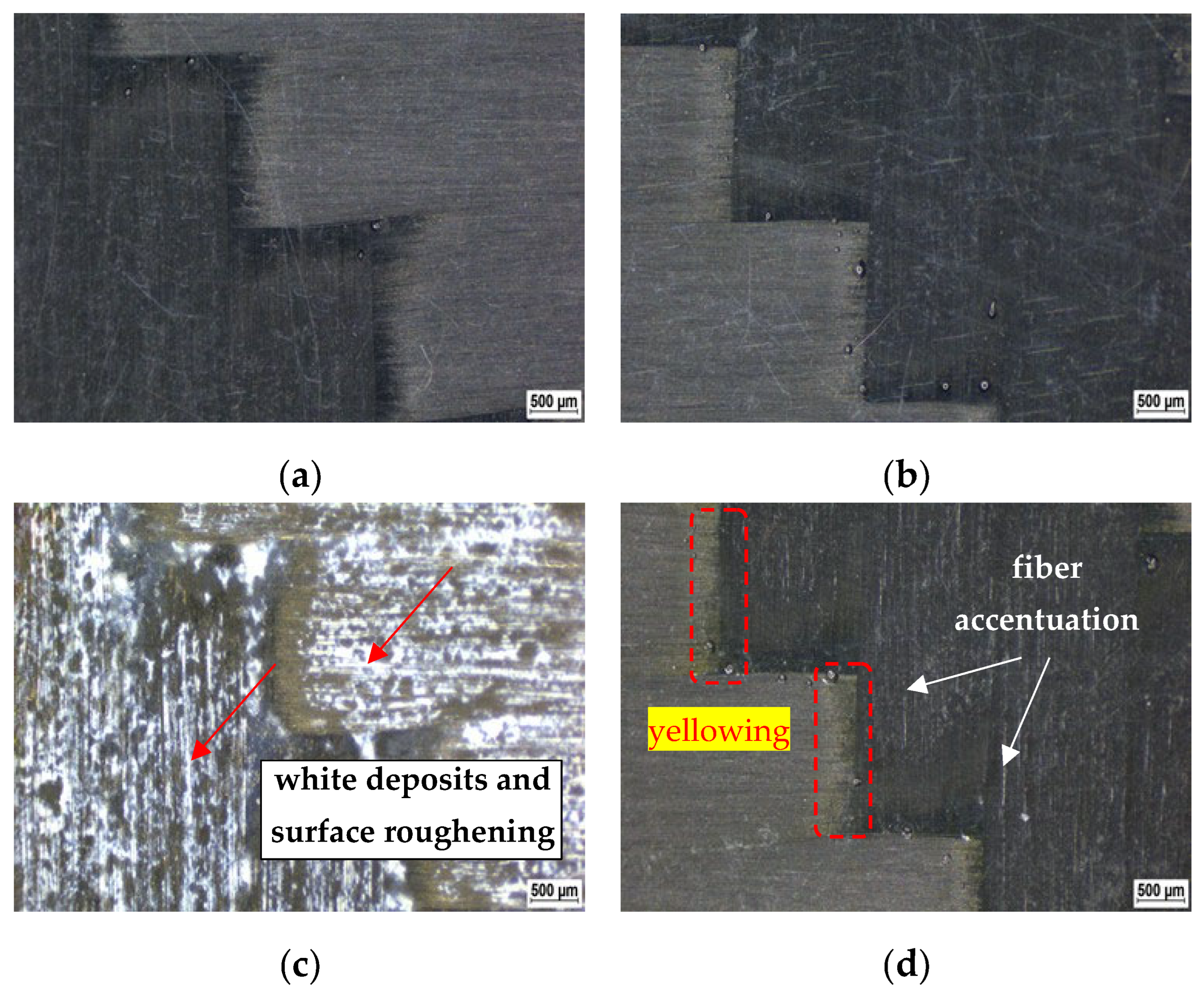
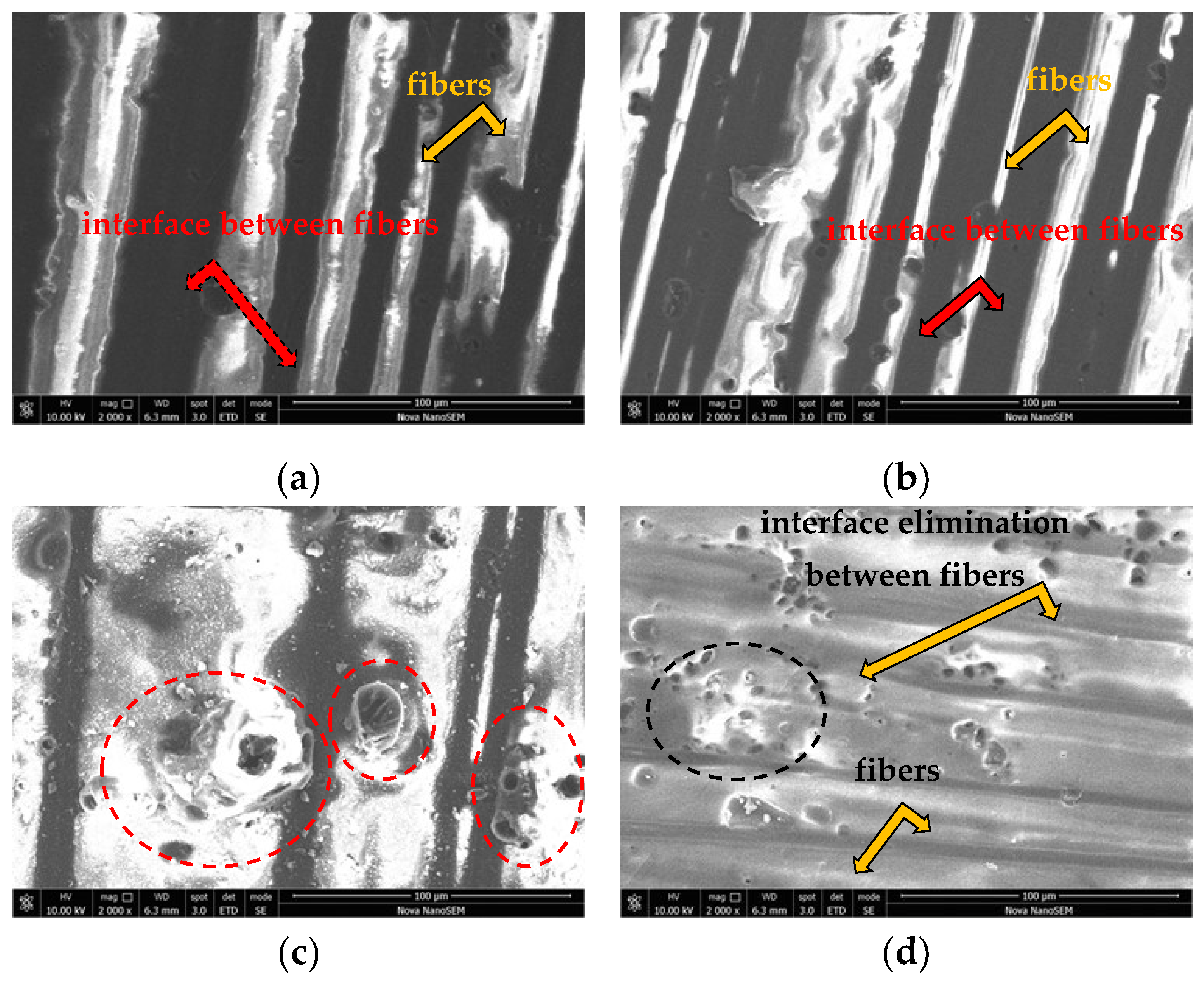
3.1. Macroscopic Observation of the Surface Changes
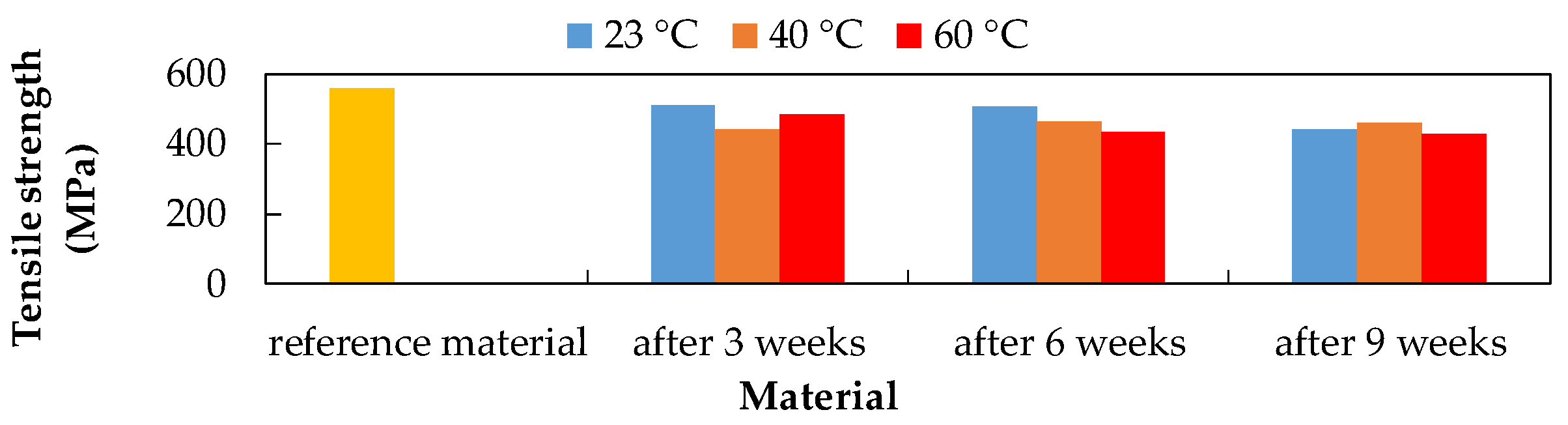
3.2. Tensile Test
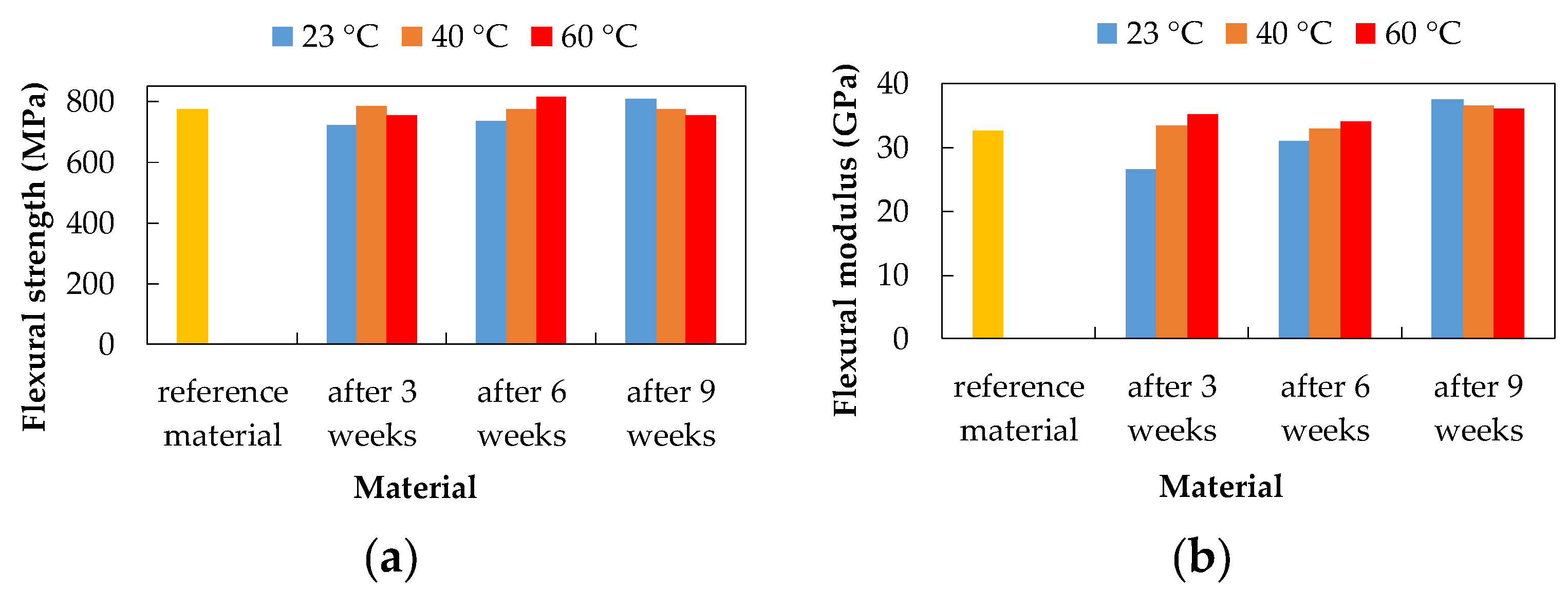
3.3. Three-Point Bending Test
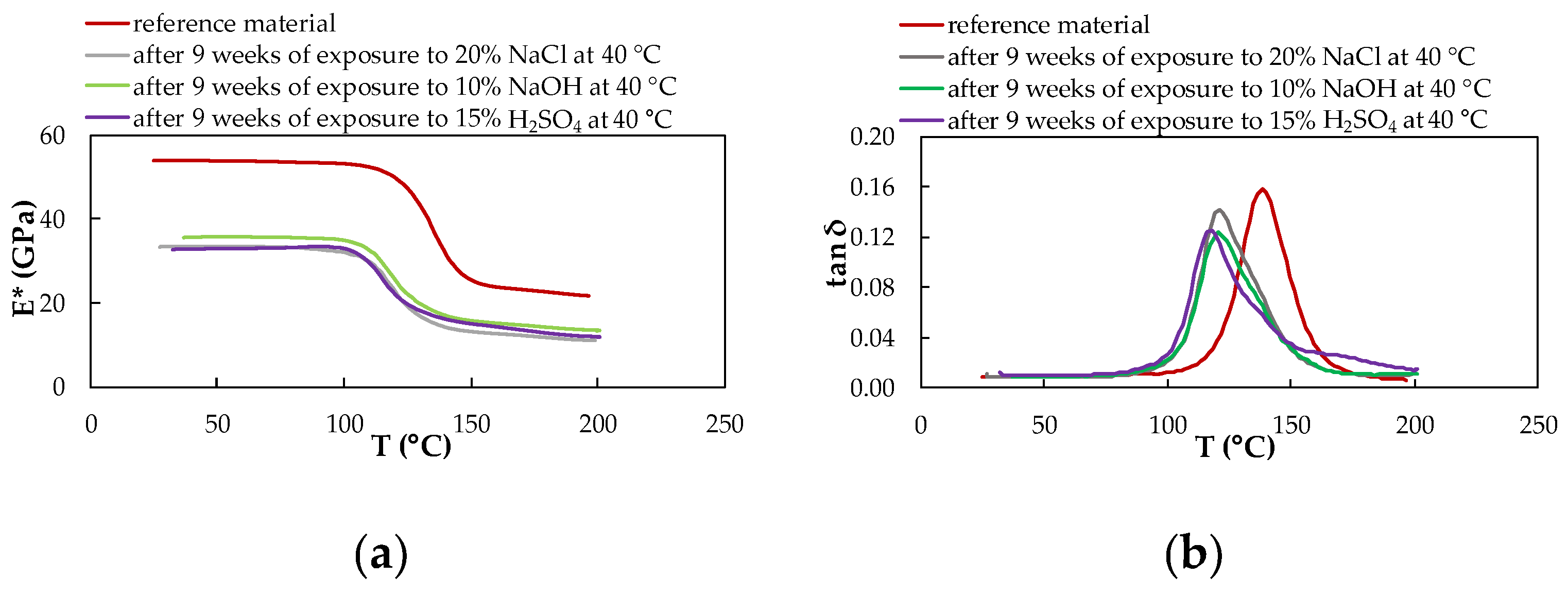
3.4. Dynamic Mechanical Analysis
4. Discussion
5. Conclusions
- Based on the observation of the experimental CFRP composite surface macrostructure through light and electron microscopy, it can be concluded that exposure to 10% NaOH and 15% H2SO4 had the greatest impact on the CFRP composite. In contrast, exposure to 20% NaCl did not leave any macroscopically visible changes on the surface of the investigated CFRP composite.
- The results of tensile and three-point bending tests showed that the influence of chemical and thermal influences on the degradation of the CFRP composite led to a decrease in mechanical properties under most exposure conditions.
- Determination of the viscoelastic properties of the experimental material by DMA to obtain information on how the chemical environments of a given temperature affect the viscoelastic properties of the material after nine weeks of exposure confirmed a synergistic influence. It is conditioned by the chemical and temperature influence of the experimental environments which is caused by degradation changes and plasticizing effect due to the water absorption causing a reduction of modulus of elasticity.
- A more pronounced occurrence of two separation relaxation events appears only at a temperature of 60 °C. A higher peak with a higher Tg value represents the formation of a bond to the reinforcement, and a lower peak with a lower Tg value represents the degradation processes of the CFRP composite, which are caused by the cleavage of the polymer chain.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Escale, L.; De Almeida, O.; Bernhart, G.; Ferrero, J.F. Comparison of the impact resistance of carbon/epoxy and carbon/peek composite laminates. In Proceedings of the ECCM15-15TH European Conference on Composite Materials, Venice, Italy, 24–28 June 2012. [Google Scholar]
- Tanks, J.; Sharp, S.; Harris, D. Charpy impact testing to assess the quality and durability of unidirectional CFRP rods. Polym. Test. 2016, 51, 63–68. [Google Scholar] [CrossRef]
- Kojnoková, T.; Nový, F.; Markovičová, L. Study of the effect of chemical solutions on impact resistance of carbon fiber reinforced polymer composite. Adv. Manuf. Repair Technol. Veh. Ind. Colloq. Proc. Žilina Žilinská Univerzita V Žiline 37 2022, 1, 58–61. [Google Scholar]
- Markovičová, L.; Zatkalíková, V.; Kojnoková, T.; Gaňa, D.; Liptáková, T. The physical-mechanical properties of low-density polyethylene films. IOP Conf. Ser. Mater. Sci. Eng. 2020, 726, 2984650. [Google Scholar] [CrossRef]
- García-Moreno, I.; Caminero, M.Á.; Rogriguez, G.P.; López-Cela, J.J. Effect of Thermal Ageing on the Impact and Flexural Damage Behaviour of Carbon Fibre-Reinforced Epoxy Laminates. Polymers 2019, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.D.; Sowntharya, L.; Kamal, K.K. Polymer-Based Composite Structures: Processing and Applications. In Composite Materials; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–36. ISBN 978-3-662-49514-8. [Google Scholar] [CrossRef]
- Kojnoková, T.; Markovičová, L.; Nový, F. Evaluation of tensile properties of carbon fiber reinforced polymers produced from commercial prepregs. Mater. Today Proc. 2022, 62, 2663–2668. [Google Scholar] [CrossRef]
- Rodriguez, L.A. Environmental Durability and Degradation of Fiber-Reinforced Bismaleimide/Quartz Composite for Aircraft Radome Applications. Dissertation Thesis, University of Miami, Miami, FL, USA, 2017. [Google Scholar]
- Bahari, A.; Nasiri, J.R. Properties of FRP composite durability. In Excellence in Concrete Construction through Innovation; Limbachiya, M.C., Kew, H.Y., Eds.; Taylor & Francis Group: London, UK, 2009; ISBN 978-0-415-47592-1. [Google Scholar]
- Minster, J. Aplikace Vláknových Polymerních Kompozitů ve Stavebnictví. Available online: http://www.csm-kompozity.wz.cz/stav.pdf (accessed on 4 January 2022).
- Amaro, A.P.B.M.; Reis, P.; Neto, M.A.; Santos, C.M. Effects of alkaline and acid solutions on glass/epoxy composites. Polym. Degrad. Stab. 2013, 98, 853–862. [Google Scholar] [CrossRef]
- Amaro, A.P.B.M.; Reis, P.; Neto, M.A.; Santos, C.M. Effect of different acid solutions on glass/epoxy composites. J. Reinf. Plast. Compos. 2013, 32, 1018–1029. [Google Scholar] [CrossRef]
- Mahmoud, M.K.; Tantawi, S.H. Effect of Strong Acids on Mechanical Properties of Glass=Polyester GRP Pipe at Normal and High Temperatures. Polym. Plast. Technol. Eng. 2003, 42, 677–688. [Google Scholar] [CrossRef]
- Hong, B.; Xian, G.; Wang, Z. Durability study of pultruded carbon fiber reinforced polymer plates subjected to water immersion. Adv. Struct. Eng. 2017, 21, 571–579. [Google Scholar] [CrossRef]
- Uthaman, A.; Xian, G.; Thomas, S.; Wang, Y. Durability of an Epoxy Resin and Its Carbon FiberReinforced Polymer Composite upon Immersion in Water, Acidic, and Alkaline Solutions. Polymers 2020, 12, 614. [Google Scholar] [CrossRef] [PubMed]
- Hammami, A.; Al-Ghuilani, N. Durability and environmental degradation of glassvinylester composites. Polym. Compos. 2004, 25, 609–616. [Google Scholar] [CrossRef]
- Muñoz, G.A. Composite Materials Processing, Applications, Characterizations. In Composite Materials; Kar, K., Ed.; Indian Institute of Technology Kanpur: Kanpur, India, 2017; ISBN 978-3-662-49514-8. [Google Scholar]
- Zhou, J.; Lucas, J.P. Hygrothermal effects of epoxy resin. Part II: Variations of glass transition temperature. Polymer 1999, 40, 5513–5522. [Google Scholar] [CrossRef]
- Parnas, L.; Katirci, N. Design of fiber-reinforced composite pressure vessels under various loading conditions. Compos. Struct. 2002, 58, 83–95. [Google Scholar] [CrossRef]
- Kim, G.; Sterkenburg, R.; Tsutsui, W. Investigating the effects of fluid intrusion on Nomex® honeycomb sandwich structures with carbon fiber facesheets. Compos. Struct. 2018, 2016, 535–549. [Google Scholar] [CrossRef]
- Silva, M.A.G.; Da Fonseca, B.S.; Biscaia, H. On estimates of durability of FRP based on accelerated tests. Compos. Struct. 2014, 116, 377–387. [Google Scholar] [CrossRef]
- Cromwell, J.R.; Harries, K.; Shahrooz, B.M. Environmental durability of externally bonded FRP materials intended for repair of concrete structures. Constr. Build. Mater. 2011, 25, 2528–2539. [Google Scholar] [CrossRef]
- Grammatikos, S.A.; Mark, E.; Mitchels, J.; Zafari, B. On the response to hygrothermal aging of pultruded FRPs used in the civil engineering sector. Mater. Des. 2016, 96, 283–295. [Google Scholar] [CrossRef]
- Li, C.; Yin, X.; Liu, Y.; Guo, R.; Xian, G. Long-term service evaluation of a pultruded carbon/glass hybrid rod exposed to elevated temperature, hydraulic pressure and fatigue load coupling. Int. J. Fatigue 2020, 134, 105480. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.-L.; Xian, G.; Wu, G.; Singh Raman, R.K.; Al-Saadi, S. Effect of sustained load and seawater and sea sand concrete environment on durability of basalt- and glass-fibre reinforced polymer (B/GFRP) bars. Corros. Sci. 2018, 138, 200–218. [Google Scholar] [CrossRef]
- Mortas, N.; Er, O.; Reis, P.N.B.; Ferreira, J.A.M. Effect of corrosive solutions on composites laminates subjected to low velocity impact loading. Compos. Struct. 2013, 108, 205–211. [Google Scholar] [CrossRef]
- Polak, P.I.S. The Influence of Moisture Absorption into Uncured Resins. J. Undergrad. Eng. Res. 2014, 6, 14. [Google Scholar]
- Bhide, S.J.; Zurale, M.M. Durability aspects of fiber reinforced composites. Durab. Build. Mater. Compon. 8 1999, 1–4, 1382–1391. [Google Scholar]
- Kumar, B.G.; Singh, R.P.; Nakamura, T. Degradation of Carbon Fiber-Reinforced Epoxy Composites by Ultraviolet Radiation and Condensation. J. Compos. Mater. 2002, 36, 2713–2733. [Google Scholar] [CrossRef]
- Kim, H.; Park, Y.H.; You, Y.J.; Moon, C.K. Durability of GFRP composite exposed to various environmental conditions. KSCE J. Civ. Eng. 2006, 10, 291–295. [Google Scholar] [CrossRef]
- Ji, Y.; Kim, Y.J. Effects of Sulfuric Acid on Durability Characteristics of CFRP Composite Sheets. J. Mater. Civ. Eng. 2017, 29, 04017159. [Google Scholar] [CrossRef]
- Dotson, D. The Effects of High Temperature on Epoxy. Available online: https://sciencing.com/effects-high-temperature-epoxy-8590977.html (accessed on 7 April 2020).
- Guermazi, N.; Elleuch, K.; Ayedi, H.F. The efect of time and aging temperature on structural and mechanical properties of pipeline coating. Mater. Des. 2010, 30, 2006–2010. [Google Scholar] [CrossRef]
- Marouani, S.; Curtil, L.; Hamelin, P. Ageing of carbon/epoxy and carbon/vinylester composites used in the reinforcement and/or the repair of civil engineering structures. Compos. Part B Eng. 2012, 43, 2020–2030. [Google Scholar] [CrossRef]
- Delasi, R.; Whiteside, J.B. Effect of Moisture on Epoxy Resins and Composites; American Society for Testing and Materials: West Conshohocken, PA, USA, 1978. [Google Scholar]
- Grace, L.R.; Altan, M.C. Three-Dimensional Anisotropic Moisture Absorption in QuartzReinforced Bismaleimide Laminates. Polym. Eng. Sci. 2014, 54, 137–146. [Google Scholar] [CrossRef]
- Ching, Y.C.; Gunathilake, T.M.S.U.; Ching, K.Y.; Chuah, C.H.; Sandu, V.; Singh, R.; Liou, N.-S. Effects of high temperature and ultraviolet radiation on polymer composites. In Durability and Life Prediction in Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Woodhead Publishing: Sawston, UK, 2019; pp. 407–426. [Google Scholar] [CrossRef]
- Phil, E.; Soutis, C. Polymer Composites in the Aerospace Industry, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]







| Research | Material | Types of Conditioning | Variables | Methodology | Conclusions |
|---|---|---|---|---|---|
| [12] | GFRP | NaOH, HCl | alkaline and acid solution, time | three-point bending test | A decrease in flexural strength and flexural modulus over time, influence of NaOH base was more aggressive compared to HCl. |
| [14] | CFRP | water | temperature | immersion and mechanical properties | Degradation and decrease of mechanical properties. |
| [20] | GFRP | hydraulic fluid, or engine oil | types of environment | absorption, three-point bending test, impact test | A greater reduction in mechanical properties due to hydraulic fluid. |
| [21] | GFRP | salt fog and immersion into salt water | time of exposure and temperature | tensile test | GFRP laminates decreased their tensile strength after immersion in salt water. |
| [22] | CFRP and GFRP | water, salt water, alkaline environment, elevated temperature, exposure to diesel fuel | type of environment, type of FRP composite, time of exposure | tensile test, short beam shear test, and bending test | An adverse effect on the overall performance of CFRP and GFRP laminates, particularly bond strength, has been noted. Immersion in salt and alkaline environment significantly affect the GFRP samples. |
| [23] | GFRP | hygrothermal conditions | temperature | moisture absorption, tensile test, dynamic mechanical analysis, SEM (scanning electron microscopy), FTIR (Fourier-transform infrared spectroscopy) | Increased temperature accelerates the absorption of moisture and moisture diffusion coefficient. No influence was observed on the tensile strength and modulus of elasticity of the samples, with the exception of aging at 80 °C. |
| [24] | pultruded carbon/glass hybrid rod | underground oil well-exposure environment of elevated temperature hydraulic pressure and fatigue load | exposure conditions | the long-term evaluation of mechanical, and thermal properties and microstructure | The short beam shear strength and interface shear strength of rods decreased with the exposure time. Higher exposed temperature and hydraulic pressure aggravated the degradation by accelerating the diffusion of water molecules into HFRP rods. |
| [25] | basalt- and glass-fiber-reinforced polymer bars | seawater and sea sand concrete (SWSSC) | synergistic effect of sustained load and corrosion environment | scanning electron microscopy | SEM results reveal the degradation mechanism of BFRP bars in stress condition in SWSSC solution. |
| Layer | Orientation | Carbon Fiber Fabric | Epoxy Resin |
|---|---|---|---|
| 1. | 0/90° | GG280T(T300) | DT121HT-42 KE |
| 2. | ±45° | ||
| 3. | 0/90° |
| Weeks of Immersion | Chemical Solution (wt. %) | Temperature (°C) |
|---|---|---|
| 3 | 20% NaCl | 23 |
| 6 | 10% NaOH | 40 |
| 9 | 15% H2SO4 | 60 |
| Chemical Solution | Temperature | Tg1 |
|---|---|---|
| - | - | 122.95 |
| 20% NaCl | 23 | 113.64 |
| 40 | 107.95 | |
| 60 | 102.27 | |
| 10% NaOH | 23 | 110.80 |
| 40 | 107.95 | |
| 60 | 93.75 | |
| 15% H2SO4 | 23 | 107.95 |
| 40 | 106.82 | |
| 60 | 96.59 |
| Chemical Solution | Temperature | Tg1 | Tg2 |
|---|---|---|---|
| - | - | 138.24 | |
| 20% NaCl | 23 | 120.59 | |
| 40 | 123.53 | ||
| 60 | 116.28 | 126.47 | |
| 10% NaOH | 23 | 126.47 | |
| 40 | 120.59 | ||
| 60 | 111.76 | 123.53 | |
| 15% H2SO4 | 23 | 120.59 | |
| 40 | 117.65 | ||
| 60 | 117.76 | 125.25 |
| Mechanical Property | 3 Weeks | 6 Weeks | 9 Weeks |
|---|---|---|---|
| Tensile strength | 20% NaCl at 40 °C by 20.62% | 10% NaOH at 60 °C by 35.13% | 15% H2SO4 at 40 °C by 34.83% |
| Flexural strength | 10% NaOH at 60 °C by 27.13% | 10% NaOH at 60 °C by 29.97% 15% H2SO4 at 60 °C by 9.14% | 10% NaOH at 60 °C by 29.96% 15% H2SO4 at 60 °C by 10.88% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kojnoková, T.; Nový, F.; Markovičová, L. The Study of Chemical and Thermal Influences of the Environment on the Degradation of Mechanical Properties of Carbon Composite with Epoxy Resin. Polymers 2022, 14, 3245. https://doi.org/10.3390/polym14163245
Kojnoková T, Nový F, Markovičová L. The Study of Chemical and Thermal Influences of the Environment on the Degradation of Mechanical Properties of Carbon Composite with Epoxy Resin. Polymers. 2022; 14(16):3245. https://doi.org/10.3390/polym14163245
Chicago/Turabian StyleKojnoková, Tatiana, František Nový, and Lenka Markovičová. 2022. "The Study of Chemical and Thermal Influences of the Environment on the Degradation of Mechanical Properties of Carbon Composite with Epoxy Resin" Polymers 14, no. 16: 3245. https://doi.org/10.3390/polym14163245






