Production of Low Molecular Weight Chitosan Using a Combination of Weak Acid and Ultrasonication Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Shrimp Shell Preparation
2.3. Characterization of Shrimp Shell Powder
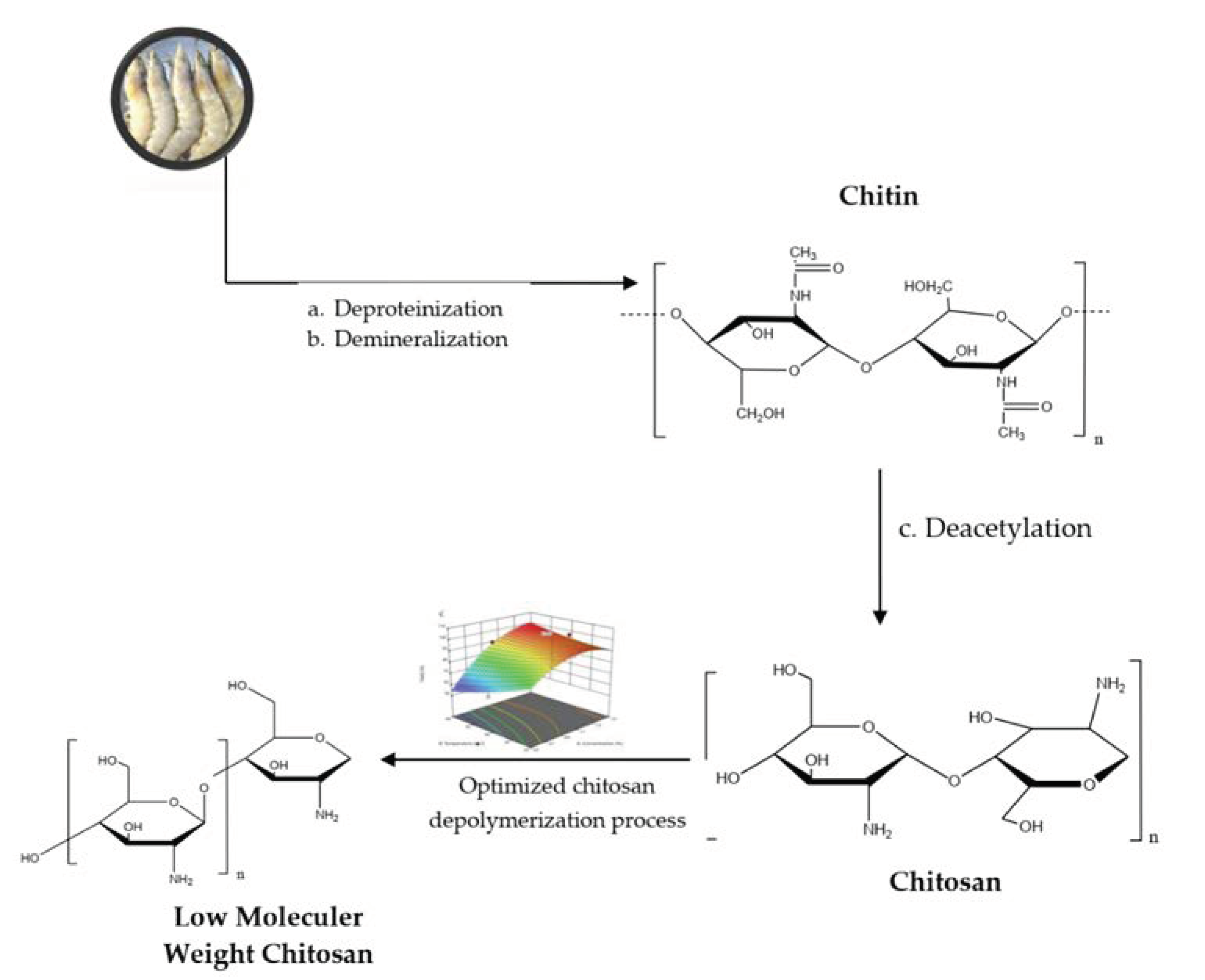
2.4. Chitosan Preparation
2.4.1. Deproteinization
2.4.2. Demineralization
2.4.3. Deacetylation
2.5. Chitosan Characterization Based on Pharmaceutical Requirement
2.6. Chitosan Depolymerization
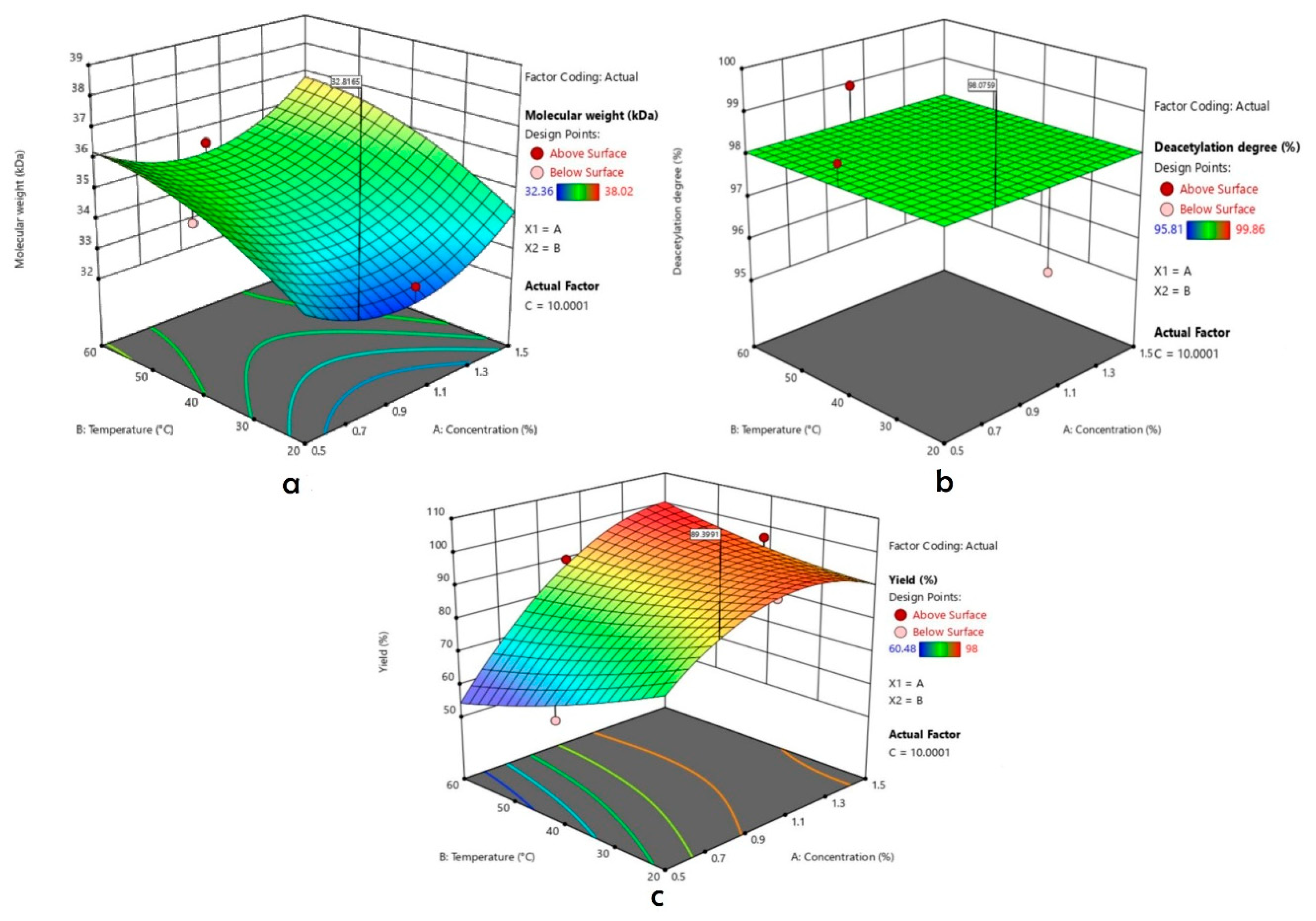
2.7. Optimization Process
2.8. Characterization of Low Molecular Weight Chitosan
2.8.1. Fourier Transform Infra-Red (FTIR) Spectroscopy
2.8.2. H-NMR Spectroscopy
2.8.3. Scanning Electron Microscope
2.8.4. Differential Scanning Calorimetry
2.8.5. X-ray Diffractometry
3. Results and Discussion
3.1. Characterization of Shrimp Shell Powder
3.2. Chitosan Characterization Based on Pharmaceutical Requirement
3.3. Depolymerization Process
3.4. Low Molecular Wight Chitosan Characterization
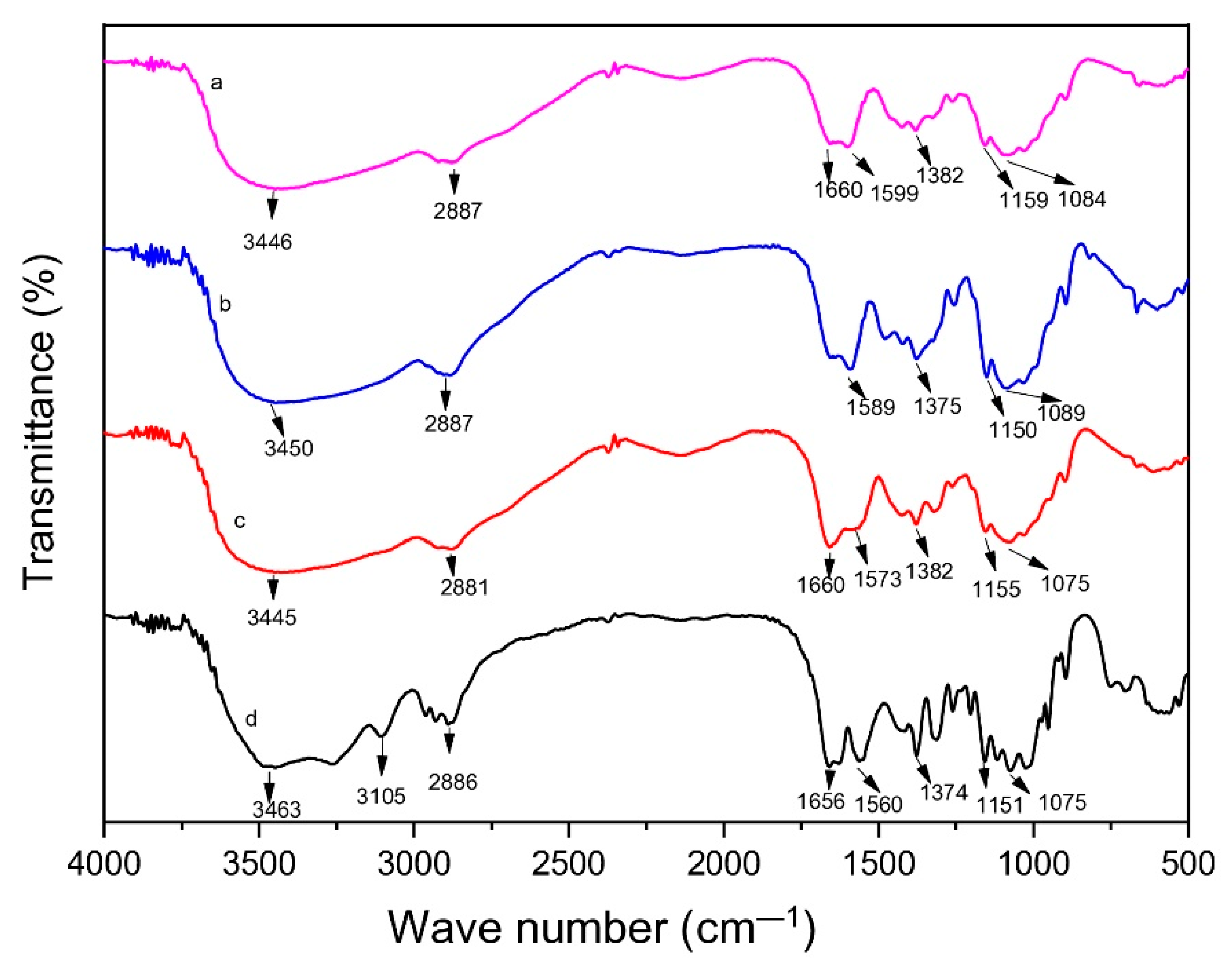
3.4.1. Fourier Transform Infra-Red (FTIR) Spectroscopy
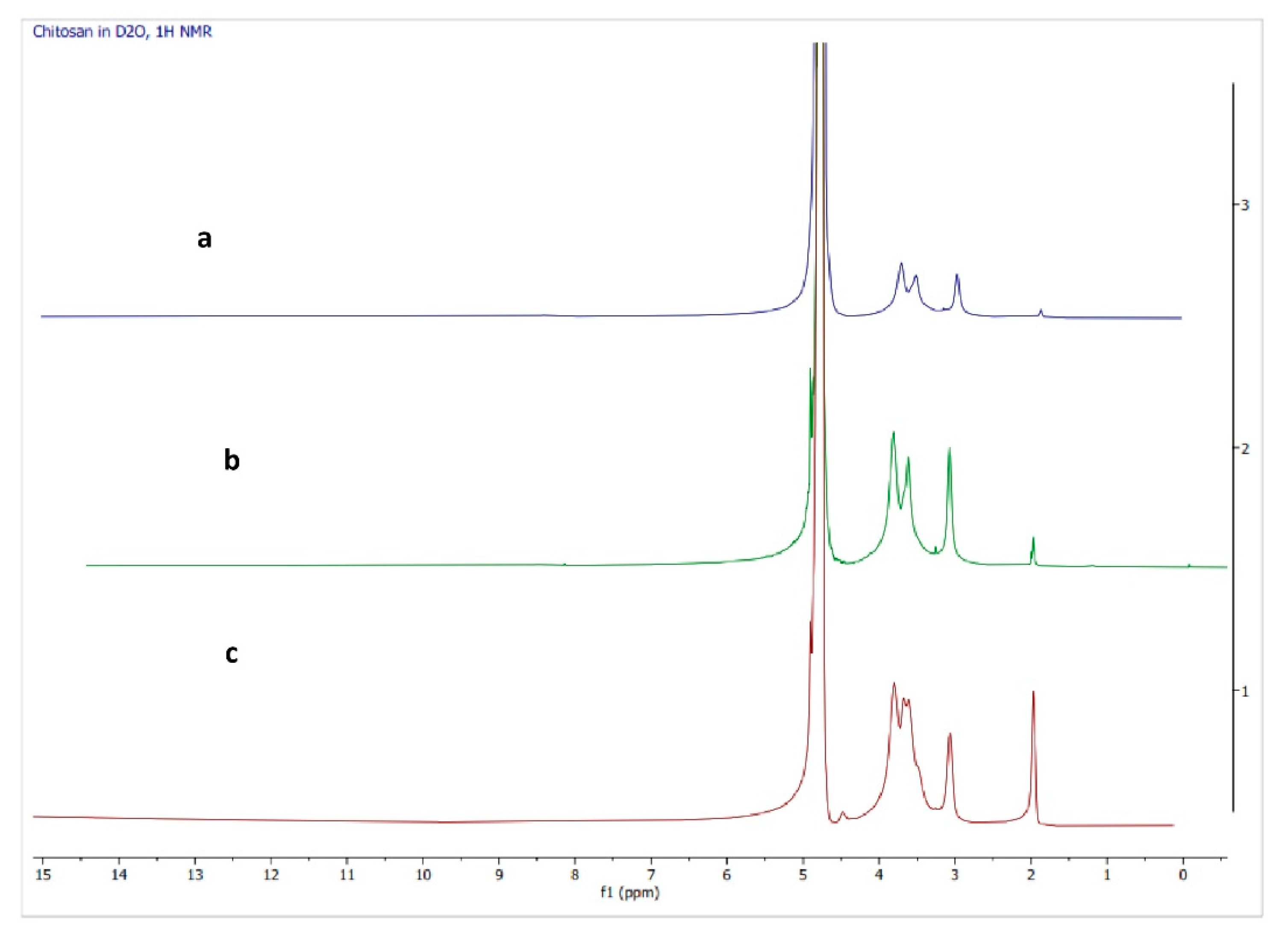
3.4.2. H-NMR Spectroscopy
3.4.3. Scanning Electron Microscope
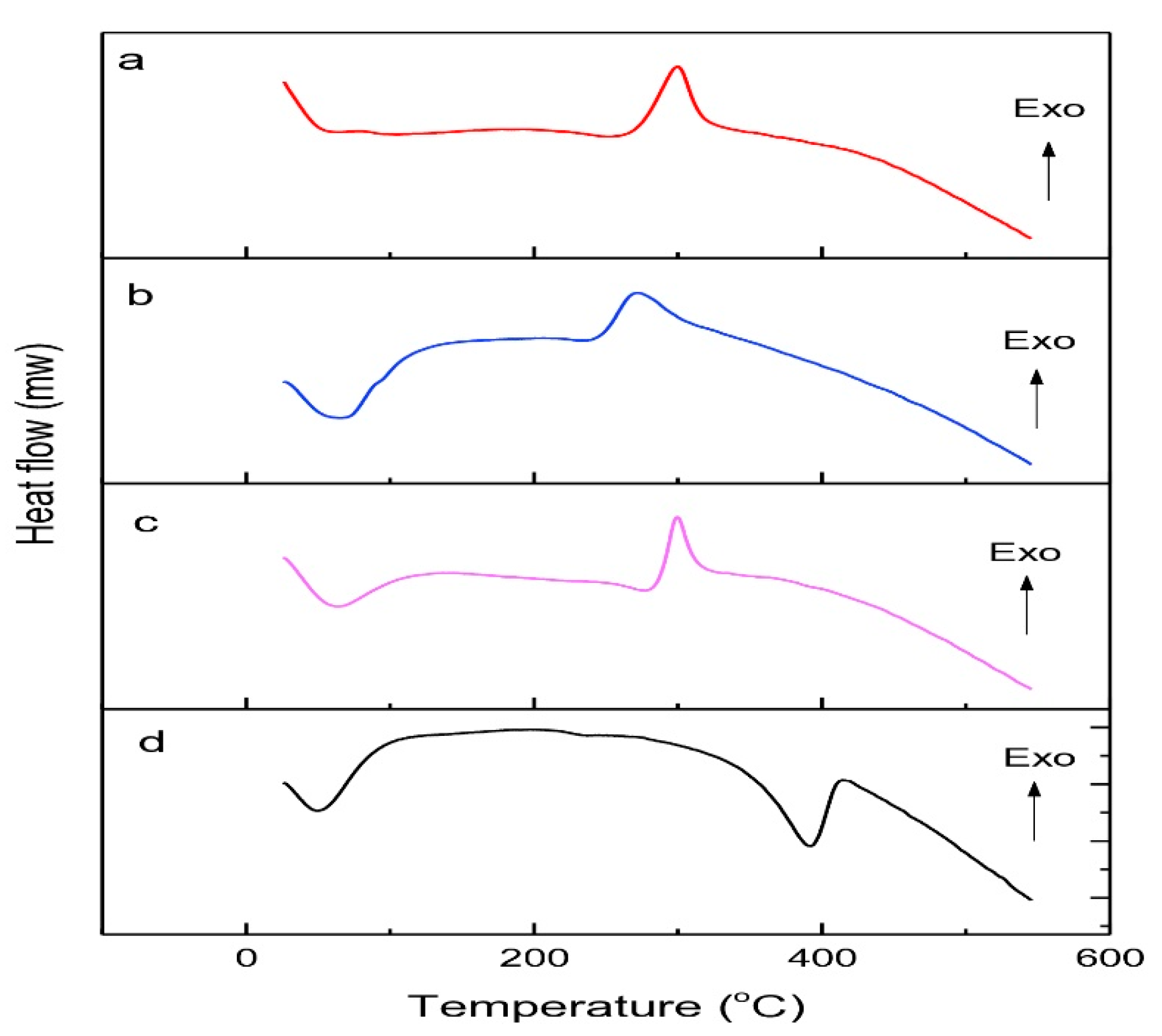
3.4.4. Thermal Analysis Using Differential Scanning Calorimetry
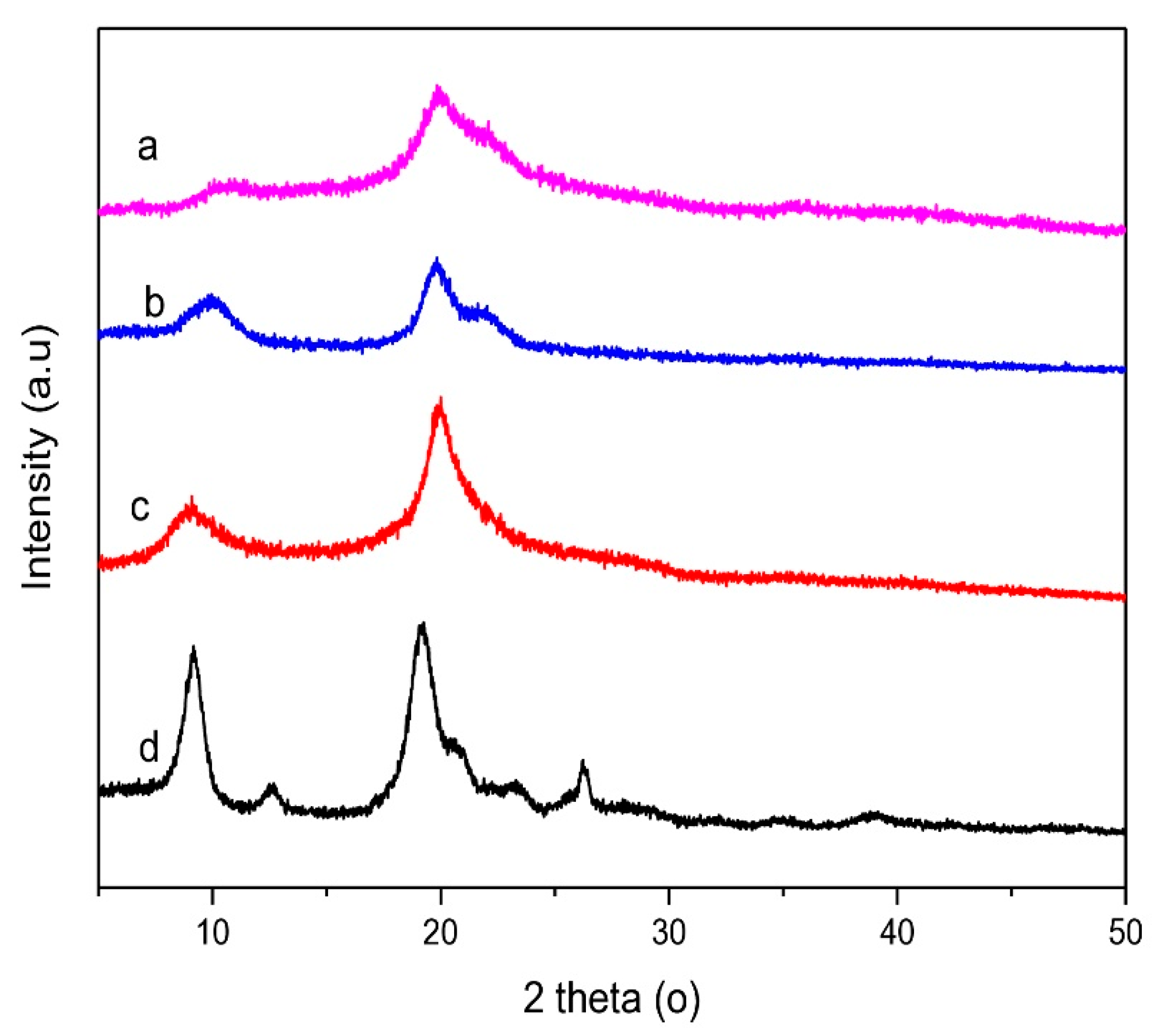
3.4.5. X-ray Diffractometry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A Review of source and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Gonçalves, C.; Ferreira, N.; Lourenço, L. Production of Low Molecular Weight Chitosan and Chitooligosaccharides (COS): A Review. Polymers 2021, 13, 2466. [Google Scholar] [CrossRef]
- Ahmad, M.; Mazoor, K.; Singh, S.; Ikram, S. Chitosan centered bionanocamposites for medical specialty and curative applicatiobs: A review. Int. J. Pharm. 2017, 529, 200–217. [Google Scholar] [CrossRef]
- Islam, N.; Dmour, I.; Taha, M.O. Degradability of chitosan micro/nanoparticles for pulmonary drug delivery. Heliyon 2019, 5, e01684. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, R.; Qin, W.; Dai, J.; Zhang, Q.; Lee, K.; Liu, Y. Physiochemical properties of gelatin fiilms containing tea polyphenol-loaded chitosan nanoparticle generated by electrospray. Mater. Des. 2020, 185, 108277. [Google Scholar] [CrossRef]
- Raorane, C.J.; Shastri, D.; Parveen, A.S.; Haldhar, R.; Raj, V. Complex Inhibits Fluconazole-Resistant Candida albicans Biofilm Formation. Antibiotics 2022, 11, 950. [Google Scholar] [CrossRef]
- Ra, V.; Kim, K.; Kim, Y.-G.; Lee, J.-H.; Lee, J. Chitosan gum arabic embedded alizarin nanocarries inhibit biofilm formation of multispecies microorganisms. Carbohydr. Polym. 2022, 284, 118959. [Google Scholar]
- Jongsri, P.; Wangsomboondee, T.; Rojsitthisak, P.; Seraypheap, K. Effect of molecular weights of chitosan coating on postharvest quality and physicochemical characteristics of mango fruit. LWT 2016, 73, 28–36. [Google Scholar] [CrossRef]
- Choi, C.; Nam, J.; Nah, J. Appilcation of chitosan and chitosan derivatives as biomaterials. J. Ind. Eng. Chem. 2016, 33, 1–10. [Google Scholar] [CrossRef]
- Chatterjee, N.S.; Dara, P.K.; Raman, S.P.; Vijayan, D.K.; Sadasivam, J.; Mathew, S.; Ravishankar, C.N.; Anandan, R. Nanoencapsulation in low-molecular-weight chitosan improves in vivo antioxidant potential of black carrot anthocyanin. J. Sci. Food Agric. 2021, 101, 5264–5271. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.; Deshpande, C.; Patil, S.; Jain, R.; Dandekar, P. Mechanistic insights into controlled depolymerization of Chitosan using H-Mordenite. Carbohydr. Polym. 2019, 230, 115600. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, H.; Zhong, Z.; Yan, N. Base-catalysed, one-step mechanochemical conversion of chitin and shrimp shells into low molecular weight chitosan. Green Chem. 2017, 19, 2783–2792. [Google Scholar] [CrossRef] [Green Version]
- Qandil, A.M.; Marji, T.J.; Al-Taani, B.M.; Khaled, A.H.; Badwan, A.A. Depolymerization of HMW into a predicted LMW chitosan and determination of the degree of deacetylation to guarantee its quality for research use. J. Excipients Food Chem. 2018, 9, 51–63. [Google Scholar]
- Hosseini-Ashtiani, N.; Tadjarodi, A.; Zare-Dorabei, R. Low molecular weight chitosan-cyanocobalamin nanoparticles for controlled delivery of ciprofloxacin: Preparation and evaluation. Int. J. Biol. Macromol. 2021, 176, 459–467. [Google Scholar] [CrossRef]
- Kapadnis, G.; Dey, A.; Dandekar, P.; Jain, R. Effect of degree of deacetylation on solubility of low-molecular-weight chitosan produced via enzymatic breakdown of chitosan. Polym. Int. 2019, 68, 1054–1063. [Google Scholar] [CrossRef]
- Soares, P.I.P.; Sousa, A.I.; Silva, J.C.; Ferreira, I.M.M.; Novo, C.M.M.; Borges, J.P. Chitosan-based nanoparticles as drug delivery systems for doxorubicin: Optimization and modelling. Carbohydr. Polym. 2016, 147, 304–312. [Google Scholar] [CrossRef]
- Iacob, A.; Lupascu, F.; Apotrosoaei, M.; Vasincu, I.; Tauser, R.; Lupascu, D.; Giusca, S.; Caruntu, I.-D.; Profire, L. Recent Biomedical Approaches for Chitosan Based Materials as Drug Delivery Nanocarriers. Pharmaceutics 2021, 13, 587. [Google Scholar] [CrossRef]
- Minh, N.C.; van Hoa, N.; Trung, T.S. Preparation, properties and application of low-molecular-weight chitosan. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Doan, V.K.; Ly, K.L.; Tran, N.M.P.; Thi Ho, T.P.; Ho, M.H.; Ngoc-Dang, N.T.; Chang, C.C.; Thu Nguyen, H.T.; Ha, P.T.; Tran, Q.N.; et al. Characterizations and antibacterial efficacy of chitosan oligomers synthesized by microwace-assisted hydrogen peroxide oxidative depolymerization method for infectious wound application. Materials 2021, 14, 16. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- de Farias, B.S.; Grundmann, D.D.R.; Rizzi, F.Z.; Martins, N.S.S.; Junior, T.R.S.C.; Pinto, L.A.D.A. Production of low molecular weight chitosan by acid and oxidative pathways: Effect on physicochemical properties. Food Res. Int. 2019, 123, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.T.; Chang, C.H.; Lin, Y.Y.; Wu, M.F.; Han, J.L.; Hsieh, K.H. Kinetic study of acid depolymerization of chitosan and effects of low molecular weight chitosan on erythrocyte rouleau formation. Carbohydr. Res. 2011, 346, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Aljbour, N.D.; Beg, M.D.; Gimbun, J. Acid Hydrolysis of Chitosan to Oligomers Using Hydrochloric Acid. Chem. Eng. Technol. 2019, 42, 1741–1746. [Google Scholar] [CrossRef]
- Savitri, E.; Juliastuti, S.R.; Handaratri, A.; Sumarno; Roesyadi, A. Degradation of chitosan by sonication in very-low-concentration acetid acid. Polym. Degrad. 2014, 110, 344–352. [Google Scholar] [CrossRef]
- Schmitz, C.; Auza, L.G.; Koberidze, D.; Rasche, S.; Fischer, R.; Bortesi, L. Conversion of Chitin to Defined Chitosan Oligomers: Current Status and Future Prospects. Mar. Drugs 2019, 17, 452. [Google Scholar] [CrossRef] [Green Version]
- Purwanto, E.; Connor, J.; Ngothai, Y. The kinetics oxidative degradatiion of chitosan in formic acid with the presence of hydrogen peroxide. IOP Conf. Ser. Mater. Sci. Eng. 2019, 703, 012041. [Google Scholar] [CrossRef]
- Alves, H.J.; Vieceli, M.; Alves, C.; Muñiz, G.I.B.; de Oliveira, C.L.P.; Feroldi, M.; Arantes, M.K. Chitosan Depolymerization and Nanochitosan Production Using a Single Physical Procedure. J. Polym. Environ. 2018, 26, 3913–3923. [Google Scholar] [CrossRef]
- Liu, H.; Bao, J.; Du, Y.; Zhou, X.; Kennedy, J.F. Effect of ultrasonic treatment on the biochemphysical properties of chitosan. Carbohydr. Polym. 2006, 64, 553–559. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Zhaliazniak, N.V.; Egorov, A.R.; Lobanov, N.N.; Volkova, O.V.; Zabodalova, L.A.; Suchkova, E.P.; Kurliuk, A.V.; Shakola, T.V.; Rubanik, V.V.; et al. Chitosan derivatives and their based nanoparticles: Ultrasonic approach to the synthesis, antimicrobial and transfection properties. Carbohydr. Polym. 2020, 242, 116478. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S.; Prodpran, T. Ultrasound-Assisted Extraction of Chitosan from Squid Pen: Molecular Characterization and Fat Binding Capacity. J. Food Sci. 2019, 84, 224–234. [Google Scholar] [CrossRef]
- Popa-Nita, S.; Lucas, J.-M.; Ladavière, C.; David, L.; Domard, A. Mechanisms Involved During the Ultrasonically Induced Depolymerization of Chitosan: Characterization and Control. Biomacromolecules 2009, 10, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Masselin, A.; Rousseau, A.; Pradeaau, S.; Fort, L.; Gueret, R.; Buon, L.; Armand, S.; Cottaz, S.; Choisnard, L.; Fort, S. Optimizing chitin depolymerization by lysozyme to long-chain oligasaccharides. Mar. Drugs. 2021, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Pratama, H.B.; Supijo, M.C. Sutopo Experimental design and response surface method in geothermal energy: A comprehensive study in probabilistic resource assessment. Geothermics 2020, 87, 101869. [Google Scholar] [CrossRef]
- Damay, J.; Duret, X.; Ghislain, T.; Lalonde, O.; Lavoie, J.-M. Steam explosion of sweet sorghum stems: Optimisation of the production of sugars by response surface methodology combined with the severity factor. Ind. Crop. Prod. 2018, 111, 482–493. [Google Scholar] [CrossRef]
- Zamri, A.I.; Latiff, N.F.; Abdullah, Q.H.; Ahmad, F. Extraction and optimization of chitosa from razor clam (Ensis arcuatus) shell by using response surface methodology (RSM). Food Res. 2020, 4, 674–678. [Google Scholar] [CrossRef]
- Li, Z.; Gerdroodbary, M.B.; Valipour, P.; Moradi, R.; Babazadeh, H. The optimization via response surface method for micro hydrogen gas actuator. Int. J. Hydrogen Energy 2019, 44, 31633–31643. [Google Scholar] [CrossRef]
- Li, Z.; Lu, D.; Gao, X. Optimization of mixture proportions by statistical experimental design using response surface method—A review. J. Build. Eng. 2020, 36, 102101. [Google Scholar] [CrossRef]
- Solanki, A.B.; Parikh, J.R.; Parikh, R.H. Formulation and optimization of piroxicam proniosomes by 3-factor, 3-level box-behnken design. AAPS PharmSciTech 2007, 8, 43–49. [Google Scholar] [CrossRef]
- Beg, S.; Akhter, S. Box–Behnken Designs and Their Applications in Pharmaceutical Product Development. In Design of Experiments for Pharmaceutical Product Development; Beg, S., Ed.; Springer: Singapore, 2021; pp. 77–85. [Google Scholar] [CrossRef]
- Rai, P.; Pandey, A.; Pandey, A. Optimization of sugar release from banana peel powder waste (BPPW) using box behnken design (BBD): BPPW to bioihydrogen conversion. Int. J. Hydrogen Energy. 2019, 44, 25505–25513. [Google Scholar] [CrossRef]
- Utami, Y.P.; Sisang, S.; Burhan, A. Pengukuran Parameter Simplisia Dan Ekstrak Etanol Daun Patikala (Etlingera elation (Jack) R. M. Sm) Asal Kabupaten Enrekang Sulawesi Selatan. Maj. Far. dan Farmakol. Sekol. Tinggi Farm. Makassar. 2020, 24, 5–10. [Google Scholar]
- Rowe, R.C.; Sheskey, P.; Quinn, M. Handbook of pharmaceutical excipients; Libros Digitales-Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Ramadhan, L.O.A.N.; Radiman, C.L.; Wahyuningrum, D.; Suendo, V.; Ahmad, L.O.; Valiyaveetiil, S. Deasetilasi Kitin secara Bertahap dan Pengaruhnya terhadap Derajat Deasetilasi serta Masa Molekul Kitosan. J. Kim. Indones. 2010, 5, 17–21. [Google Scholar]
- Lubis, H. Pemeriksaan Kandungan Logam Merkuri, Timbal, dan Kadmium dalam Daging Rajungan Segar yang Berasal dari TPI Gabion Belawan Secara Spektrofotometri Serapat Aton. Maj. Kedokt. Nusant. 2008, 41, 39–47. [Google Scholar]
- Rismana, E.; Rosidah, I.; Bunga, O.; Yunianto, P.; Erna, E. Pengujian Stabilitas Sediaan Luka Bakar Berbahan Baku Aktif Kitosan/Ekstral Pegagan (Centella Asiatica). J. Kim. Terap. Indones 2015, 17, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Irnawati; Riyanto, S.; Martono, S.; Rohman, A. Optimasi Metode Ultrasonik untuk Ekstraksi Minyak Biji Labu Kuning (Cucurbita maxima) The Optimization of Ultrasonic Method for the Extraction of Pumpkin seed (Cucurbita maxima). Indonesian J. Chemom. Pharm. Anal. 2021, 1, 42–51. [Google Scholar]
- Kasaai, M.R. Determination of the degree of N-acetylation for chitin and chitosan by various NMR spectroscopy techniques: A review. Carbohydr. Polym. 2010, 79, 801–810. [Google Scholar] [CrossRef]
- Vadackova, P.; Vranikova, B.; Petra, S.; Ales, F.; Jan, E.; Jan, M. Evaluation and comparison of three types of spray dried coprocessed excipient Avicel® for direct compression. Biomed Res. Int. 2018, 2018, 2739428. [Google Scholar]
- Arjuna, A.; Natsir, S.; Khumaerah, A.A.; Yulianty, R. Modifikasi Serat Limbah Kubis Menjadi Nanokristalin Selulosa Melalui Metode Hidrolisis Asam. J. Farm. Galen. 2018, 4, 119–125. [Google Scholar] [CrossRef]
- Sogias, I.A.; Khutoryanskiy, V.V.; Williams, A.C. Exploring the Factors Affecting the Solubility of Chitosan in Water. Macromol. Chem. Phys. 2009, 211, 426–433. [Google Scholar] [CrossRef]
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical extraction and modification of chitin and chitosan from shrimp shells. Eur. Polym. J. 2021, 159, 110709. [Google Scholar] [CrossRef]
- Srinivasan, H.; Kanayairam, V.; Ravichandran, R. Chitin and chitosan preparation from shrimp shells Penaerus monodon and its human ovarium cancar cell line, PA-1. Int. J. Biol. Macromol. 2018, 107 Pt A, 662–667. [Google Scholar] [CrossRef]
- Yen, M.-T.; Yang, J.-H.; Mau, J.-L. Physicochemical characterization of chitin and chitosan from crab shells. Carbohydr. Polym. 2009, 75, 15–21. [Google Scholar] [CrossRef]
- Poverenov, E.; Rips, H.A.; Zaitev, Y.; Bar, V.; Danay, O.; Horev, B.; Saiz, C.B.; McHugh, T.; Rodov, V. Potential of chitosan from mushroom waste to enhace quality and storability of fresh-cut-melons. Food Chem. 2018, 268, 233–241. [Google Scholar] [CrossRef]
- Ghormade, V.; Pathan, E.K.; Deshpande, M.V. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017, 104, 1415–1421. [Google Scholar] [CrossRef]
- Ioelovich, M. Crystallinity and Hydrophility of Chitin and Chitosan. Res. Rev. J. Chem. 2014, 3, 7–14. [Google Scholar]


| No | Materials | Source/Manufacturer |
|---|---|---|
| 1 | Vaname shrimp shell | PT Yanagi (Kendari, Indonesia) |
| 2 | Commercial chitosan | Sigma-Aldrich (Saint.Louis, MO, USA) |
| 3 | Formic acid | CV Multi Usaha Mandiri (Sidoarjo, Indonesia) |
| 4 | Acetic acid | CV Sumber Rejeki (Makassar-Indonesia) |
| 5 | Sodium hydroxide | CV Sumber Rejeki (Makassar-Indonesia) |
| 6 | Hydrochloric acid | CV Sumber Rejeki (Makassar-Indonesia) |
| 7 | ethanol 96% | CV Sumber Rejeki (Makassar-Indonesia) |
| 8 | deionized water | CV Sumber Rejeki (Makassar-Indonesia) |
| 9 | Potato Dextrose Agar | Merck KGaA (Darmstads, Germany) |
| 10 | Staphilococcus aureus ATCC25923 | Laboratory of Biomedic, Faculty of Medicine, Halu Oleo University, Kendari, Indonesia |
| 11 | Escherichia coli ATCC 35218 | Laboratory of Biomedic, Faculty of Medicine, Halu Oleo University, Kendari, Indonesia |
| 12 | Salmonella sp. ATCC 14028 | Laboratory of Biomedic, Faculty of Medicine, Halu Oleo University, Kendari, Indonesia |
| Level | Chitosan Concentration (%) | Temperature (°C) | Time (Minute) |
|---|---|---|---|
| Low (−1) | 0.5 | 20 | 10 |
| Middle (0) | 1 | 40 | 20 |
| High (+1) | 1.5 | 60 | 30 |
| No | Run Order | Chitosan Concentration (%) | Temperature (°C) | Time (Minute) |
|---|---|---|---|---|
| 1 | 11 | 0 | −1 | +1 |
| 2 | 7 | −1 | 0 | +1 |
| 3 | 8 | +1 | 0 | +1 |
| 4 | 16 | 0 | 0 | 0 |
| 5 | 14 | 0 | 0 | 0 |
| 6 | 6 | +1 | 0 | −1 |
| 7 | 2 | +1 | −1 | 0 |
| 8 | 15 | 0 | 0 | 0 |
| 9 | 17 | 0 | 0 | 0 |
| 10 | 9 | 0 | −1 | −1 |
| 11 | 12 | 0 | +1 | +1 |
| 12 | 13 | 0 | 0 | 0 |
| 13 | 3 | −1 | +1 | 0 |
| 14 | 4 | +1 | +1 | 0 |
| 15 | 10 | 0 | +1 | −1 |
| 16 | 1 | −1 | −1 | 0 |
| 17 | 5 | −1 | 0 | −1 |
| Specification | Shrimp Shell Powder |
|---|---|
| Organoleptic | Coarse powder, slightly yellowish, strong shrimp shell odour, less tasty |
| Solubility in water | 5% |
| Solubility in 96% ethanol | 3% |
| Ash content | 14.14% |
| Water content | 54.57% |
| Fat content | 1.81% |
| Protein content | 14.92% |
| Specification | Isolated Chitosan | Pharmaceutical Specification of Chitosan | Reference |
|---|---|---|---|
| Organoleptic properties | Odourless, creamy white powder | Odourless, white or creamy white, or flakes | Rowe, 2009 |
| Particle size | 4.3702 µm (PI = 0.679) | <30 µm | Rowe, 2009 |
| pH | 4.68 | 4.0–6.0 | Rowe, 2009 |
| Moisture content | 5.09% | ≤5% | USP-36, NF-31 |
| Solubility: | PhEur-5 | ||
| Water | Very slightly soluble | Sparingly Soluble | |
| Ethanol | Practically Insoluble | Practically Insoluble | |
| Molecular weight | 57,543.99 Dalton | ≤1,000,000 | USP-36, NF-31 |
| Degree of deacetylation | 95.95% | 70–95% | USP-36, NF-31 |
| Loss on drying | 0.37% | ≤10% | Rowe, 2009 |
| Ash content | 0.87% | <1% | Rowe, 2009 |
| Heavy metal Lead (Pb) Cadmium (Cd) Mercury (Hg) | 0.0002 mg/L 0.0004 mg/L <0.0001 mg/L | ≤40 ppm | Rowe, 2009 |
| Microbial contamination | SNI, 2013 | ||
| Escherecia coli | <3 MPM/g | <3 MPM/g | |
| Salmonella | - | ||
| Total plate count (Bacteria) | 270 colony/g | Maximum 1 × 103 colony/g | |
| Total plate count (fungi) | 350 colony/g | ||
| Yield | 26% | ||
| Nynhidrine test | Purple |
| No | Factor | Response | ||||
|---|---|---|---|---|---|---|
| Concentration (%) | Temperature (°C) | Time (Minute) | Molecular Weight (kDa) | Deacetylation Degree (%) | Yield (%) | |
| 1 | 1 | 20 | 30 | 35.48 | 96.69 | 92.90 |
| 2 | 0.5 | 40 | 30 | 35.48 | 99.44 | 69.68 |
| 3 | 1.5 | 40 | 30 | 38.02 | 99.01 | 95.4 |
| 4 | 1 | 40 | 20 | 36.31 | 97.85 | 84.5 |
| 5 | 1 | 40 | 20 | 33.88 | 99.13 | 98 |
| 6 | 1.5 | 40 | 10 | 36.67 | 96.77 | 96.70 |
| 7 | 1.5 | 20 | 20 | 36.3 | 99.61 | 86.20 |
| 8 | 1 | 40 | 20 | 34.67 | 95.81 | 88.10 |
| 9 | 1 | 40 | 20 | 34.67 | 96.82 | 94.30 |
| 10 | 1 | 20 | 10 | 33.11 | 96.16 | 95.40 |
| 11 | 1 | 60 | 30 | 32.36 | 96.70 | 93.70 |
| 12 | 1 | 40 | 20 | 33.88 | 99.27 | 98 |
| 13 | 0.5 | 60 | 20 | 34.67 | 99.86 | 63.84 |
| 14 | 1.5 | 60 | 20 | 36.31 | 97.86 | 97.50 |
| 15 | 1 | 60 | 10 | 35.48 | 98.96 | 89.8 |
| 16 | 0.5 | 20 | 20 | 34.67 | 98.76 | 87.28 |
| 17 | 0.5 | 40 | 10 | 35.11 | 98.59 | 60.48 |
| Samples | The Cristalinity Index (%) |
|---|---|
| Low molecular weight chitosan | 36.64 |
| Native chitosan | 42.01 |
| Commercial chitosan | 42.90 |
| Chitin | 61.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suryani, S.; Chaerunisaa, A.Y.; Joni, I.M.; Ruslin, R.; Ramadhan, L.O.A.N.; Wardhana, Y.W.; Sabarwati, S.H. Production of Low Molecular Weight Chitosan Using a Combination of Weak Acid and Ultrasonication Methods. Polymers 2022, 14, 3417. https://doi.org/10.3390/polym14163417
Suryani S, Chaerunisaa AY, Joni IM, Ruslin R, Ramadhan LOAN, Wardhana YW, Sabarwati SH. Production of Low Molecular Weight Chitosan Using a Combination of Weak Acid and Ultrasonication Methods. Polymers. 2022; 14(16):3417. https://doi.org/10.3390/polym14163417
Chicago/Turabian StyleSuryani, Suryani, Anis Yohana Chaerunisaa, I. Made Joni, Ruslin Ruslin, La Ode Ahmad Nur Ramadhan, Yoga Windhu Wardhana, and Sitti Hadijah Sabarwati. 2022. "Production of Low Molecular Weight Chitosan Using a Combination of Weak Acid and Ultrasonication Methods" Polymers 14, no. 16: 3417. https://doi.org/10.3390/polym14163417
APA StyleSuryani, S., Chaerunisaa, A. Y., Joni, I. M., Ruslin, R., Ramadhan, L. O. A. N., Wardhana, Y. W., & Sabarwati, S. H. (2022). Production of Low Molecular Weight Chitosan Using a Combination of Weak Acid and Ultrasonication Methods. Polymers, 14(16), 3417. https://doi.org/10.3390/polym14163417







