The Processing and Electrical Properties of Isotactic Polypropylene/Copper Nanowire Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
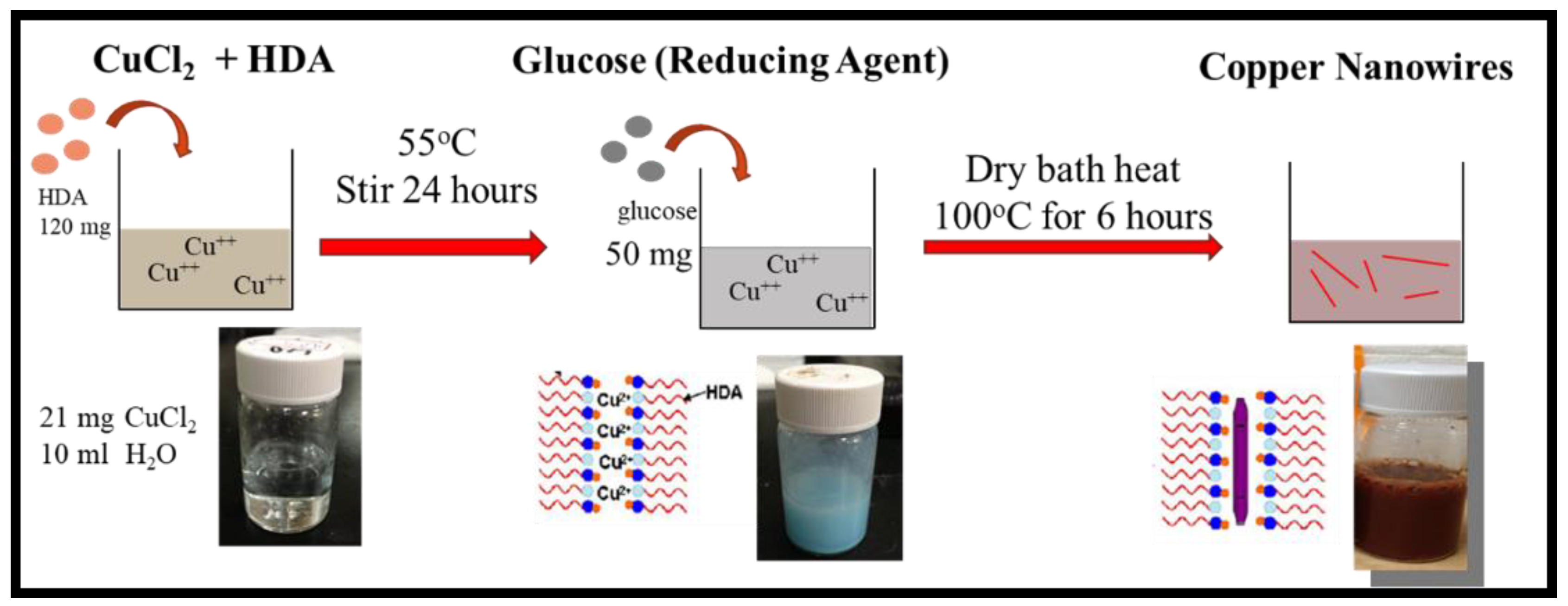
2.2. Copper Nanowires Preparation
2.3. Preparation of i-PP/CuNWs Composites
2.4. Characterization
2.5. Data Analysis
3. Results and Discussion
3.1. The UV-Vis Absorption Spectra for CuNWs Solutions
3.2. Transcrystallization of i-PP/CuNWs Composites
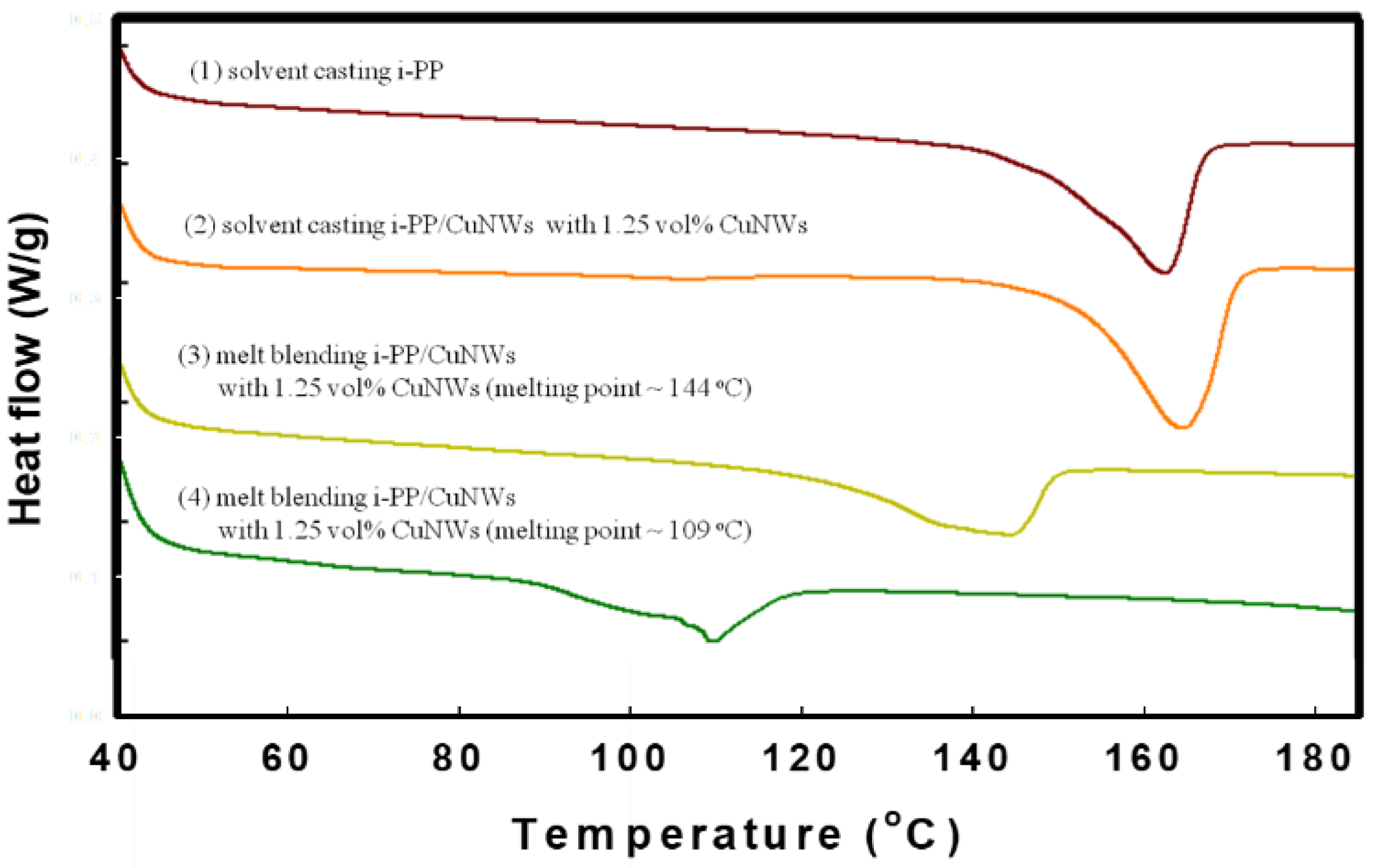
3.3. DSC Thermal Analysis for i-PP/CuNWs Composites
3.4. XRD Analysis for i-PP/CuNWs Composites
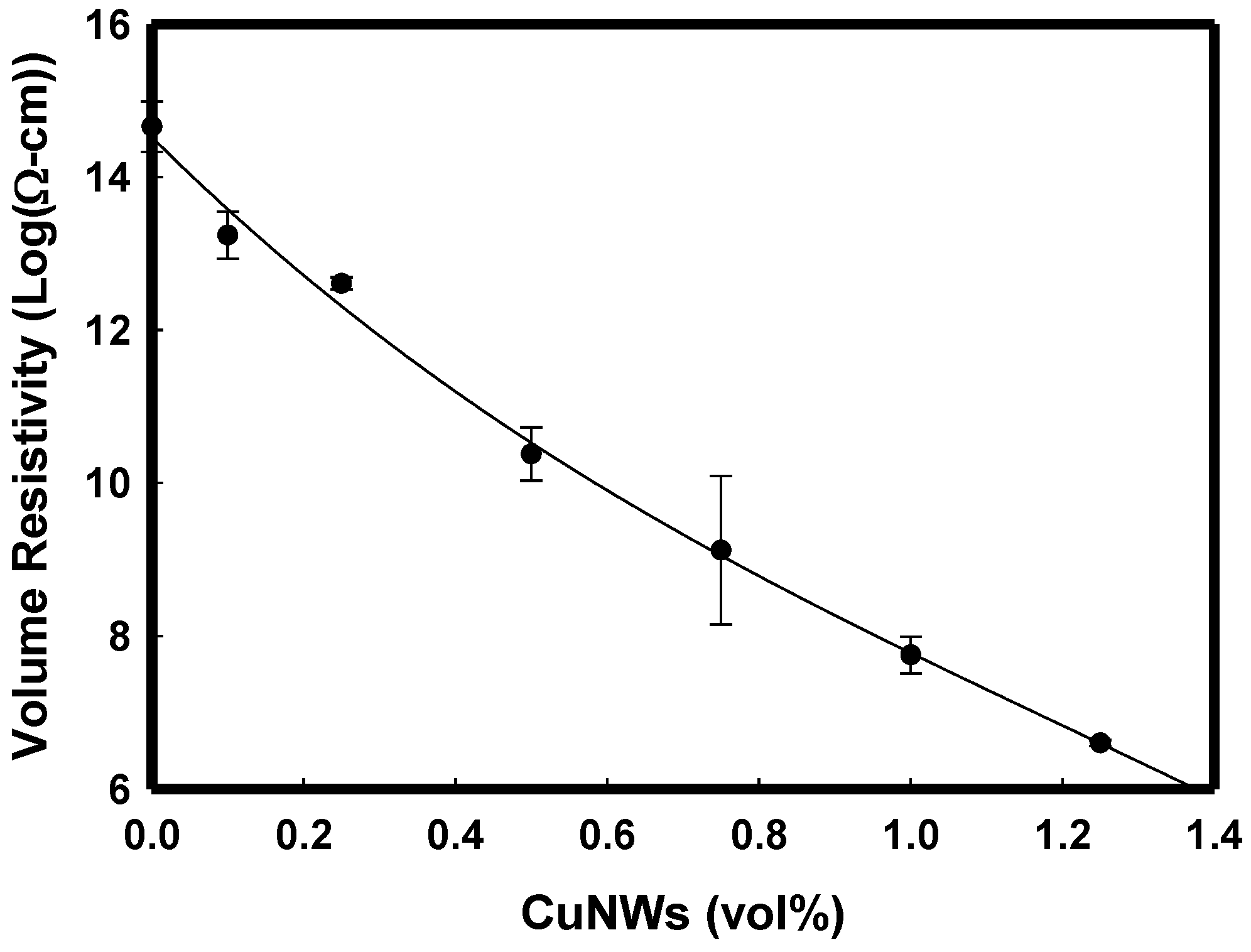
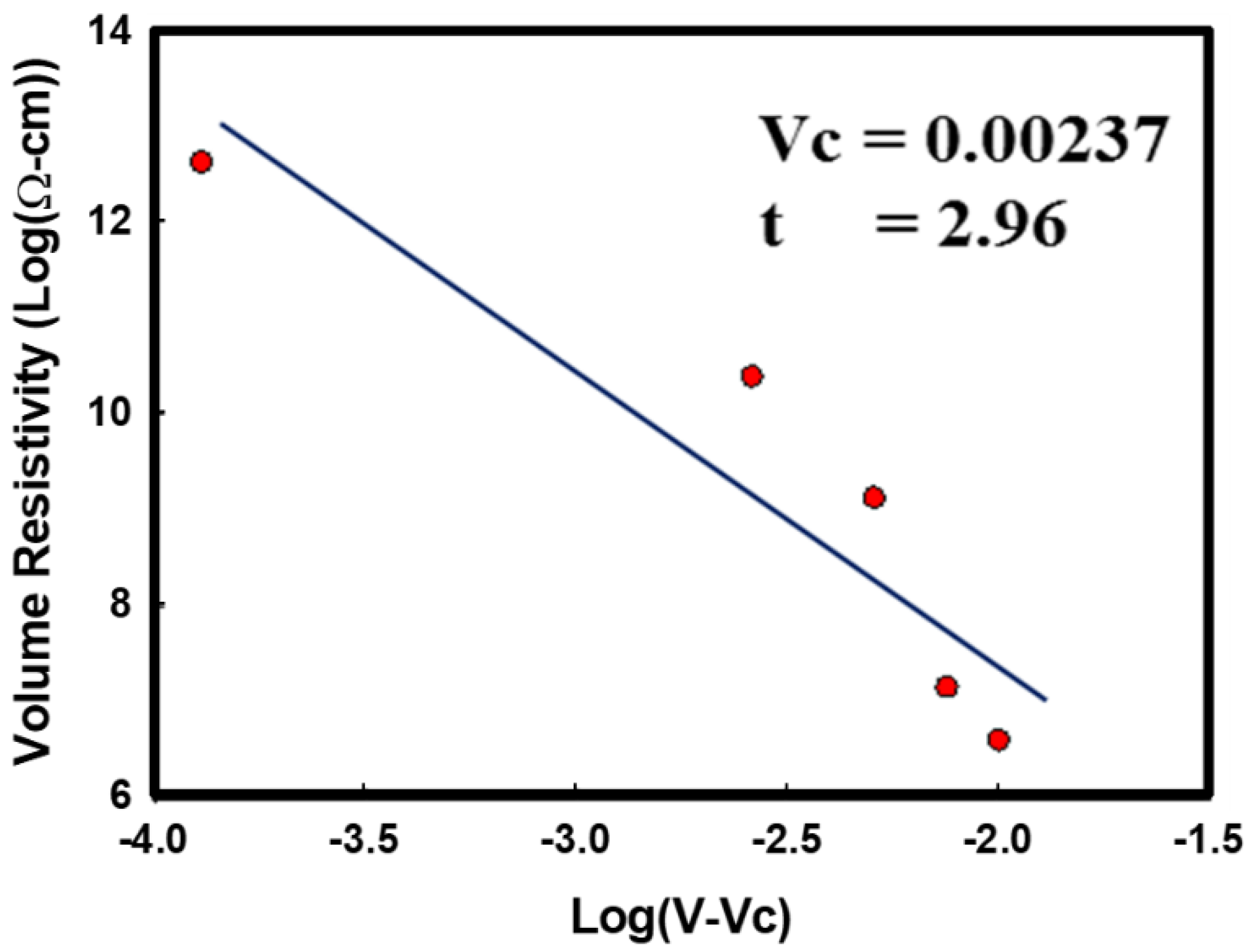
3.5. Electrical Volume Resistivity Measurement for i-PP/CuNWs Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nwabunma, D.; Kyu, T.; Wiley, I. Polyolefin Composites; Wiley-Interscience: Hoboken, NJ, USA, 2008. [Google Scholar]
- Karian, H.G. Handbook of Polypropylene and Polypropylene Composites; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- Karger-Kocsis, J.; Bárány, T.; SpringerLink. Polypropylene Handbook: Morphology, Blends and Composites, 1st ed.; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Maddah, H.A. Polypropylene as a Promising Plastic: A Review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Harekrushna, S.; Rabiranjan, M.; Chiranjit, D.; Mutlu, O.; Subash Chandra, M. High Density Polyethylene (HDPE) and Polypropylene (PP) Polyblend: An Experimental Approach; B P International: West Bengal, India, 2019; Volume 1. [Google Scholar]
- Bailey, A.G. Static Electricity. AccessScience. 2021. [Google Scholar] [CrossRef]
- Khazhinsky, M.G. 9-Protecting against electrostatic discharge (ESD) in complementary metal oxide semiconductor (CMOS) integrated circuits (ICs) manufactured using silicon-on-insulator (SOI) technology. In Silicon-On-Insulator (SOI) Technology; Woodhead Publishing: Cambridge, UK, 2014; pp. 243–271. [Google Scholar]
- Amarasekera, J. Conductive plastics for electrical and electronic applications. Reinf. Plast. 2005, 49, 38–41. [Google Scholar] [CrossRef]
- Gulrez, S.K.H.; Ali Mohsin, M.E.; Shaikh, H.; Anis, A.; Pulose, A.M.; Yadav, M.K.; Qua, E.H.P.; Al-Zahrani, S.M. A review on electrically conductive polypropylene and polyethylene. Polym. Compos. 2014, 35, 900–914. [Google Scholar] [CrossRef]
- Liu, H.; Thostenson, E.T. 6.11 Conductive Nanocomposites for Multifunctional Sensing Applications. In Comprehensive Composite Materials II; Beaumont, P.W.R., Zweben, C.H., Eds.; Elsevier: Oxford, UK, 2018; pp. 315–351. [Google Scholar]
- Gelves, G.A.; Lin, B.; Sundararaj, U.; Haber, J.A. Low Electrical Percolation Threshold of Silver and Copper Nanowires in Polystyrene Composites. Adv. Funct. Mater. 2006, 16, 2423–2430. [Google Scholar] [CrossRef]
- Nam, V.B.; Lee, D. Copper Nanowires and Their Applications for Flexible, Transparent Conducting Films: A Review. Nanomaterials 2016, 6, 47. [Google Scholar] [CrossRef]
- Esumi, K.; Suzuki, A.; Yamahira, A.; Torigoe, K. Role of Poly(amidoamine) Dendrimers for Preparing Nanoparticles of Gold, Platinum, and Silver. Langmuir 2000, 16, 2604–2608. [Google Scholar] [CrossRef]
- Yu, D.; Yam, V.W.-W. Controlled Synthesis of Monodisperse Silver Nanocubes in Water. J. Am. Chem. Soc. 2004, 126, 13200–13201. [Google Scholar] [CrossRef]
- Liu, G.; Cai, W.; Liang, C. Trapeziform Ag Nanosheet Arrays Induced by Electrochemical Deposition on Au-Coated Substrate. Cryst. Growth Des. 2008, 8, 2748–2752. [Google Scholar] [CrossRef]
- Gedanken, A. Using sonochemistry for the fabrication of nanomaterials. Ultrason. Sonochem. 2004, 11, 47–55. [Google Scholar] [CrossRef]
- Mansour, H.H.; Eid, M.; El-Arnaouty, M.B. Effect of silver nanoparticles synthesized by gamma radiation on the cytotoxicity of doxorubicin in human cancer cell lines and experimental animals. Hum. Exp. Toxicol. 2018, 37, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Julkarnain, M.; Mondal, A.; Rahman, M.M.; Rana, M.S. Preparation and Properties of Chemically Reduced Cu and Ag Nanoparticles. In Proceedings of the International Conference on Mechanical, Industrial and Materials Engineering 2013 (ICMIME2013), Rajshahi, Bangladesh, 1–3 November 2013. [Google Scholar]
- Luo, M.; Zhou, M.; da Silva, R.R.; Tao, J.; Figueroa-Cosme, L.; Gilroy, K.D.; Peng, H.-C.; He, Z.; Xia, Y. Pentatwinned Cu Nanowires with Ultrathin Diameters below 20 nm and Their Use as Templates for the Synthesis of Au-Based Nanotubes. ChemNanoMat 2017, 3, 190–195. [Google Scholar] [CrossRef]
- Ravi Kumar, D.V.; Kim, I.; Zhong, Z.; Kim, K.; Lee, D.; Moon, J. Cu(ii)–alkyl amine complex mediated hydrothermal synthesis of Cu nanowires: Exploring the dual role of alkyl amines. Phys. Chem. Chem. Phys. 2014, 16, 22107–22115. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Liu, Y.; Li, J.; Iyer, S.; Reynolds, L. Doping Dependent Magnetic Behavior in MBE Grown GaAs1-xSbx Nanowires. Sci. Rep. 2020, 10, 8995. [Google Scholar] [CrossRef]
- Peng, H.; Xie, C.; Schoen, D.T.; Cui, Y. Large Anisotropy of Electrical Properties in Layer-Structured In2Se3 Nanowires. Nano Lett. 2008, 8, 1511–1516. [Google Scholar] [CrossRef]
- Wang, Z. Transmission electron microscopy of shape-controlled nanocrystals and their assemblies. J. Phys. Chem. B 2000, 104, 1153–1175. [Google Scholar] [CrossRef]
- Jin, M.; He, G.; Zhang, H.; Zeng, J.; Xie, Z.; Xia, Y. Shape-Controlled Synthesis of Copper Nanocrystals in an Aqueous Solution with Glucose as a Reducing Agent and Hexadecylamine as a Capping Agent. Angew. Chem. Int. Ed. 2011, 50, 10560–10564. [Google Scholar] [CrossRef]
- Jia, B.; Qin, M.; Zhang, Z.; Chu, A.; Zhang, L.; Liu, Y.; Qu, X. The influence of reagents on the preparation of Cu nanowires by tetradecylamine-assisted hydrothermal method. J. Mater. Sci. 2013, 48, 4073–4080. [Google Scholar] [CrossRef]
- Southall, N.T.; Dill, K.A.; Haymet, A.D.J. A View of the Hydrophobic Effect. J. Phys. Chem. B 2002, 106, 521–533. [Google Scholar] [CrossRef]
- Lin, Y.; Bilotti, E.; Bastiaansen, C.W.M.; Peijs, T. Transparent semi-crystalline polymeric materials and their nanocomposites: A review. Polym. Eng. Sci. 2020, 60, 2351–2376. [Google Scholar] [CrossRef]
- Billon, N.; Haudin, J. Influence of transcrystallinity on DSC analysis of polymers: Experimental and theoretical aspects. J. Therm. Anal. Calorim. 1994, 42, 679–696. [Google Scholar] [CrossRef]
- Pompe, G.; Mäder, E. Experimental detection of a transcrystalline interphase in glass-fibre/polypropylene composites. Compos. Sci. Technol. 2000, 60, 2159–2167. [Google Scholar] [CrossRef]
- Wang, B.; Lin, F.-h.; Li, X.-y.; Ji, X.-r.; Liu, S.-x.; Han, X.-j.; Yuan, Z.-q.; Luo, J. Transcrystallization of Isotactic Polypropylene/Bacterial Cellulose Hamburger Composite. Polymers 2019, 11, 508. [Google Scholar] [CrossRef]
- Nishi, T.; Wang, T.T. Melting Point Depression and Kinetic Effects of Cooling on Crystallization in Poly(vinylidene fluoride)-Poly(methyl methacrylate) Mixtures. Macromolecules 1975, 8, 909–915. [Google Scholar] [CrossRef]
- Flory, P.J.; Vrij, A. Melting Points of Linear-Chain Homologs. The Normal Paraffin Hydrocarbons. J. Am. Chem. Soc. 1963, 85, 3548–3553. [Google Scholar] [CrossRef]
- Tiwari, A.D.; Mishra, A.K.; Mishra, S.B.; Kuvarega, A.T.; Mamba, B.B. Stabilisation of silver and copper nanoparticles in a chemically modified chitosan matrix. Carbohydr. Polym. 2013, 92, 1402–1407. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Circelli, T.; Waymouth, R.M. Crystallization of the α and γ Forms of Isotactic Polypropylene as a Tool To Test the Degree of Segregation of Defects in the Polymer Chains. Macromolecules 2002, 35, 3622–3629. [Google Scholar] [CrossRef]
- Cho, Y.-S.; Huh, Y.-D. Synthesis of ultralong copper nanowires by reduction of copper-amine complexes. Mater. Lett. 2009, 63, 227–229. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef]
- Han, J.-W.; Lohn, A.; Kobayashi, N.P.; Meyyappan, M. Evolutional Transformation of Copper Oxide Nanowires to Copper Nanowires by a Reduction Technique. Mater. Express 2011, 1, 176–180. [Google Scholar] [CrossRef]
- Lux, F. Models proposed to explain the electrical conductivity of mixtures made of conductive and insulating materials. J. Mater. Sci. 1993, 28, 285–301. [Google Scholar] [CrossRef]
- Glatz-Reichenbach, J. FEATURE ARTICLE Conducting Polymer Composites. J. Electroceram. 1999, 3, 329–346. [Google Scholar]
- Weber, M.; Kamal, M.R. Estimation of the volume resistivity of electrically conductive composites. Polym. Compos. 1997, 18, 711–725. [Google Scholar] [CrossRef]
- Al-Saleh, M.H.; Sundararaj, U. A review of vapor grown carbon nanofiber/polymer conductive composites. Carbon 2009, 47, 2–22. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Zhang, T.; Zhao, M.; Chang, C.-S.; Sue, H.-J. Preparation of thermally conductive but electrically insulated polypropylene containing copper nanowire. Polymer 2021, 236, 124317. [Google Scholar] [CrossRef]
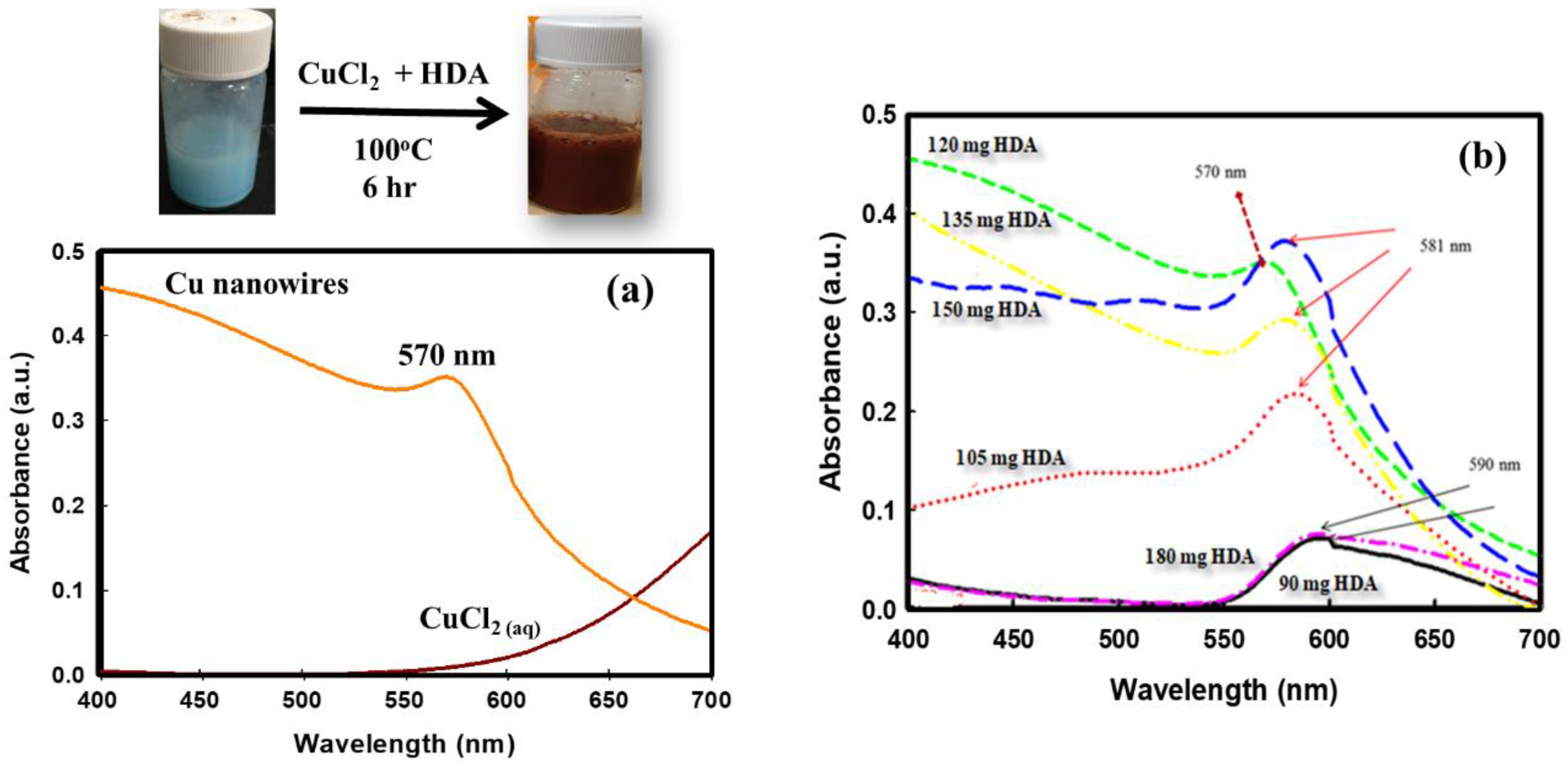
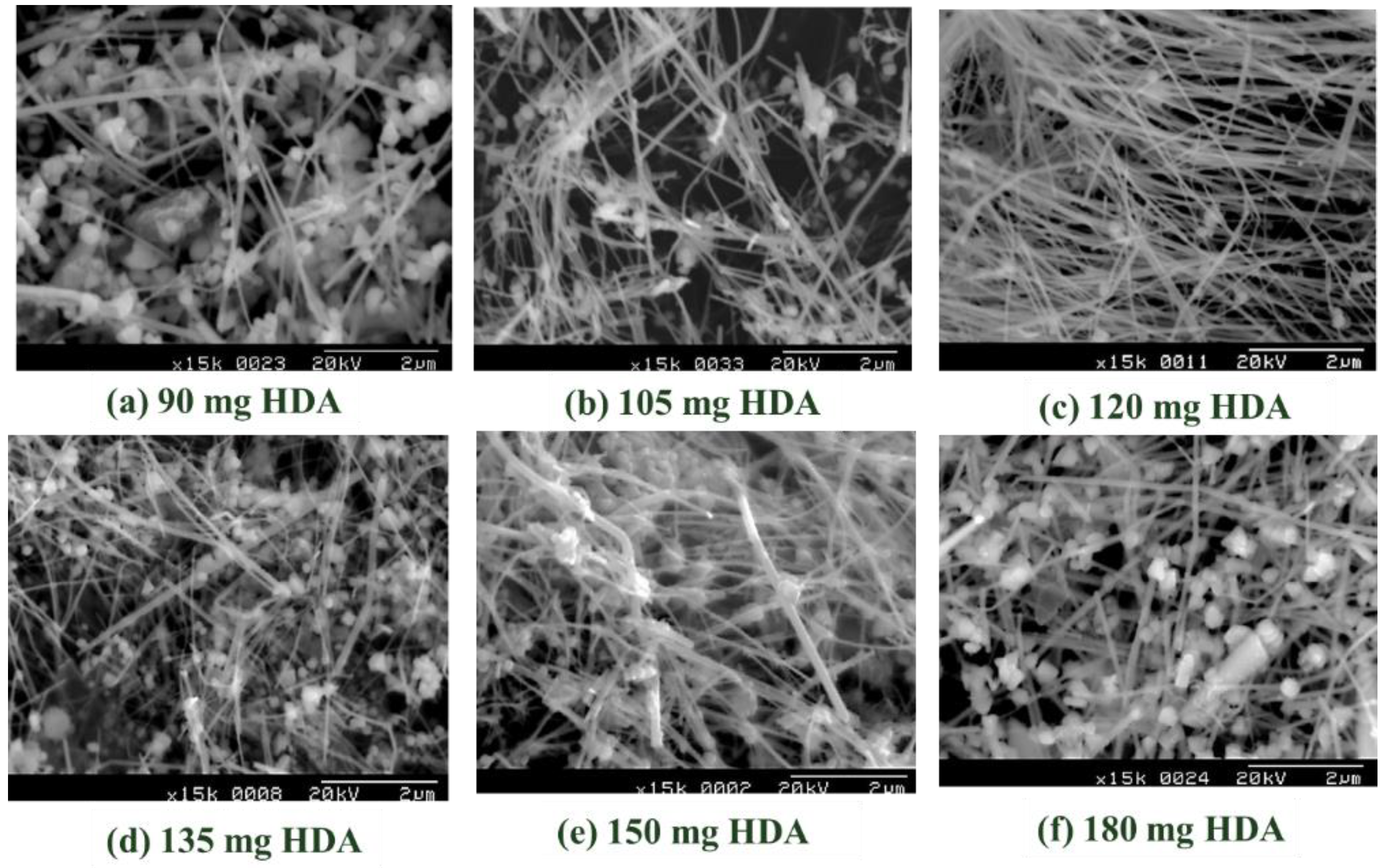





| HAD Content (mg) | Absorbance Peak (nm) | Average Dia. CuNWs (nm) |
|---|---|---|
| 90 | 581 | 110 ± 22 a |
| 105 | 570 | 85 ± 20 b,d,e |
| 120 | 570 | 66 ± 16 c,d |
| 135 | 581 | 80 ± 18 d,e |
| 150 | 581 | 92 ± 21 e |
| 180 | 590 | 115 ± 18 a |
| CuNWs (vol%) | Tm (°C) | ∆H (J/g) | Normalized ∆H (J/g) | Tc (°C) |
|---|---|---|---|---|
| 0.00 | 162.5 | 64.8 | 64.8 | 111.5 |
| 0.10 | 161.1 | 63.2 | 63.8 | 111.7 |
| 0.50 | 160.7 | 54.4 | 57.1 | 112.6 |
| 0.75 | 157.7 | 46.8 | 50.3 | 112.8 |
| 1.00 | 153.1 | 49.4 | 54.4 | 114.9 |
| 1.25 | 144.2 | 50.1 | 56.4 | 116.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, P.-W.; Jaihao, C.; Pan, L.-C.; Tsai, P.-W.; Huang, C.-S.; Brangule, A.; Zarkov, A.; Kareiva, A.; Wang, H.-T.; Yang, J.-C. The Processing and Electrical Properties of Isotactic Polypropylene/Copper Nanowire Composites. Polymers 2022, 14, 3369. https://doi.org/10.3390/polym14163369
Lu P-W, Jaihao C, Pan L-C, Tsai P-W, Huang C-S, Brangule A, Zarkov A, Kareiva A, Wang H-T, Yang J-C. The Processing and Electrical Properties of Isotactic Polypropylene/Copper Nanowire Composites. Polymers. 2022; 14(16):3369. https://doi.org/10.3390/polym14163369
Chicago/Turabian StyleLu, Po-Wen, Chonlachat Jaihao, Li-Chern Pan, Po-Wei Tsai, Ching-Shuan Huang, Agnese Brangule, Aleksej Zarkov, Aivaras Kareiva, Hsin-Ta Wang, and Jen-Chang Yang. 2022. "The Processing and Electrical Properties of Isotactic Polypropylene/Copper Nanowire Composites" Polymers 14, no. 16: 3369. https://doi.org/10.3390/polym14163369
APA StyleLu, P.-W., Jaihao, C., Pan, L.-C., Tsai, P.-W., Huang, C.-S., Brangule, A., Zarkov, A., Kareiva, A., Wang, H.-T., & Yang, J.-C. (2022). The Processing and Electrical Properties of Isotactic Polypropylene/Copper Nanowire Composites. Polymers, 14(16), 3369. https://doi.org/10.3390/polym14163369









