Improving the Ionic Conductivity of PEGDMA-Based Polymer Electrolytes by Reducing the Interfacial Resistance for LIBs
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Instrumentations and Measurements
2.3. Synthesis of PEGDMA 1000
2.4. Preparation of PEs on the LFP Cathode and Half-Cell
3. Results and Discussion
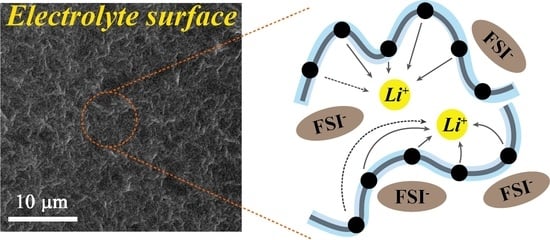
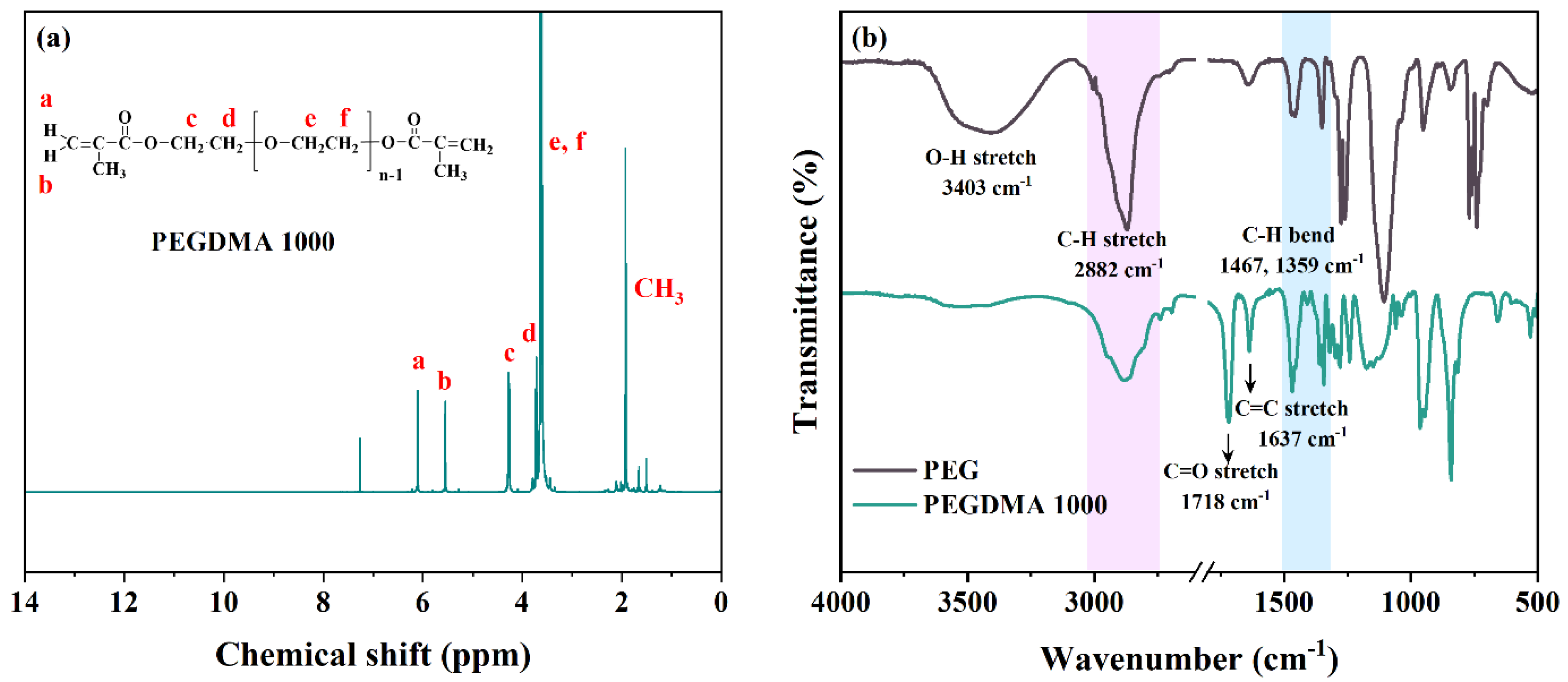
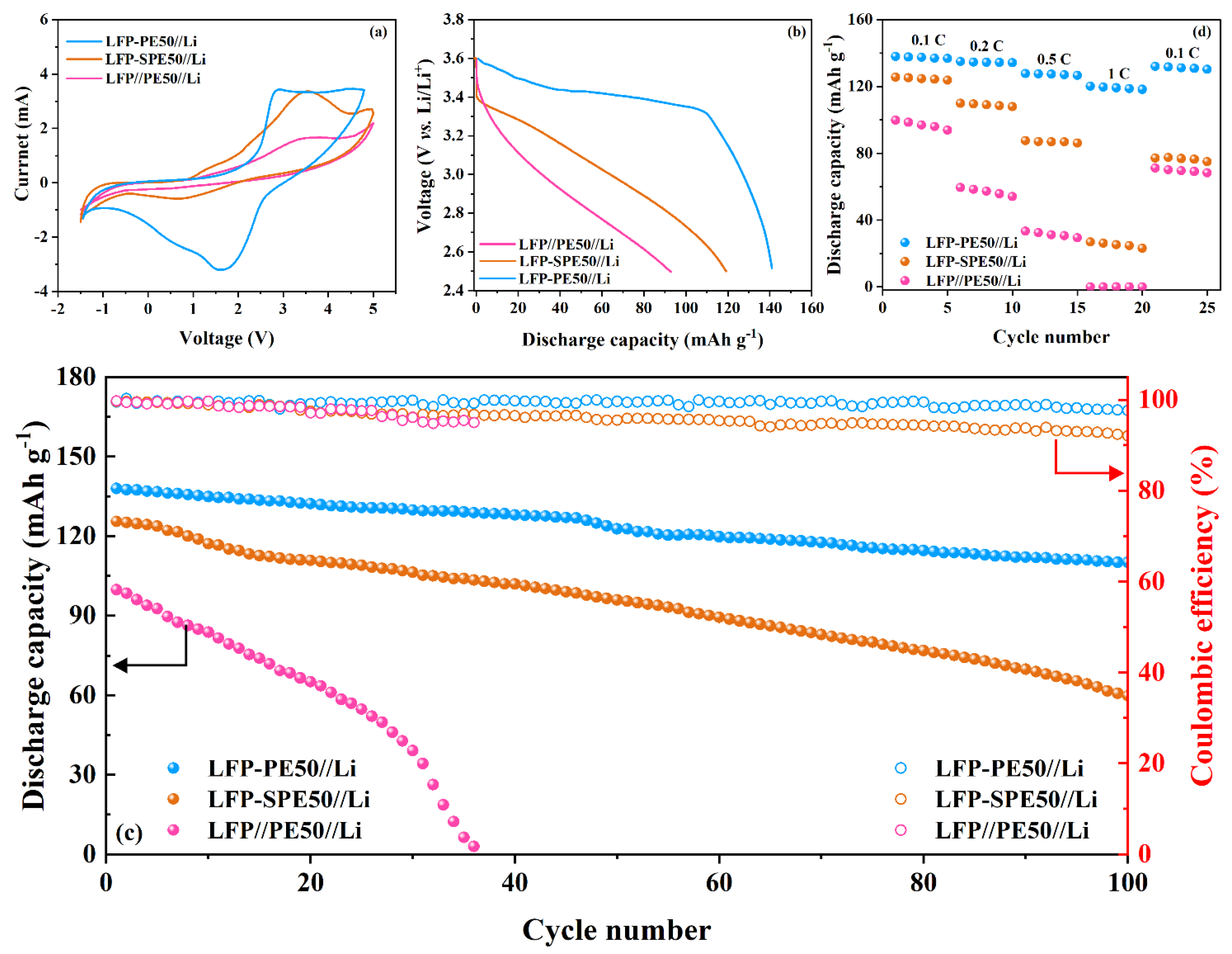
3.1. Characteristics and Physiochemical Properties of PEGDMA 1000 and Polymer Electrolytes
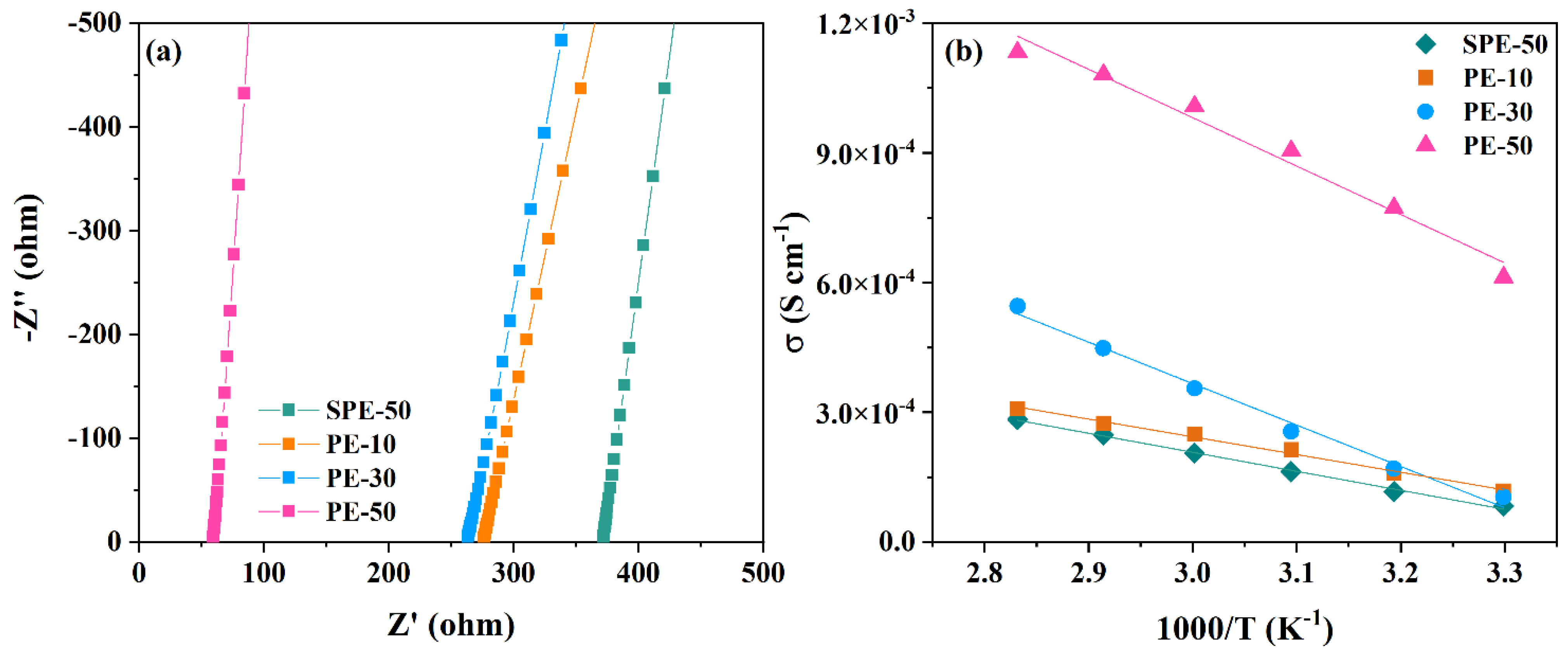
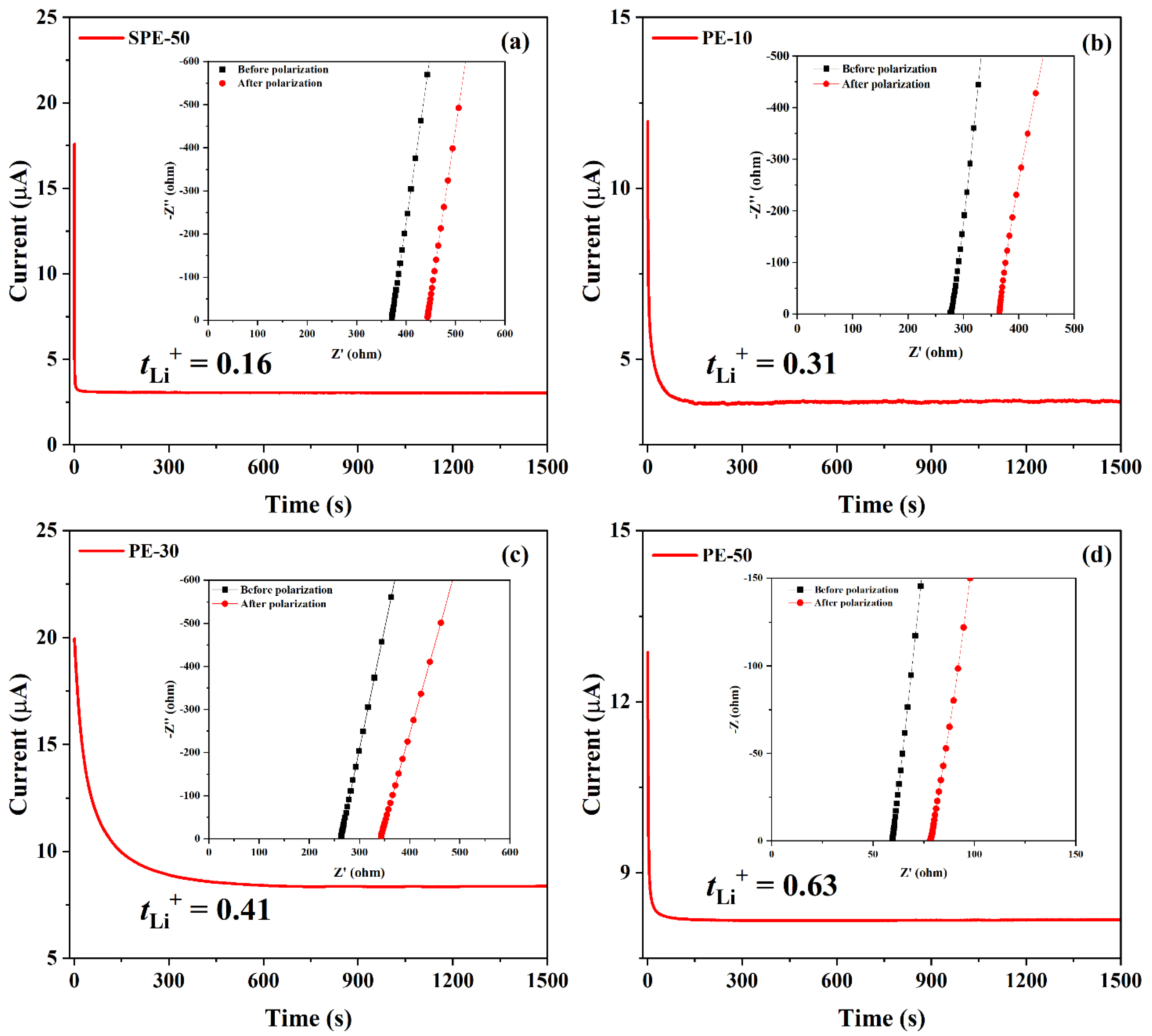
3.2. Electrochemical Properties of PEs and Coin Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Edge, J.S.; O’Kane, S.; Prosser, R.; Kirkaldy, N.D.; Patel, A.N.; Hales, A.; Ghosh, A.; Ai, W.; Chen, J.; Yang, J. Lithium Ion Battery Degradation: What You Need to Know. Phys. Chem. Chem. Phys. 2021, 23, 8200–8221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Courtier, N.E.; Zhang, Z.; Liu, K.; Bailey, J.J.; Boyce, A.M.; Richardson, G.; Shearing, P.R.; Kendrick, E.; Brett, D.J.L. A Review of Lithium-Ion Battery Electrode Drying: Mechanisms and Metrology. Adv. Energy Mater. 2022, 12, 2102233. [Google Scholar] [CrossRef]
- Baum, Z.J.; Bird, R.E.; Yu, X.; Ma, J. Lithium-Ion Battery Recycling-Overview of Techniques and Trends. ACS Energy Lett. 2022, 7, 712–719. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, L.; Li, Q.; Wang, K. A Comprehensive Review on the State of Charge Estimation for Lithium-Ion Battery Based on Neural Network. Int. J. Energy Res. 2022, 46, 5423–5440. [Google Scholar] [CrossRef]
- Arya, A.; Sharma, A.L. A Glimpse on All-Solid-State Li-Ion Battery (ASSLIB) Performance Based on Novel Solid Polymer Electrolytes: A Topical Review. J. Mater. Sci. 2020, 55, 6242–6304. [Google Scholar] [CrossRef]
- Duffner, F.; Kronemeyer, N.; Tübke, J.; Leker, J.; Winter, M.; Schmuch, R. Post-Lithium-Ion Battery Cell Production and Its Compatibility with Lithium-Ion Cell Production Infrastructure. Nat. Energy 2021, 6, 123–134. [Google Scholar] [CrossRef]
- Lv, W.; Zhu, C.; Chen, J.; Ou, C.; Zhang, Q.; Zhong, S. High Performance of Low-Temperature Electrolyte for Lithium-Ion Batteries Using Mixed Additives. Chem. Eng. J. 2021, 418, 129400. [Google Scholar] [CrossRef]
- Huang, W.; Feng, X.; Han, X.; Zhang, W.; Jiang, F. Questions and Answers Relating to Lithium-Ion Battery Safety Issues. Cell Rep. Phys. Sci. 2021, 2, 100285. [Google Scholar] [CrossRef]
- Niu, H.; Wang, L.; Guan, P.; Zhang, N.; Yan, C.; Ding, M.; Guo, X.; Huang, T.; Hu, X. Recent Advances in Application of Ionic Liquids in Electrolyte of Lithium Ion Batteries. J. Energy Storage 2021, 40, 102659. [Google Scholar] [CrossRef]
- Hubble, D.; Brown, D.E.; Zhao, Y.; Fang, C.; Lau, J.; McCloskey, B.D.; Liu, G. Liquid Electrolyte Development for Low-Temperature Lithium-Ion Batteries. Energy Environ. Sci. 2022, 15, 550–578. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Zhou, Y.; Liang, Z.; Tavajohi, N.; Li, B.; Li, T. Solid Polymer Electrolytes with High Conductivity and Transference Number of Li Ions for Li-Based Rechargeable Batteries. Adv. Sci. 2021, 8, 2003675. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for Lithium-Ion Battery Safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Yu, H.; Ding, Z.; Liu, Y.; Lu, J.; Lavorgna, M.; Wu, J.; Liu, X. Review on Polymer-Based Composite Electrolytes for Lithium Batteries. Front. Chem. 2019, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Jiang, Y.; Pan, Y.; Li, S.; Wang, J.; He, Y.; Xia, Y.; Liu, Z.; Chen, G.Z. Lithium Bis(Fluorosulfony)Imide-Lithium Hexafluorophosphate Binary-Salt Electrolytes for Lithium-Ion Batteries: Aluminum Corrosion Behaviors and Electrochemical Properties. ChemistrySelect 2018, 3, 1954–1960. [Google Scholar] [CrossRef]
- Bushkova, O.V.; Yaroslavtseva, T.V.; Dobrovolsky, Y.A. New Lithium Salts in Electrolytes for Lithium-Ion Batteries (Review). Russ. J. Electrochem. 2017, 53, 677–699. [Google Scholar] [CrossRef]
- Han, H.B.; Zhou, S.S.; Zhang, D.J.; Feng, S.W.; Li, L.F.; Liu, K.; Feng, W.F.; Nie, J.; Li, H.; Huang, X.J.; et al. Lithium Bis(Fluorosulfonyl)Imide (LiFSI) as Conducting Salt for Nonaqueous Liquid Electrolytes for Lithium-Ion Batteries: Physicochemical and Electrochemical Properties. J. Power Sources 2011, 196, 3623–3632. [Google Scholar] [CrossRef]
- Duan, J.; Tang, X.; Dai, H.; Yang, Y.; Wu, W.; Wei, X.; Huang, Y. Building Safe Lithium-Ion Batteries for Electric Vehicles: A Review; Springer: Singapore, 2020; Volume 3, ISBN 0123456789. [Google Scholar]
- Xi, G.; Xiao, M.; Wang, S.; Han, D.; Li, Y.; Meng, Y. Polymer-Based Solid Electrolytes: Material Selection, Design, and Application. Adv. Funct. Mater. 2021, 31, 2007598. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, X.; Ma, Z.; Mei, P.; Xiao, W.; You, Q.; Zhang, Y. Development of the PEO Based Solid Polymer Electrolytes for All-Solid State Lithium Ion Batteries. Polymers 2018, 10, 1237. [Google Scholar] [CrossRef]
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G. Polymer Electrolytes for Lithium-Based Batteries: Advances and Prospects. Chem 2019, 5, 2326–2352. [Google Scholar] [CrossRef]
- Ngai, K.S.; Ramesh, S.; Ramesh, K.; Juan, J.C. A Review of Polymer Electrolytes: Fundamental, Approaches and Applications. Ionics 2016, 22, 1259–1279. [Google Scholar] [CrossRef]
- Deng, K.; Zeng, Q.; Wang, D.; Liu, Z.; Qiu, Z.; Zhang, Y.; Xiao, M.; Meng, Y. Single-Ion Conducting Gel Polymer Electrolytes: Design, Preparation and Application. J. Mater. Chem. A 2020, 8, 1557–1577. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.-Q.; Shen, L.; Liu, Q.; Ma, J.-B.; Lv, W.; He, Y.-B.; Yang, Q.-H. Progress and Perspective of Ceramic/Polymer Composite Solid Electrolytes for Lithium Batteries. Adv. Sci. 2020, 7, 1903088. [Google Scholar] [CrossRef] [PubMed]
- Bachman, J.C.; Muy, S.; Grimaud, A.; Chang, H.-H.; Pour, N.; Lux, S.F.; Paschos, O.; Maglia, F.; Lupart, S.; Lamp, P.; et al. Inorganic Solid-State Electrolytes for Lithium Batteries: Mechanisms and Properties Governing Ion Conduction. Chem. Rev. 2016, 116, 140–162. [Google Scholar] [CrossRef]
- Fan, P.; Liu, H.; Marosz, V.; Samuels, N.T.; Suib, S.L.; Sun, L.; Liao, L. High Performance Composite Polymer Electrolytes for Lithium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2101380. [Google Scholar] [CrossRef]
- Park, K.H.; Bai, Q.; Kim, D.H.; Oh, D.Y.; Zhu, Y.; Mo, Y.; Jung, Y.S. Design Strategies, Practical Considerations, and New Solution Processes of Sulfide Solid Electrolytes for All-Solid-State Batteries. Adv. Energy Mater. 2018, 8, 1800035. [Google Scholar] [CrossRef]
- Hikima, K.; Phuc, N.H.H.; Tsukasaki, H.; Mori, S.; Muto, H.; Matsuda, A. High Ionic Conductivity of Multivalent Cation Doped Li6PS5Cl Solid Electrolytes Synthesized by Mechanical Milling. RSC Adv. 2020, 10, 22304–22310. [Google Scholar] [CrossRef]
- Yi, L.; Zou, C.; Chen, X.; Liu, J.; Cao, S.; Tao, X.; Zang, Z.; Liu, L.; Chang, B.; Shen, Y.; et al. One-Step Synthesis of PVDF-HFP/PMMA-ZrO2Gel Polymer Electrolyte to Boost the Performance of a Lithium Metal Battery. ACS Appl. Energy Mater. 2022, 5, 7317–7327. [Google Scholar] [CrossRef]
- Hosseinioun, A.; Paillard, E. In Situ Crosslinked PMMA Gel Electrolyte from a Low Viscosity Precursor Solution for Cost-Effective, Long Lasting and Sustainable Lithium-Ion Batteries. J. Membr. Sci. 2020, 594, 117456. [Google Scholar] [CrossRef]
- Liu, C.; Sacci, R.L.; Sahore, R.; Veith, G.M.; Dudney, N.J.; Chen, X.C. Polyacrylonitrile-Based Electrolytes: How Processing and Residual Solvent Affect Ion Transport and Stability. J. Power Sources 2022, 527, 231165. [Google Scholar] [CrossRef]
- An, Y.; Han, X.; Liu, Y.; Azhar, A.; Na, J.; Nanjundan, A.K.; Wang, S.; Yu, J.; Yamauchi, Y. Progress in Solid Polymer Electrolytes for Lithium-Ion Batteries and Beyond. Small 2022, 18, 2103617. [Google Scholar] [CrossRef]
- Elizalde, F.; Amici, J.; Trano, S.; Vozzolo, G.; Aguirresarobe, R.; Versaci, D.; Bodoardo, S.; Mecerreyes, D.; Sardon, H.; Bella, F. Self-Healable Dynamic Poly(Urea-Urethane) Gel Electrolyte for Lithium Batteries. J. Mater. Chem. A 2022, 10, 12588–12596. [Google Scholar] [CrossRef]
- Ma, C.; Cui, W.; Liu, X.; Ding, Y.; Wang, Y. In Situ Preparation of Gel Polymer Electrolyte for Lithium Batteries: Progress and Perspectives. InfoMat 2022, 4, e12232. [Google Scholar] [CrossRef]
- Guo, C.; Cao, Y.; Li, J.; Li, H.; Kumar, S.; Oleksandr, S.; Chen, F. Solvent-Free Green Synthesis of Nonflammable and Self-Healing Polymer Film Electrolytes for Lithium Metal Batteries ✩. Appl. Energy 2022, 323, 119571. [Google Scholar] [CrossRef]
- Amici, J.; Torchio, C.; Versaci, D.; Dessantis, D.; Marchisio, A.; Caldera, F.; Bella, F.; Francia, C.; Bodoardo, S. Nanosponge-Based Composite Gel Polymer Electrolyte for Safer Li-O2 Batteries. Polymers 2021, 13, 1625. [Google Scholar] [CrossRef]
- Yang, P.; Gao, X.; Tian, X.; Shu, C.; Yi, Y.; Liu, P.; Wang, T.; Qu, L.; Tian, B.; Li, M. Upgrading Traditional Organic Electrolytes toward Future Lithium Metal Batteries: A Hierarchical Nano-SiO2-Supported Gel Polymer Electrolyte. ACS Energy Lett. 2020, 5, 1681–1688. [Google Scholar] [CrossRef]
- Choi, J.; Zabihi, O.; Varley, R.J.; Fox, B.; Naebe, M. Enhancement of Ionic Conduction and Mechanical Properties for All-Solid-State Polymer Electrolyte Systems through Ionic and Physical Bonding. Mater. Today Chem. 2022, 23, 100663. [Google Scholar] [CrossRef]
- Oh, B.-K.; Jung, W.-I.; Kim, D.-W.; Rhee, H.-W. Preparation of UV Curable Gel Polymer Electrolytes and Their Electrochemical Properties. Bull. Korean Chem. Soc. 2002, 23, 683–687. [Google Scholar] [CrossRef]
- Mindemark, J.; Lacey, M.J.; Bowden, T.; Brandell, D. Beyond PEO—Alternative Host Materials for Li+-Conducting Solid Polymer Electrolytes. Prog. Polym. Sci. 2018, 81, 114–143. [Google Scholar] [CrossRef]
- Devaux, D.; Bouchet, R.; Glé, D.; Denoyel, R. Mechanism of Ion Transport in PEO/LiTFSI Complexes: Effect of Temperature, Molecular Weight and End Groups. Solid State Ion. 2012, 227, 119–127. [Google Scholar] [CrossRef]
- Wang, H.; Sheng, L.; Yasin, G.; Wang, L.; Xu, H.; He, X. Reviewing the Current Status and Development of Polymer Electrolytes for Solid-State Lithium Batteries. Energy Storage Mater. 2020, 33, 188–215. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Liu, X.; Zhong, H.; Xu, H.; Xu, Z.; Shao, H.; Ding, F. Suppression of Lithium Dendrite Formation by Using LAGP-PEO (LiTFSI) Composite Solid Electrolyte and Lithium Metal Anode Modified by PEO (LiTFSI) in All-Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2017, 9, 13694–13702. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yao, Y.; Ou, J.; Li, M.; Luo, D.; Dou, H.; Li, Z.; Amine, K.; Yu, A.; Chen, Z. A Review of Composite Solid-State Electrolytes for Lithium Batteries: Fundamentals, Key Materials and Advanced Structures. Chem. Soc. Rev. 2020, 49, 8790–8839. [Google Scholar] [CrossRef] [PubMed]
- Atik, J.; Diddens, D.; Thienenkamp, J.H.; Brunklaus, G.; Winter, M.; Paillard, E. Cation-Assisted Lithium-IonT Ransport for High-Performance PEO-Based Ternary Solid Polymer Electrolytes. Angew. Chem. Int. Ed. 2021, 60, 11919–11927. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Xu, B.; Grundish, N.S.; Xia, Y.; Li, Y.; Lu, C.; Liu, Y.; Wu, N.; Goodenough, J.B. Li2S6-Integrated PEO-Based Polymer Electrolytes for All-Solid-State Lithium-Metal Batteries. Angew. Chem. 2021, 133, 17842–17847. [Google Scholar] [CrossRef]
- Jin, L.; Jang, G.; Lim, H.; Zhang, W.; Kim, W.; Jang, H. An In Situ Polymeric Electrolyte with Low Interfacial Resistance on Electrodes for Lithium-Ion Batteries. Adv. Mater. Interfaces 2022, 9, 2101958. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Chen, Y.; Wang, P.; Zhang, H.; He, X. PEO Based Polymer-Ceramic Hybrid Solid Electrolytes: A Review. Nano Converg. 2021, 8, 1–12. [Google Scholar] [CrossRef]
- You, L.; Duan, K.; Zhang, G.; Song, W.; Yang, T.; Song, X.; Wang, S.; Liu, J. N, N-Dimethylformamide Electrolyte Additive Via a Blocking Strategy Enables High-Performance Lithium-Ion Battery under High Temperature. J. Phys. Chem. C 2019, 123, 5942–5950. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Hussein, M.Z. Poly(Lactic Acid)/Poly(Ethylene Glycol) Polymer Nanocomposites: Effects of Graphene Nanoplatelets. Polymers 2014, 6, 93–104. [Google Scholar] [CrossRef]
- Shameli, K.; Ahmad, M.B.; Jazayeri, S.D.; Sedaghat, S.; Shabanzadeh, P.; Jahangirian, H.; Mahdavi, M.; Abdollahi, Y. Synthesis and Characterization of Polyethylene Glycol Mediated Silver Nanoparticles by the Green Method. Int. J. Mol. Sci. 2012, 13, 6639–6650. [Google Scholar] [CrossRef]
- Xu, G.; Shangguan, X.; Dong, S.; Zhou, X.; Cui, G. Key Scientific Issues in Formulating Blended Lithium Salts Electrolyte for Lithium Batteries. Angew. Chem. Int. Ed. 2019, 17, 955. [Google Scholar] [CrossRef]
- Xue, Z.; He, D.; Xie, X. Poly(Ethylene Oxide)-Based Electrolytes for Lithium-Ion Batteries. J. Mater. Chem. A 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
- Jin, L.; Ahmed, F.; Ryu, T.; Yoon, S.; Zhang, W.; Lee, Y.; Kim, D.; Jang, H.; Kim, W. Highly Conductive and Flexible Gel Polymer Electrolyte with Bis(Fluorosulfonyl)Imide Lithium Salt via UV Curing for Li-Ion Batteries. Membranes 2019, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Arora, N.; Paul, K.; Kumar, R.; Kumar, R. FTIR and Rheological Studies of PMMA-Based Nano-Dispersed Gel Polymer Electrolytes Incorporated with LiBF4 and SiO2. Ionics 2019, 25, 1495–1503. [Google Scholar] [CrossRef]
- Aldalur, I.; Martinez-Ibañez, M.; Piszcz, M.; Rodriguez-Martinez, L.M.; Zhang, H.; Armand, M. Lowering the Operational Temperature of All-Solid-State Lithium Polymer Cell with Highly Conductive and Interfacially Robust Solid Polymer Electrolytes. J. Power Sources 2018, 383, 144–149. [Google Scholar] [CrossRef]
- Fullerton-Shirey, S.K.; Maranas, J.K. Effect of LiClO4 on the Structure and Mobility of PEO-Based Solid Polymer Electrolytes. Macromolecules 2009, 42, 2142–2156. [Google Scholar] [CrossRef]
- Chen, L.; Fu, J.; Lu, Q.; Shi, L.; Li, M.; Dong, L.; Xu, Y.; Jia, R. Cross-Linked Polymeric Ionic Liquids Ion Gel Electrolytes by in Situ Radical Polymerization. Chem. Eng. J. 2019, 378, 122245. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, L.; Jang, G.; Lim, H.; Zhang, W.; Park, S.; Jeon, M.; Jang, H.; Kim, W. Improving the Ionic Conductivity of PEGDMA-Based Polymer Electrolytes by Reducing the Interfacial Resistance for LIBs. Polymers 2022, 14, 3443. https://doi.org/10.3390/polym14173443
Jin L, Jang G, Lim H, Zhang W, Park S, Jeon M, Jang H, Kim W. Improving the Ionic Conductivity of PEGDMA-Based Polymer Electrolytes by Reducing the Interfacial Resistance for LIBs. Polymers. 2022; 14(17):3443. https://doi.org/10.3390/polym14173443
Chicago/Turabian StyleJin, Lei, Giseok Jang, Hyunmin Lim, Wei Zhang, Sungjun Park, Minhyuk Jeon, Hohyoun Jang, and Whangi Kim. 2022. "Improving the Ionic Conductivity of PEGDMA-Based Polymer Electrolytes by Reducing the Interfacial Resistance for LIBs" Polymers 14, no. 17: 3443. https://doi.org/10.3390/polym14173443