In Vivo Evaluation of PCL Vascular Grafts Implanted in Rat Abdominal Aorta
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polymer Composition
2.2. Vascular Scaffold Fabrication
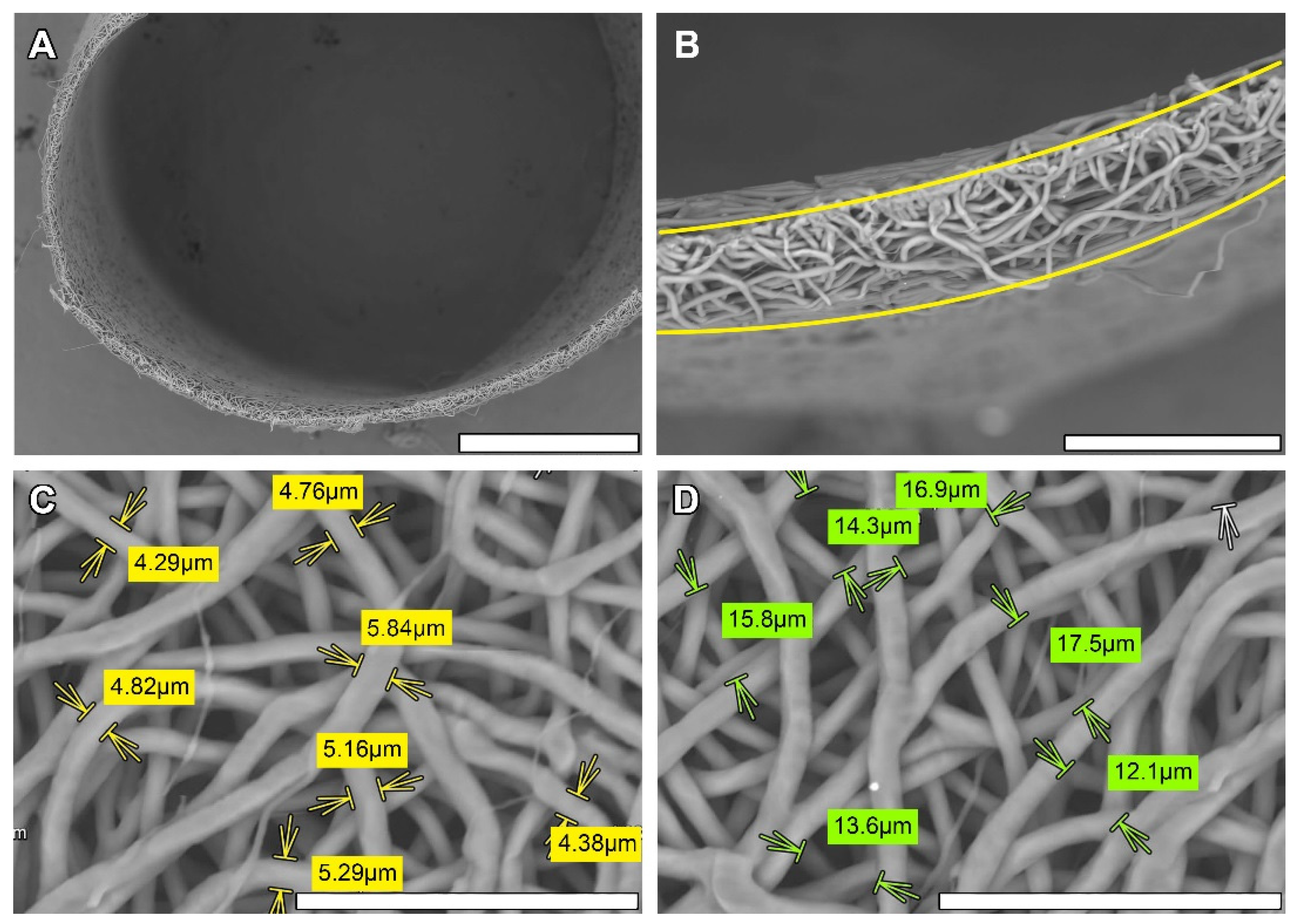
2.3. Scanning Electronic Microscopy (SEM)
2.4. Graft Sterilization
2.5. In Vivo Implantation
2.5.1. Premedication
2.5.2. Preoperational Procedures
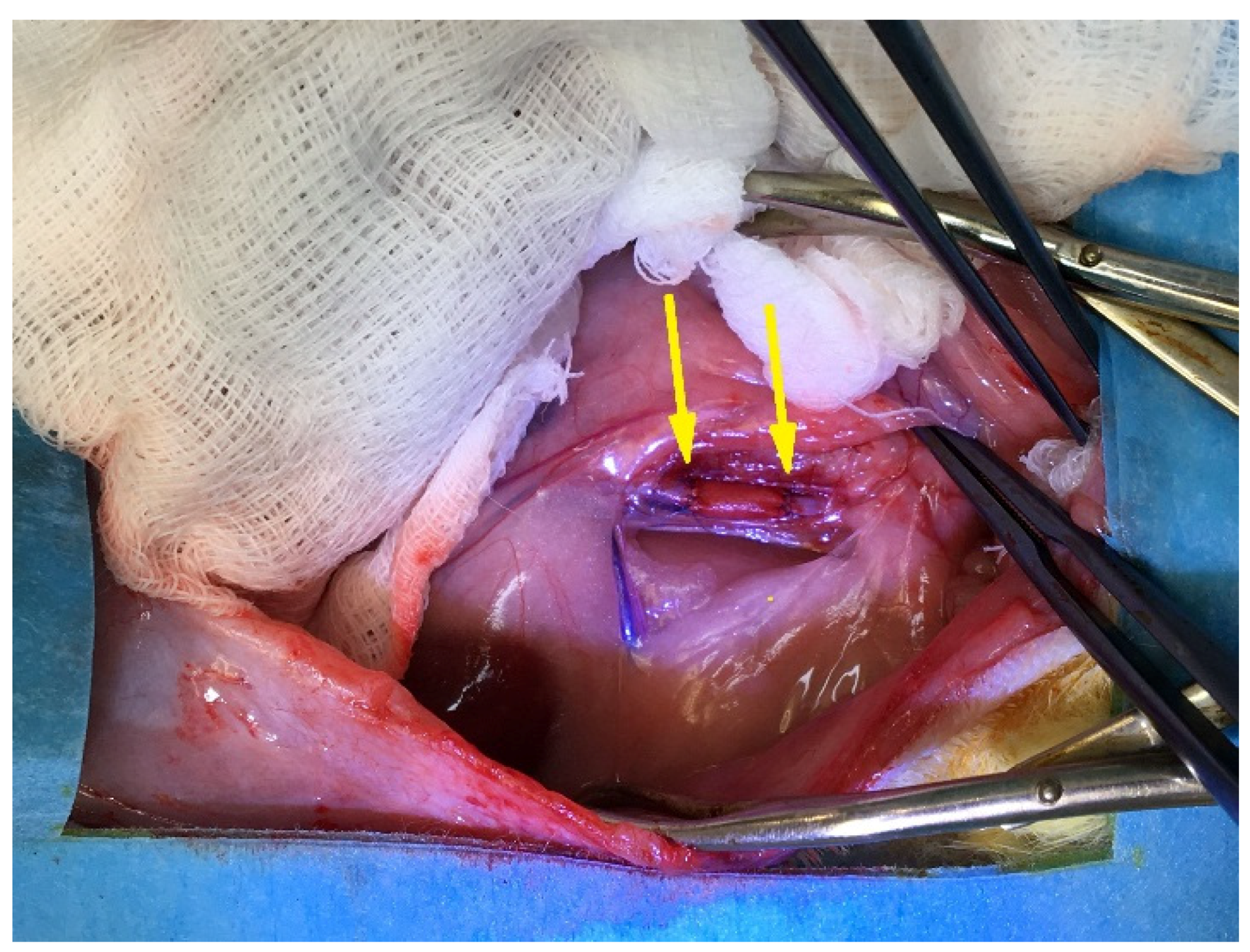
2.5.3. Surgery
2.5.4. Postoperative Period
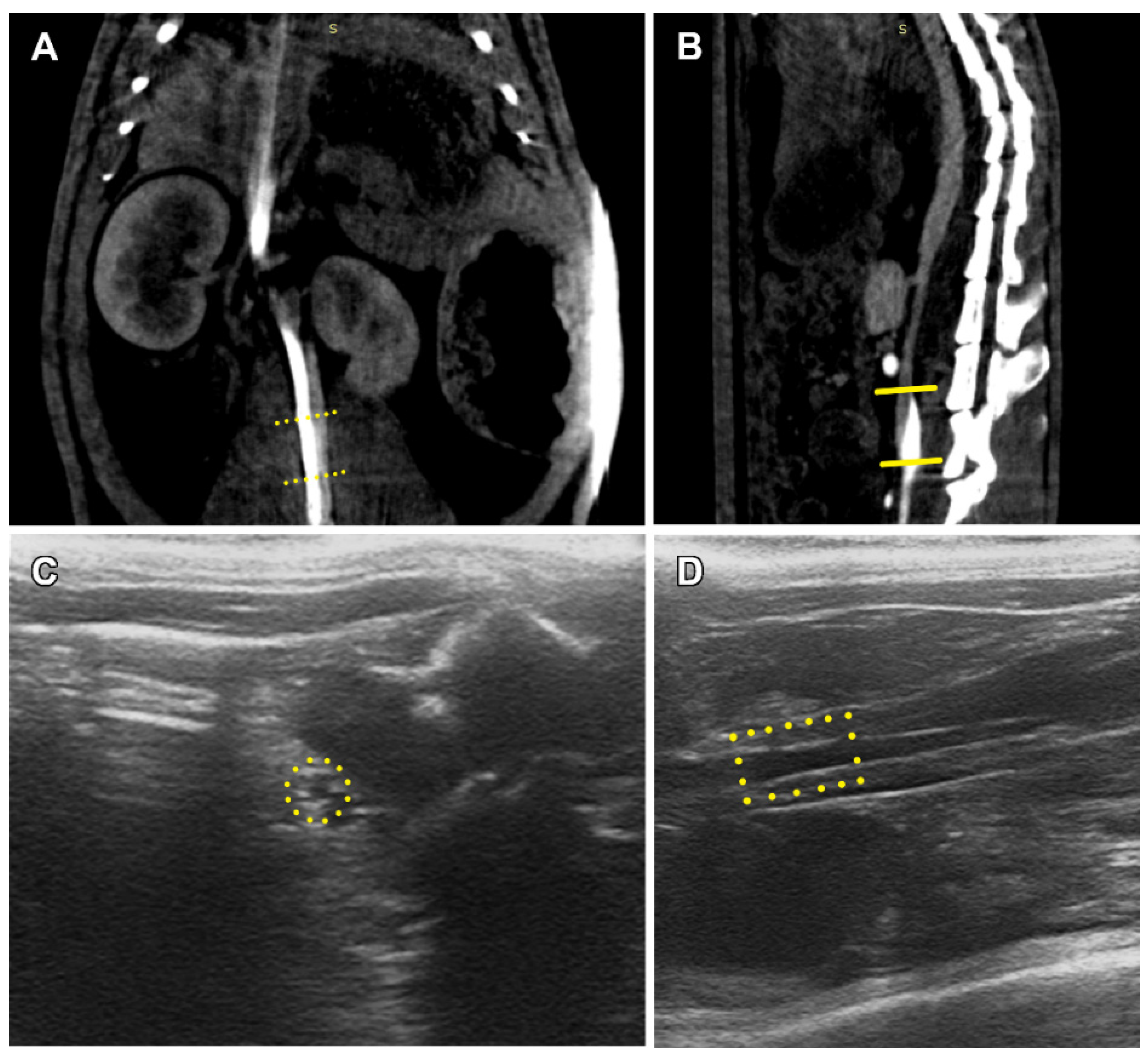
2.6. Angiography
2.7. Ultrasound Diagnostic
2.8. Euthanasia and Autopsy
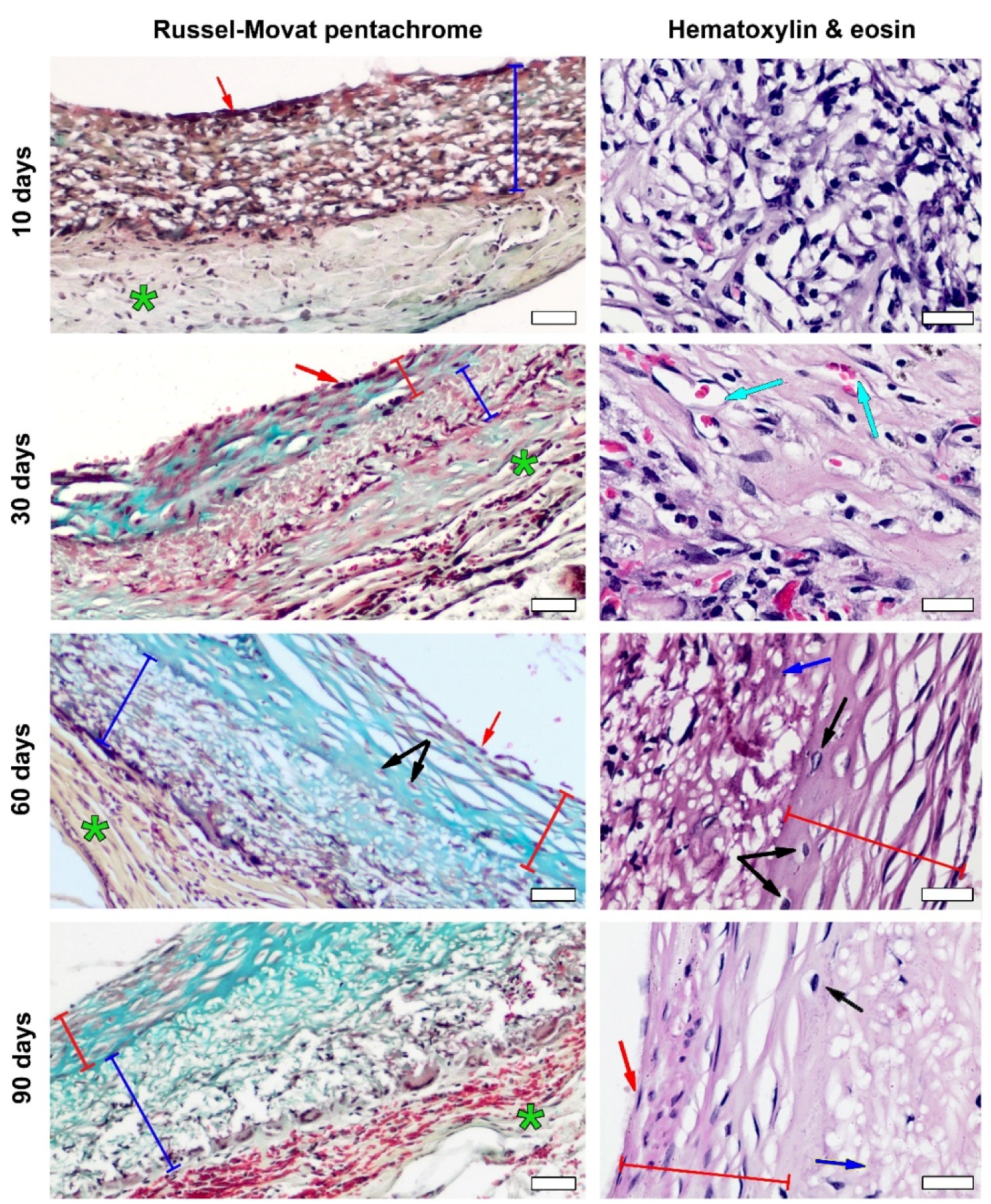
2.9. Histology
2.10. Statistical Analysis
3. Results
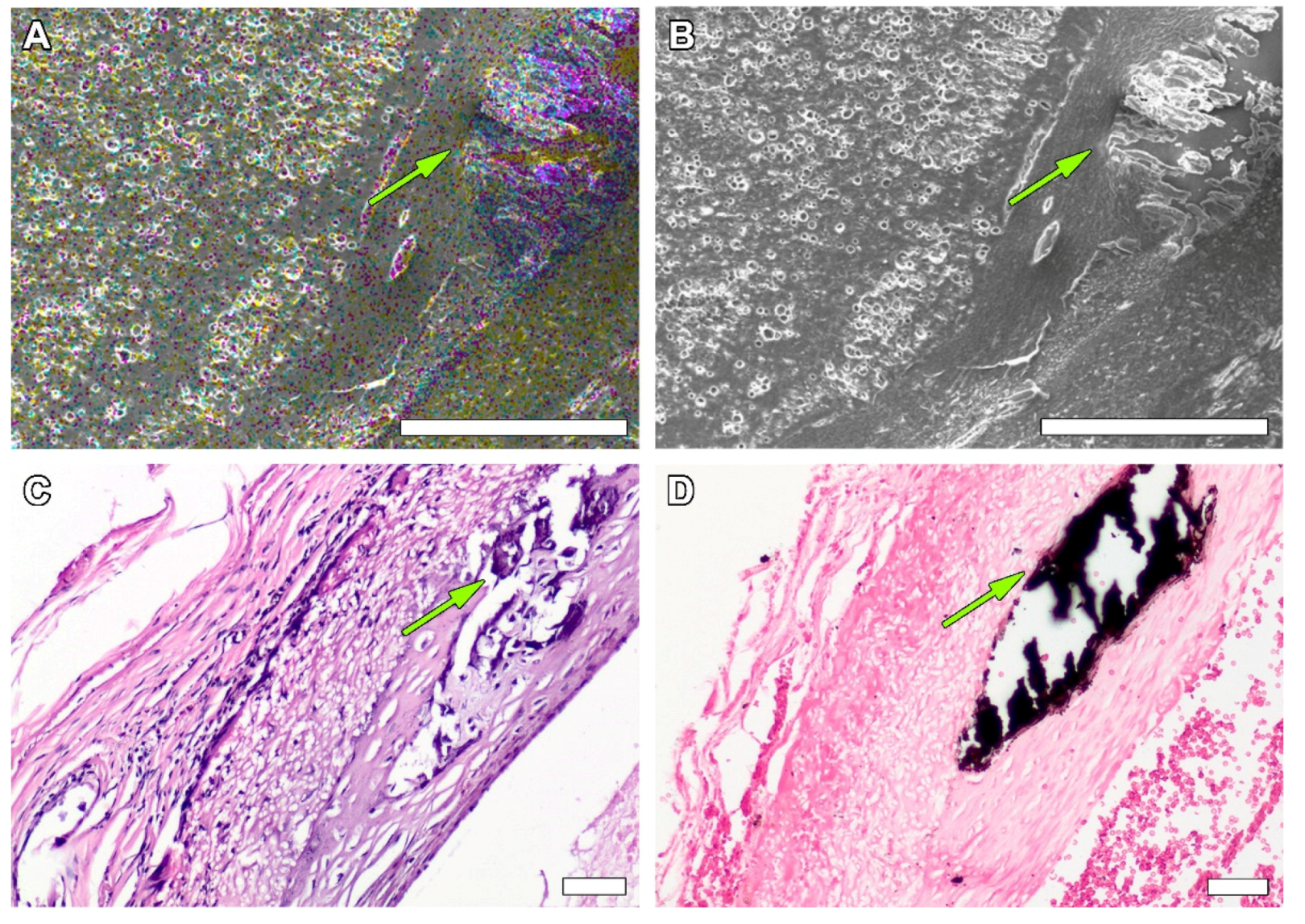
3.1. Non-Implanted Scaffolds
3.2. PCL Graft Observation in Dynamics
3.3. PCL Graft Transformation in Dynamics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leal, B.B.; Wakabayashi, N.; Oyama, K.; Kamiya, H.; Braghirolli, D.I.; Pranke, P. Vascular tissue engineering: Polymers and methodologies for small caliber vascular grafts. Front. Cardiovasc. Med. 2021, 7, 376. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, Y.; Li, Q.; Turng, L.S. Artificial small-diameter blood vessels: Materials, fabrication, surface modification, mechanical properties, and bioactive functionalities. J. Mater. Chem. B 2020, 8, 1801–1822. [Google Scholar] [CrossRef] [PubMed]
- Shinoka, T. What is the best material for extracardiac Fontan operation? J. Thorac Cardiovasc Surg. 2017, 153, 1551–1552. [Google Scholar] [CrossRef]
- Furdella, K.J.; Higuchi, S.; Behrangzade, A.; Kim, K.; Wagner, W.R.; Vande Geest, J.P. In-vivo assessment of a tissue engineered vascular graft computationally optimized for target vessel compliance. Acta Biomater. 2021, 123, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Rickel, A.P.; Deng, X.; Engebretson, D.; Hong, Z. Electrospun nanofiber scaffold for vascular tissue engineering. Mater. Sci. Eng. Part C 2021, 129, 112373. [Google Scholar] [CrossRef] [PubMed]
- Duijvelshoff, R.; Van Engeland, N.C.; Gabriels, K.M.; Söntjens, S.H.; Smits, A.I.; Dankers, P.Y.; Bouten, C.V. Host Response and Neo-Tissue Development during Resorption of a Fast Degrading Supramolecular Electrospun Arterial Scaffold. Bioengineering 2018, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Lutter, G.; Puehler, T.; Cyganek, L.; Seiler, J.; Rogler, A.; Herberth, T.; Knueppel, P.; Gorb, S.N.; Sathananthan, J.; Sellers, S.; et al. Biodegradable Poly-ε-Caprolactone Scaffolds with ECFCs and iMSCs for Tissue-Engineered Heart Valves. Int. J. Mol. Sci. 2022, 23, 527. [Google Scholar] [CrossRef]
- Stowell, C.E.; Wang, Y. Quickening: Translational design of resorbable synthetic vascular grafts. Biomaterials 2018, 173, 71–86. [Google Scholar] [CrossRef]
- Matsuzaki, Y.; John, K.; Shoji, T.; Shinoka, T. The evolution of tissue engineered vascular graft technologies: From preclinical trials to advancing patient care. Appl. Sci. 2019, 9, 1274. [Google Scholar] [CrossRef]
- Ground, M.; Waqanivavalagi, S.; Walker, R.; Milsom, P.; Cornish, J. Models of immunogenicity in preclinical assessment of tissue engineered heart valves. Acta Biomater. 2021, 133, 102–113. [Google Scholar] [CrossRef]
- Hiob, M.A.; Crouch, G.W.; Weiss, A.S. Elastomers in vascular tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhou, X.; Wei, Y.; Zhang, Q.; Wang, T.; Zhu, M.; Li, W.; Huang, R.; Liu, R.; Chen, J.; et al. Small-diameter hybrid vascular grafts composed of polycaprolactone and polydioxanone fibers. Sci. Rep. 2017, 7, 3615. [Google Scholar] [CrossRef] [PubMed]
- Zavan, B.; Gardin, C.; Guarino, V.; Rocca, T.; Cruz Maya, I.; Zanotti, F.; Ferroni, L.; Brunello, G.; Chachques, J.-C.; Ambrosio, L.; et al. Electrospun PCL-Based Vascular Grafts: In Vitro Tests. Nanomaterials 2021, 11, 751. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Jeong, S.I.; Shim, K.M.; Jang, K.; Park, J.S.; Lim, Y.M.; Kang, S.S. Polymers. In Vivo Evaluation of Gamma-Irradiated and Heparin-Immobilized Small-Diameter Polycaprolactone Vascular Grafts with VEGF in Aged Rats. Polymers 2022, 14, 1265. [Google Scholar] [CrossRef] [PubMed]
- Talacua, H.; Smits, A.I.; Muylaert, D.E.; van Rijswijk, J.W.; Vink, A.; Verhaar, M.C.; Driessen-Mol, A.; van Herwerden, L.A.; Bouten, C.V.; Kluin, J.; et al. In Situ Tissue Engineering of Functional Small-Diameter Blood Vessels by Host Circulating Cells Only. Tissue Eng. Part A 2015, 21, 2583–2594. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, X.; Yang, L.; Sun, L.; Mu, S.; Zong, H.; Zhang, Q. Evaluation of remodeling and regeneration of electrospun PCL/fibrin vascular grafts in vivo. Mater. Sci. Eng. C 2021, 118, 111441. [Google Scholar] [CrossRef]
- Wu, Y.; Qin, Y.; Wang, Z.; Wang, J.; Zhang, C.; Li, C.; Kong, D. The regeneration of macro-porous electrospun poly (ɛ-caprolactone) vascular graft during long-term in situ implantation. J. Biomed. Mater. Res. B 2018, 106, 1618–1627. [Google Scholar] [CrossRef]
- Pektok, E.; Nottelet, B.; Tille, J.-C.; Gurny, R.; Kalangos, A.; Moeller, M.; Walpoth, B.H. Degradation and healing character-istics of small-diameter poly (ε-caprolactone) vascular grafts in the rat systemic arterial circulation. Circulation 2008, 118, 2563–2570. [Google Scholar] [CrossRef]
- De Valence, S.; Tille, J.-C.; Mugnai, D.; Mrowczynski, W.; Gurny, R.; Möller, M.; Walpoth, B.H. Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model. Biomaterials 2012, 33, 38–47. [Google Scholar] [CrossRef]
- Dokuchaeva, A.A.; Timchenko, T.P.; Karpova, E.V.; Vladimirov, S.V.; Soynov, I.A.; Zhuravleva, I.Y. Effects of Electrospinning Parameter Adjustment on the Mechanical Behavior of Poly-ε-caprolactone Vascular Scaffolds. Polymers 2022, 14, 349. [Google Scholar] [CrossRef]
- Pereira, L.M.; Bezerra, D.G.; Mandarim-de-Lacerda, C.A. Aortic wall remodeling in rats with nitric oxide deficiency treated by enalapril or verapamil. Pathol.-Res. Pract. 2004, 200, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xiong, G.Z.; Luo, D.Y.; Zou, Q.Q.; Wang, Y.Y.J.; Bi, G.S. Daxx ameliorates abdominal aortic aneurysm through inhibiting the TGF-β1-mediated PI3K/AKT/ID2 signaling pathway. Eur. J. Inflamm. 2022, 20, 1721727X221091532. [Google Scholar] [CrossRef]
- Lusiantari, R.; Pramaningtyas, M.D.; Nurmasitoh, T.; Pattimura, R.H.; Dewanti, A. Shortening tends to increase aortic foam cell count and wall thickness in male Wistar rats. Univ. Med. 2018, 37, 13–18. [Google Scholar] [CrossRef]
- Bezie, Y.; Lacolley, P.; Laurent, S.; Gabella, G. Connection of smooth muscle cells to elastic lamellae in aorta of spontaneously hypertensive rats. Hypertension 1998, 32, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, Y.; Iwaki, R.; Reinhardt, J.W.; Chang, Y.C.; Miyamoto, S.; Kelly, J.; Zbinden, J.; Blum, K.; Mirhaidari, G.; Ulziibayar, A.; et al. The effect of pore diameter on neo-tissue formation in electrospun biodegradable tissue-engineered arterial grafts in a large animal model. Acta Biomater. 2020, 115, 176–184. [Google Scholar] [CrossRef]
- Garg, K.; Pullen, N.A.; Oskeritzian, C.A.; Ryan, J.J.; Bowlin, G.L. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials 2013, 34, 4439–4451. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, Y.; Wang, J.; Yang, X.; Wu, Y.; Wang, K.; Gao, X.; Li, D.; Li, Y.; Zheng, X.-L.; et al. The effect of thick fibers and large pores of electrospun poly (ε-caprolactone) vascular grafts on macrophage polarization and arterial regeneration. Biomaterials 2014, 35, 5700–5710. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.; Sant, S.; Nichol, J.W.; Khabiry, M.; Traversa, E.; Khademhosseini, A. Controlling the porosity of fibrous scaffolds by modulating the fiber diameter and packing density. J. Biomed. Mater. Res. A 2011, 96, 566–574. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, C.; Liu, Y.; Lu, T.; Wu, Z. Strategies in cell-free tissue-engineered vascular grafts. J. Biomed. Mater. Res. A 2020, 108, 426–445. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, F.; Guo, Z.; Qiang, Z. Tissue-engineered vascular grafts and regeneration mechanisms. J. Mol. Cell. Cardiol. 2021, 165, 40–53. [Google Scholar] [CrossRef]
- Klopfleisch, R.; Jung, F. The pathology of the foreign body reaction against biomaterials. J. Biomed. Mater. Res. A 2017, 105, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, I.; Sadikoglu, G.T. Design parameters for electrospun biodegradable vascular grafts. J. Ind. Text. 2018, 47, 2205–2227. [Google Scholar] [CrossRef]
- Sadeghipour, A.; Babaheidarian, P. Making Formalin-Fixed, Paraffin Embedded Blocks. In Biobanking; Methods in Molecular Biology Serie; Yong, W., Ed.; Humana Press: New York, NY, USA, 2019; Volume 1897, pp. 253–268. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dokuchaeva, A.A.; Mochalova, A.B.; Timchenko, T.P.; Podolskaya, K.S.; Pashkovskaya, O.A.; Karpova, E.V.; Ivanov, I.A.; Filatova, N.A.; Zhuravleva, I.Y. In Vivo Evaluation of PCL Vascular Grafts Implanted in Rat Abdominal Aorta. Polymers 2022, 14, 3313. https://doi.org/10.3390/polym14163313
Dokuchaeva AA, Mochalova AB, Timchenko TP, Podolskaya KS, Pashkovskaya OA, Karpova EV, Ivanov IA, Filatova NA, Zhuravleva IY. In Vivo Evaluation of PCL Vascular Grafts Implanted in Rat Abdominal Aorta. Polymers. 2022; 14(16):3313. https://doi.org/10.3390/polym14163313
Chicago/Turabian StyleDokuchaeva, Anna A., Aleksandra B. Mochalova, Tatyana P. Timchenko, Kseniya S. Podolskaya, Oxana A. Pashkovskaya, Elena V. Karpova, Ilya A. Ivanov, Natalya A. Filatova, and Irina Yu Zhuravleva. 2022. "In Vivo Evaluation of PCL Vascular Grafts Implanted in Rat Abdominal Aorta" Polymers 14, no. 16: 3313. https://doi.org/10.3390/polym14163313






