Double-Network Polymer Electrolytes with Ionic Liquids for Lithium Metal Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Composite Polymer Electrolytes
2.3. Measurements
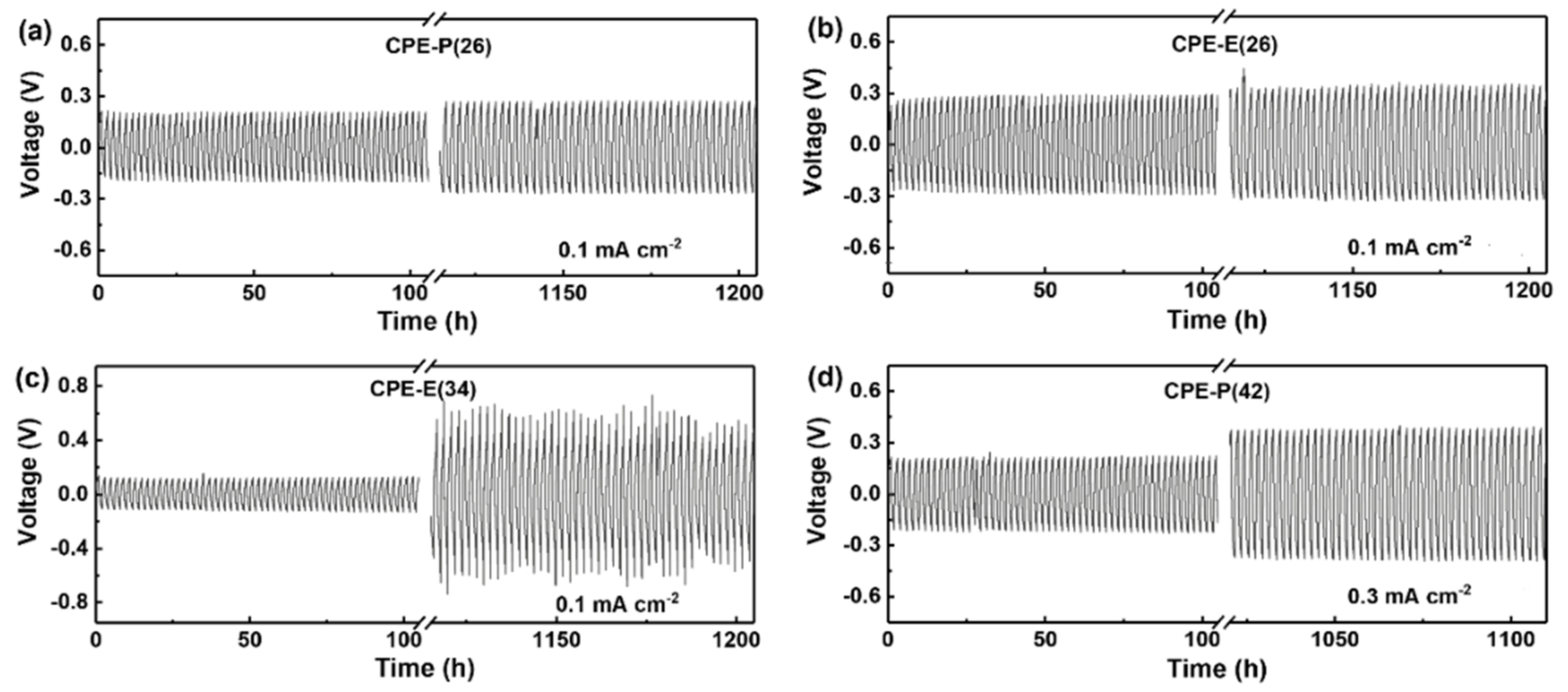
3. Results and Discussion
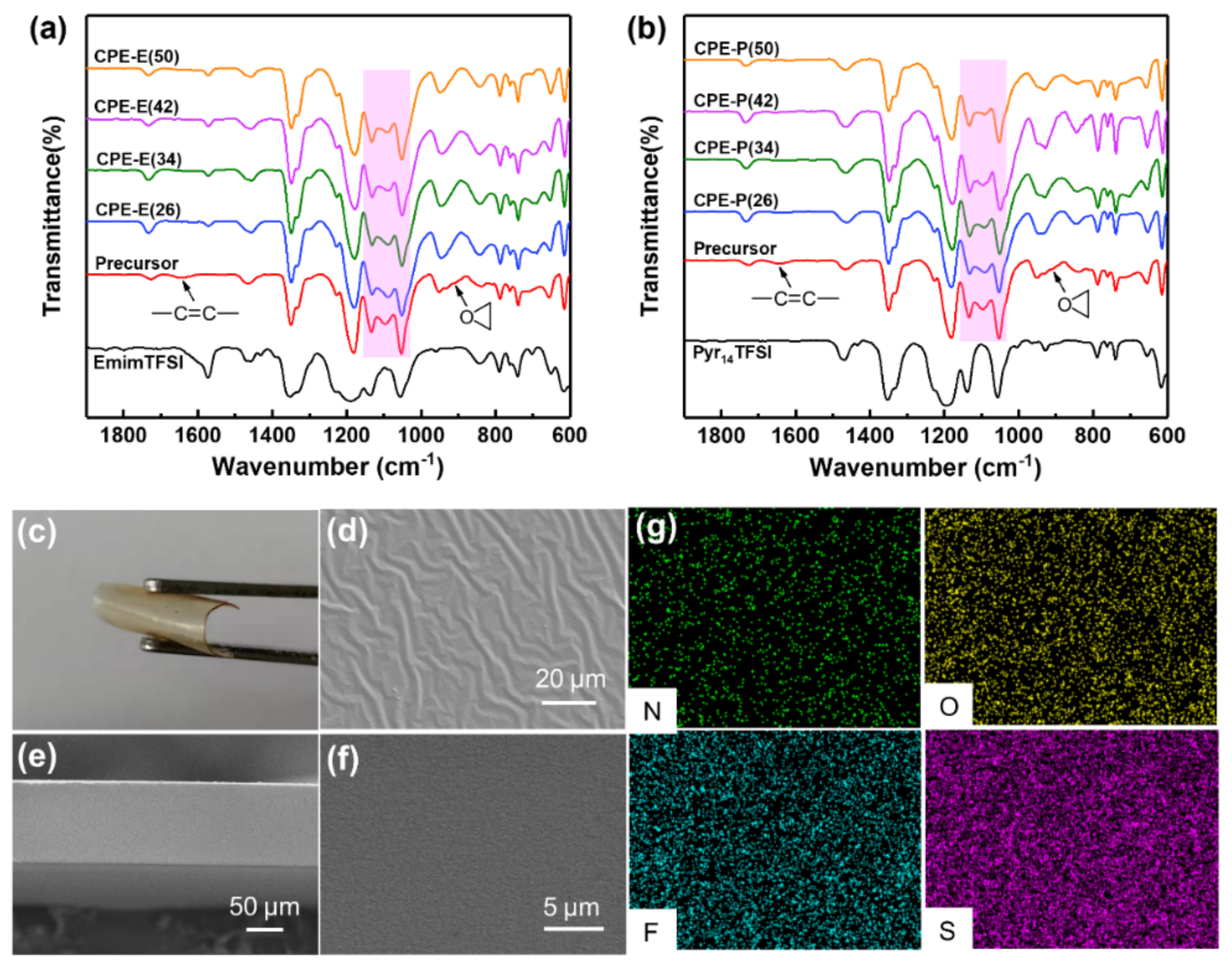
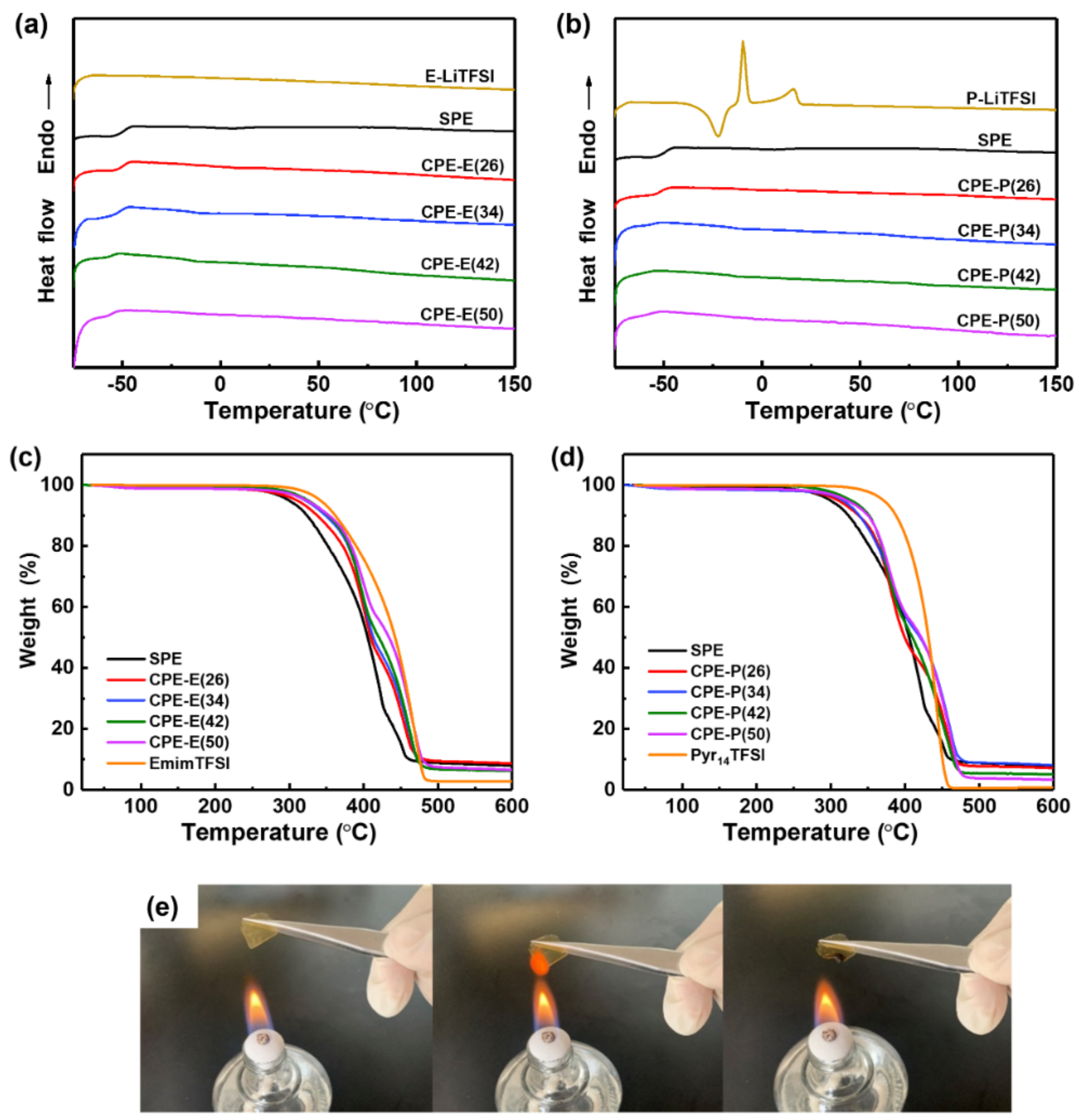
3.1. Preparation and Characterization of the Composite Polymer Electrolytes (CPEs)
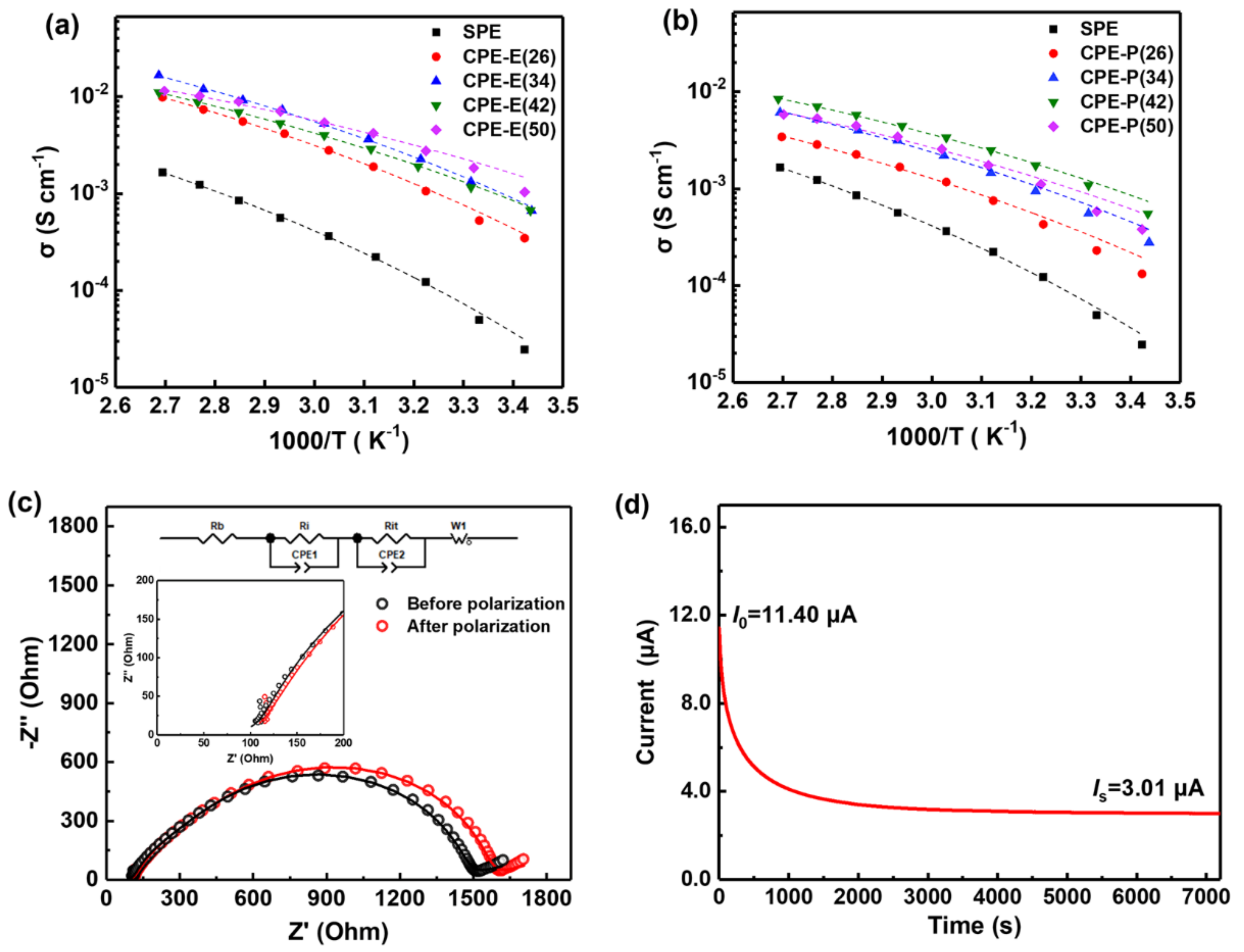
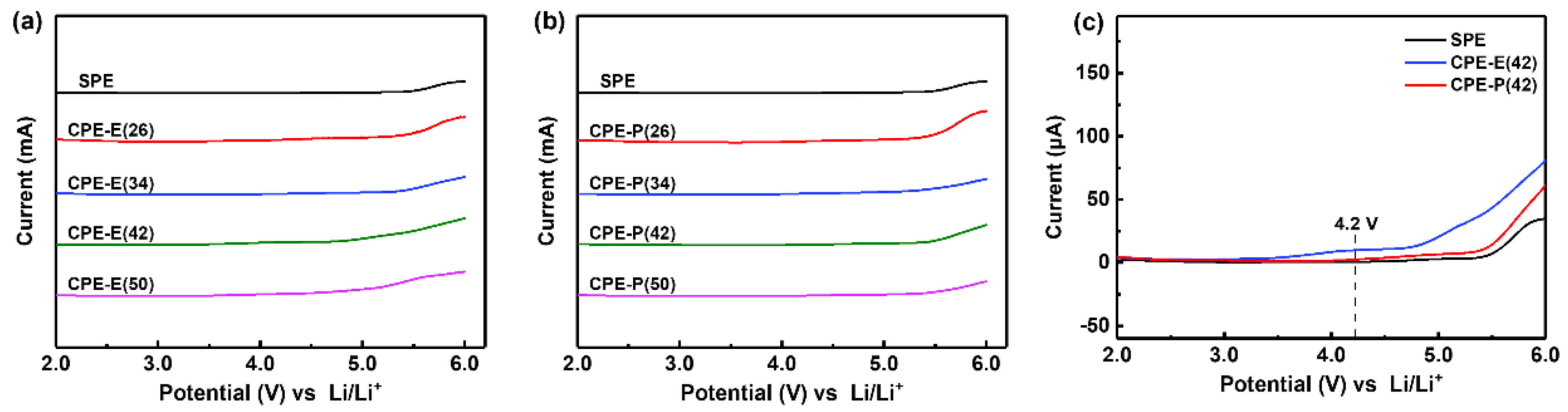
3.2. Electrochemical Properties of the Composite Polymer Electrolytes (CPEs)
3.3. Study of the Resistance to Lithium Dendrite Growth of the CPEs
3.4. Performance of Li/LiFePO4 Batteries
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, W.; Wang, J.L.; Ding, F.; Chen, X.L.; Nasybutin, E.; Zhang, Y.H.; Zhang, J.G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Lu, Y.X.; Rong, X.H.; Hu, Y.S.; Chen, L.Q.; Li, H. Research and development of advanced battery materials in China. Energy Storage Mater. 2019, 23, 144–153. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Wei, F.; Zhang, J.G.; Zhang, Q. A review of solid electrolyte interphases on lithium metal anode. Adv. Sci. 2016, 3, 1500213. [Google Scholar] [CrossRef]
- Vishnugopi, B.S.; Verma, A.; Mukherjee, P.P. Morphology-safety implications of interfacial evolution in lithium metal anodes. J. Phys. Chem. C 2020, 124, 16784–16795. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Y.; Li, C.Y. Dendrite-free, wide temperature range lithium metal batteries enabled by hybrid network ionic liquids. Energy Storage Mater. 2020, 29, 273–280. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Zhao, C.Z.; Huang, J.Q.; Zhang, Q. Recent advances in energy chemical engineering of next-generation lithium batteries. Engineering 2018, 4, 831–847. [Google Scholar] [CrossRef]
- Tikekar, M.D.; Choudhury, S.; Tu, Z.Y.; Archer, L.A. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 2016, 1, 16114. [Google Scholar] [CrossRef]
- Barai, P.; Higa, K.; Srinivasan, V. Lithium dendrite growth mechanisms in polymer electrolytes and prevention strategies. Phys. Chem. Chem. Phys. 2017, 19, 20493–20505. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.J.; Yi, J.; He, P.; Zhou, H.S. Solid-state electrolytes for lithium-ion batteries: Fundamentals, challenges and perspectives. Electrochem. Energy Rev. 2019, 2, 574–605. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.S.; Wu, Q.X.; Ma, W.Q.; Yang, L.S. Polyethylene oxide-Based composites as solid-state polymer electrolytes for lithium metal batteries: A mini review. Front. Chem. 2020, 8, 640. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.C.; Wu, J.Y.; Zhou, M.Y.; Liu, W.; Zhou, H.H. Advanced electrolyte design for stable lithium metal anode: From liquid to solid. Nano Energy 2021, 80, 105516. [Google Scholar] [CrossRef]
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G.X. Polymer electrolytes for lithium-based batteries: Advances and prospects. Chem 2019, 5, 2326–2352. [Google Scholar] [CrossRef]
- Zhang, X.W.; Daigle, J.C.; Zaghib, K. Comprehensive review of polymer architecture for all-solid-state lithium rechargeable batteries. Materials 2020, 13, 2488. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chu, X.; Wang, Y.H.; Zhang, H.T.; Yang, W.Q. Three-dimensional polymer networks for solid-state electrochemical energy storage. Chem. Eng. J. 2020, 391, 123548. [Google Scholar] [CrossRef]
- Pan, Q.W.; Smith, D.M.; Qi, H.; Wang, S.J.; Li, C.Y. Hybrid electrolytes with controlled network structures for lithium metal batteries. Adv. Mater. 2015, 27, 5995–6001. [Google Scholar] [CrossRef]
- Choudhury, S. A Highly Reversible Toom-Temperature Lithium Metal Battery Based on Cross-Linked Hairy Nanoparticles; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 35–57. [Google Scholar]
- Zhang, Y.; Lu, W.; Cong, L.; Liu, J.; Sun, L.; Mauger, A.; Julien, C.M.; Xie, H.; Liu, J. Cross-linking network based on poly(ethylene oxide): Solid polymer electrolyte for room temperature lithium battery. J. Power Sources 2019, 420, 63–72. [Google Scholar] [CrossRef]
- Moreno, J.S.; Jeremias, S.; Moretti, A.; Panero, S.; Passerini, S.; Scrosati, B.; Appetecchi, G.B. Ionic liquid mixtures with tunable physicochemical properties. Electrochim. Acta 2015, 151, 599–608. [Google Scholar] [CrossRef]
- Lewandowski, A.; Swiderska-Mocek, A. Ionic liquids as electrolytes for Li-ion batteries-An overview of electrochemical studies. J. Power Sources 2009, 194, 601–609. [Google Scholar] [CrossRef]
- Osada, I.; de Vries, H.; Scrosati, B.; Passerini, S. Ionic-liquid-based polymer electrolytes for battery applications. Angew. Chem. Int. Edit 2016, 55, 500–513. [Google Scholar] [CrossRef]
- Lee, S.; Park, K.; Koo, B.; Park, C.; Jang, M.; Lee, H.; Lee, H. Safe, Stable cycling of lithium metal batteries with low-viscosity, fire-retardant locally concentrated ionic liquid electrolytes. Adv. Funct. Mater. 2020, 30, 2003132. [Google Scholar] [CrossRef]
- Huo, H.; Zhao, N.; Sun, J.; Du, F.; Li, Y.; Guo, X. Composite electrolytes of polyethylene oxides/garnets interfacially wetted by ionic liquid for room-temperature solid-state lithium battery. J. Power Sources 2017, 372, 1–7. [Google Scholar] [CrossRef]
- Zhou, Y.D.; Wang, X.E.; Zhu, H.J.; Armand, M.; Forsyth, M.; Greene, G.W.; Pringle, J.M.; Howlett, P.C. Ternary lithium-salt organic ionic plastic crystal polymer composite electrolytes for high voltage, all-solid-state batteries. Energy Storage Mater. 2018, 15, 407–414. [Google Scholar] [CrossRef]
- Cheng, X.B.; Hou, T.Z.; Zhang, R.; Peng, H.J.; Zhao, C.Z.; Huang, J.Q.; Zhang, Q. Dendrite-free lithium deposition induced by uniformly distributed lithium ions for efficient lithium metal batteries. Adv. Mater. 2016, 28, 2888–2895. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.; Tiemblo, P.; Garcia, N.; Garcia-Calvo, O.; Fedeli, E.; Kvasha, A.; Urdampilleta, I. High performance polymer/ionic liquid thermoplastic solid electrolyte prepared by solvent free processing for solid state lithium metal batteries. Membranes 2018, 8, 55. [Google Scholar] [CrossRef] [Green Version]
- Karuppasamy, K.; Rhee, H.W.; Reddy, P.A.; Gupta, D.; Mitu, L.; Polu, A.R.; Shajan, X.S. Ionic liquid incorporated nanocomposite polymer electrolytes for rechargeable lithium ion battery: A way to achieve improved electrochemical and interfacial properties. J. Ind. Eng. Chem. 2016, 40, 168–176. [Google Scholar] [CrossRef]
- Xie, Z.K.; Wu, Z.J.; An, X.W.; Yoshida, A.; Wang, Z.D.; Hao, X.G.; Abudula, A.; Guan, G.Q. Bifunctional ionic liquid and conducting ceramic co-assisted solid polymer electrolyte membrane for quasi-solid-state lithium metal batteries. J. Membrane Sci. 2019, 586, 122–129. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Becking, J.; Stan, M.C.; Lenoch, A.; Bieker, P.; Kolek, M.; Winter, M. Wetting phenomena and their effect on the electrochemical performance of surface-tailored lithium metal electrodes in contact with cross-linked polymeric electrolytes. Angew. Chem. Int. Edit. 2020, 59, 17145–17153. [Google Scholar] [CrossRef]
- Shen, C.; Huang, Y.B.; Yang, J.R.; Chen, M.J.; Liu, Z.P. Unraveling the mechanism of ion and electron migration in composite solid-state electrolyte using conductive atomic force microscopy. Energy Storage Mater. 2021, 39, 271–277. [Google Scholar] [CrossRef]
- Pan, Q.; Barbash, D.; Smith, D.K.; Qi, H.; Gleeson, S.E.; Li, C.Y. Correlating electrode–electrolyte interface and battery performance in hybrid solid polymer electrolyte-based lithium metal batteries. Adv. Energy Mater. 2017, 7, 1701231. [Google Scholar] [CrossRef]
- Nair, J.R.; Destro, M.; Bella, F.; Appetecchi, G.B.; Gerbaldi, C. Thermally cured semi-interpenetrating electrolyte networks (s-IPN) for safe and aging-resistant secondary lithium polymer batteries. J. Power Sources 2016, 306, 258–267. [Google Scholar] [CrossRef]
- Nair, J.R.; Colo, F.; Kazzazi, A.; Moreno, M.; Bresser, D.; Lin, R.Y.; Bella, F.; Meligrana, G.; Fantini, S.; Simonetti, E.; et al. Room temperature ionic liquid (RTIL)-based electrolyte cocktails for safe, high working potential Li-based polymer batteries. J. Power Sources 2019, 412, 398–407. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Bruce, P.G.; Evans, J.; Vincent, C.A. Conductivity and transference number measurements on polymer electrolytes. Solid State Ion. 1988, 28–30, 918–922. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Dautzenberg, G.; Scrosati, B. A new class of advanced polymer electrolytes and their relevance in plastic-like, rechargeable lithium batteries. J. Electrochem. Soc. 1996, 143, 6–12. [Google Scholar] [CrossRef]
- Fan, W.; Li, N.W.; Zhang, X.L.; Zhao, S.Y.; Cao, R.; Yin, Y.Y.; Xing, Y.; Wang, J.N.; Guo, Y.G.; Li, C.J. A dual-salt gel polymer electrolyte with 3D cross-linked polymer network for dendrite-free lithium metal batteries. Adv. Sci. 2018, 5, 1800559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayakumar, V.; Diddens, D.; Heuer, A.; Kurungot, S.; Winter, M.; Nair, J.R. Dioxolanone-anchored poly(allyl ether)-based cross-linked dual-salt polymer electrolytes for high-voltage lithium metal batteries. Acs Appl. Mater. Inter. 2020, 12, 567–579. [Google Scholar] [CrossRef]
- Lim, J.Y.; Kang, D.A.; Kim, N.U.; Lee, J.M.; Kim, J.H. Bicontinuously crosslinked polymer electrolyte membranes with high ion conductivity and mechanical strength. J. Membrane Sci. 2019, 589, 117250. [Google Scholar] [CrossRef]
- Yang, G.; Song, Y.D.; Wang, Q.; Zhang, L.B.; Deng, L.J. Review of ionic liquids containing, polymer/inorganic hybrid electrolytes for lithium metal batteries. Mater. Design 2020, 190, 108563. [Google Scholar] [CrossRef]
- Kale, S.B.; Nirmale, T.C.; Khupse, N.D.; Kale, B.B.; Kulkarni, M.V.; Pavitran, S.; Gosavi, S.W. Cellulose-derived flame-retardant solid polymer electrolyte for lithium-ion batteries. Acs Sustain. Chem. Eng. 2021, 9, 1559–1567. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Montanino, M.; Carewska, M.; Moreno, M.; Alessandrini, F.; Passerini, S. Chemical-physical properties of bis(perfluoroalkylsulfonyl)imide-based ionic liquids. Electrochim. Acta 2011, 56, 1300–1307. [Google Scholar] [CrossRef]
- Qian, J.; Jin, B.Y.; Li, Y.Y.; Zhan, X.L.; Hou, Y.; Zhang, Q.H. Research progress on gel polymer electrolytes for lithium-sulfur batteries. J. Energy Chem. 2021, 56, 420–437. [Google Scholar] [CrossRef]
- Chiu, L.-L.; Chung, S.-H. A poly(ethylene oxide)/lithium bis(trifluoromethanesulfonyl)imide-loated polypropylene membrane for a high-loading lithium-sulfur battery. Polymers 2021, 13, 535. [Google Scholar] [CrossRef] [PubMed]
- Chiu, L.-L.; Chung, S.-H. Composite gel-polymer electrolyte for high-loading polysulfide cathodes. J. Mater. Chem. A 2022, 10, 13719–13726. [Google Scholar] [CrossRef]
- Henderson, W.A.; Passerini, S. Phase behavior of ionic liquid-LiX mixtures: Pyrrolidinium cations and TFSI- anions. Chem. Mater. 2004, 16, 2881–2885. [Google Scholar] [CrossRef]
- Nadherna, M.; Dominko, R.; Hanzel, D.; Reiter, J.; Gaberscek, M. Electrochemical behavior of Li2FeSiO4 with ionic liquids at elevated temperature. J. Electrochem. Soc. 2009, 156, A619–A626. [Google Scholar] [CrossRef]
- Wohde, F.; Balabajew, M.; Roling, B. Li+ transference numbers in liquid electrolytes obtained by very-low-frequency impedance spectroscopy at variable electrode distances. J. Electrochem. Soc. 2016, 163, A714–A721. [Google Scholar] [CrossRef]
- Lassegues, J.C.; Grondin, J.; Talaga, D. Lithium solvation in bis(trifluoromethanesulfonyl)imide-based ionic liquids. Phy. Chem. Chem. Phy. 2006, 8, 5629–5632. [Google Scholar] [CrossRef]
- Kobayashi, K.; Pagot, G.; Vezzu, K.; Bertasi, F.; Di Noto, V.; Tominaga, Y. Effect of plasticizer on the ion-conductive and dielectric behavior of poly(ethylene carbonate)-based Li electrolytes. Polym. J. 2021, 53, 149–155. [Google Scholar] [CrossRef]
- Choi, H.; Kim, H.W.; Kim, J.K.; Lim, Y.J.; Kim, Y.; Ahn, J.H. Nanocomposite quasi-solid-state electrolyte for high-safety lithium batteries. Nano Res. 2017, 10, 3092–3102. [Google Scholar] [CrossRef]
- Li, X.W.; Zhang, Z.X.; Li, S.J.; Yang, L.; Hirano, S. Polymeric ionic liquid-plastic crystal composite electrolytes for lithium ion batteries. J. Power Sources 2016, 307, 678–683. [Google Scholar] [CrossRef]
- Shin, J.H.; Henderson, W.A.; Tizzani, C.; Passerini, S.; Jeong, S.S.; Kim, K.W. Characterization of solvent-free polymer electrolytes consisting of ternary PEO-LiTFSI-PYR14 TFSI. J. Electrochem. Soc. 2006, 153, A1649–A1654. [Google Scholar] [CrossRef]
- Li, X.W.; Zhang, Z.X.; Li, S.J.; Yang, K.H.; Yang, L. Polymeric ionic liquid-ionic plastic crystal all-solid-state electrolytes for wide operating temperature range lithium metal batteries. J. Mater. Chem. A 2017, 5, 21362–21369. [Google Scholar] [CrossRef]




| Samples | Tg (°C) | Td (°C) | σ (20 °C, mS cm−1) | σ (60 °C, mS cm−1) | |
|---|---|---|---|---|---|
| SPE | −46.0 | 302 | 0.026 | 0.40 | 0.14 |
| CPE-E(26) | −49.3 | 316 | 0.41 | 3.12 | 0.092 |
| CPE-E(34) | −50.9 | 326 | 0.83 | 5.50 | 0.082 |
| CPE-E(42) | −54.7 | 328 | 0.80 | 4.18 | 0.084 |
| CPE-E(50) | −55.1 | 330 | 1.06 | 5.60 | 0.080 |
| CPE-P(26) | −51.3 | 317 | 0.15 | 1.26 | 0.088 |
| CPE-P(34) | −55.2 | 327 | 0.29 | 2.36 | 0.079 |
| CPE-P(42) | −58.5 | 328 | 0.60 | 3.61 | 0.082 |
| CPE-P(50) | −57.4 | 330 | 0.39 | 2.63 | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Ning, Y.; Jiang, Y.; Li, G.; Pan, Q. Double-Network Polymer Electrolytes with Ionic Liquids for Lithium Metal Batteries. Polymers 2022, 14, 3435. https://doi.org/10.3390/polym14173435
Zhu C, Ning Y, Jiang Y, Li G, Pan Q. Double-Network Polymer Electrolytes with Ionic Liquids for Lithium Metal Batteries. Polymers. 2022; 14(17):3435. https://doi.org/10.3390/polym14173435
Chicago/Turabian StyleZhu, Chenjing, Yi Ning, Yizhi Jiang, Guangji Li, and Qiwei Pan. 2022. "Double-Network Polymer Electrolytes with Ionic Liquids for Lithium Metal Batteries" Polymers 14, no. 17: 3435. https://doi.org/10.3390/polym14173435







