Measuring the Pores’ Structure in P3HT Organic Polymeric Semiconductor Films Using Interface Electrolyte/Organic Semiconductor Redox Injection Reactions and Bulk Space-Charge
Abstract
:1. Introduction
2. Methods and Materials
2.1. ER-EIS for the Electronic Structure Spectroscopy of Real Organic Semiconductors
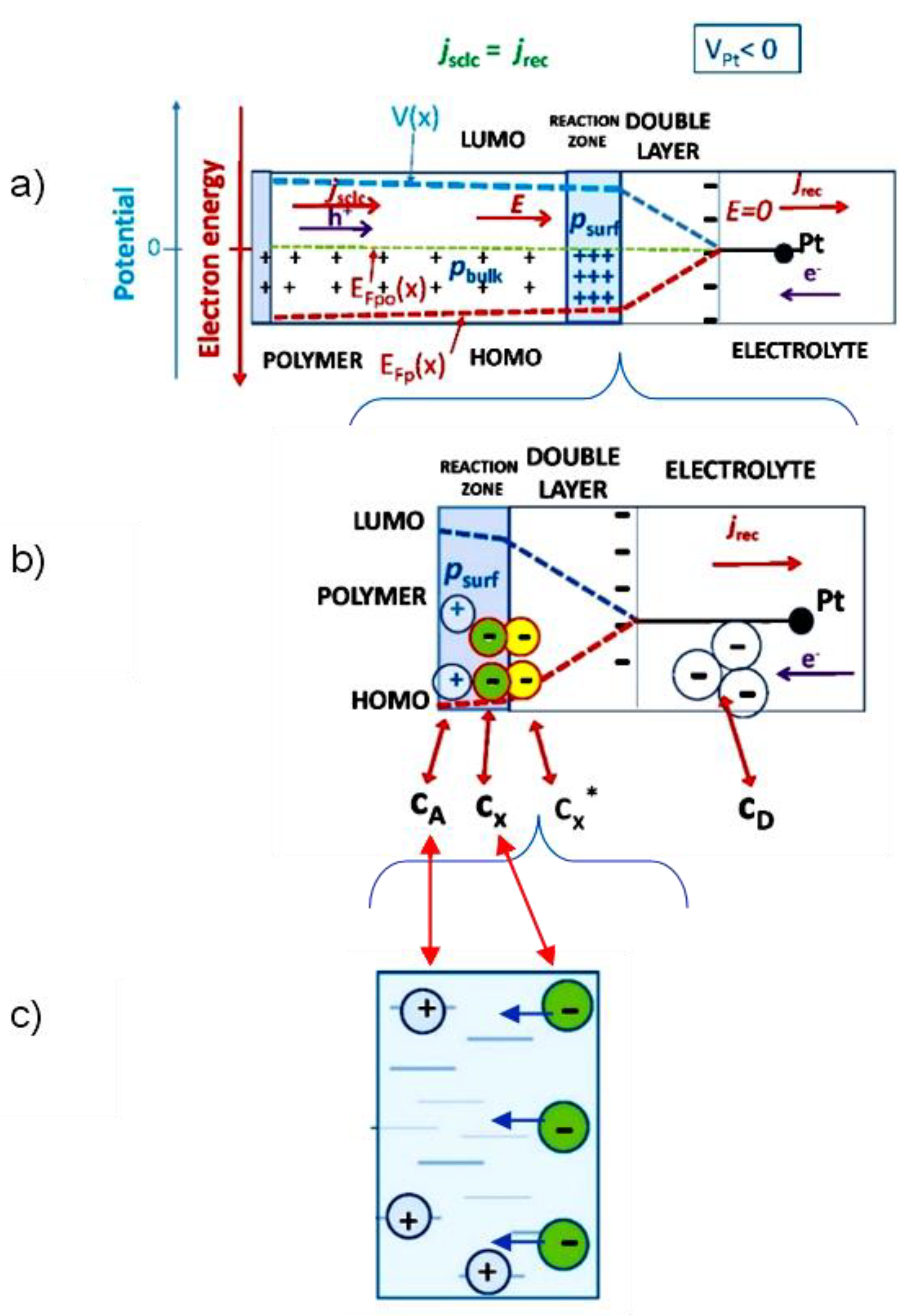
2.2. Is the Existence of Pores Influencing the ER-EIS Results?
2.3. Useful ER-EIS Outputs—Effective Rate Constant
2.4. How It Is with Compensating Ions with ER-EIS?
2.5. Materials and Apparatus
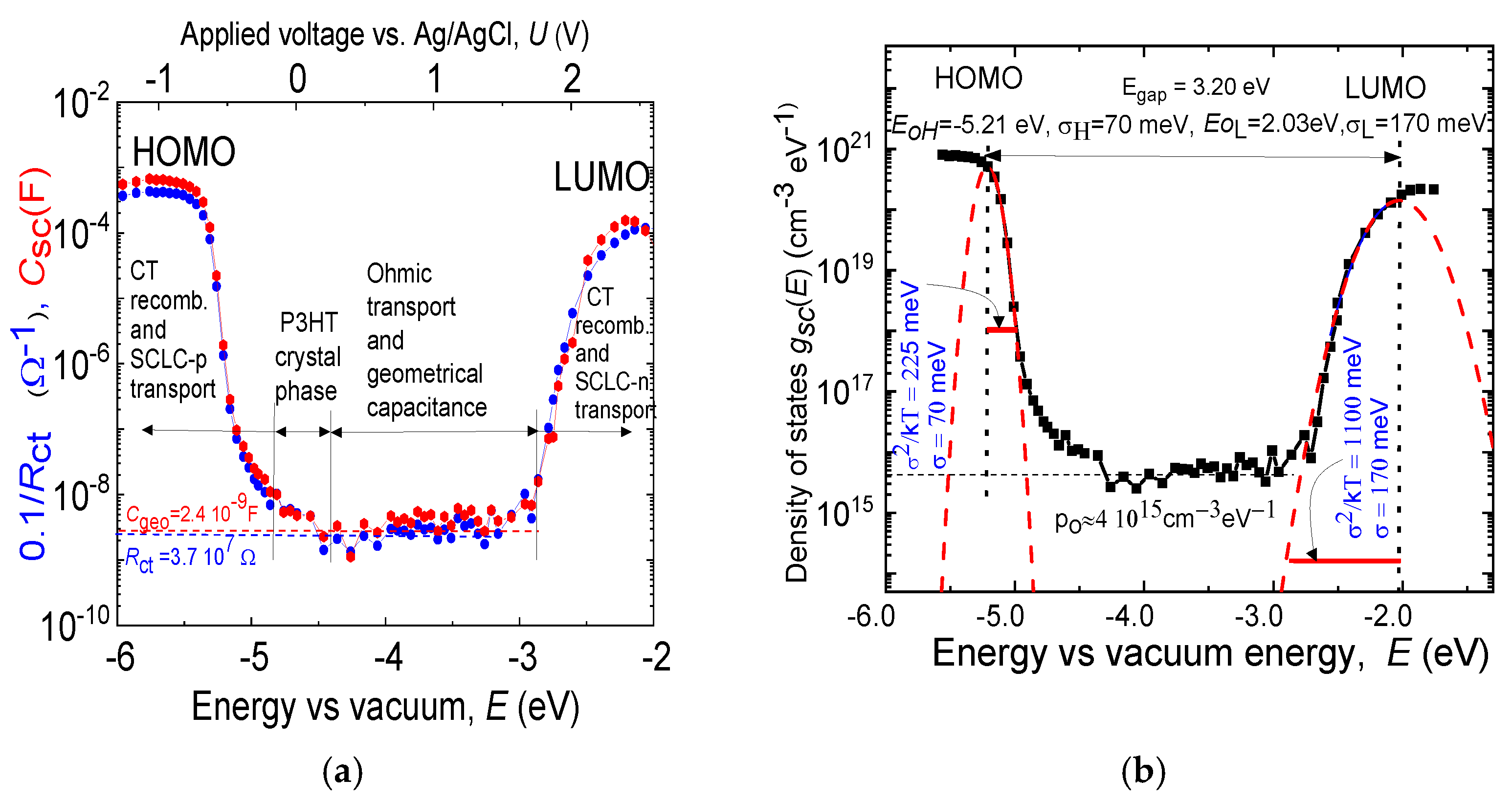
3. Results
3.1. Pores Structure
3.2. Rate Equations for the Bimolecular Preexponential Rate of Heterogeneous Interface Electrolyte/OS
3.3. Counterions Intercalation
4. Discussion and Conclusions
- The ER-EIS method may be used with advantage in the field of OS for the elucidation of:
- -
- The electronic structure (DOS) and statistics of occupation of transport bands (HOMO and LUMO),
- -
- The electronic structure of both intrinsic and extrinsic defect states,
- -
- The electronic structure of excited states in D-A systems, so crucial for organic solar-cell functioning,
- The effect of injection of high current densities by redox interface reactions in the bulk of OS with built-in pores structure may be very interesting for the design of new devices of organic electronics and energy storing.
- The present paper dispelled concerns associated with the use of the method, due to the pore structure existence and penetration of ions. It may be also concluded the ER-EIS method is not in any contradiction with Marcus’ CT D-A transport theory.
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kohler, A.; Bässler, H. Electronic Processes in Organic Semiconductor: An Introduction; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2015; p. 171, 175. [Google Scholar]
- Bässler, H. Charge transport in disordered organic photoconductors–A Monte-Carlo simulation study. Phys. Stat. Sol. 1993, 175, 15–56. [Google Scholar] [CrossRef]
- Gregg, A. Charged defects in soft semiconductors and their influence on organic photovoltaics. Soft Matter 2009, 5, 2989. [Google Scholar] [CrossRef]
- Liu, X.; Fahlman, M. Electronic Structure Characterization of Soft Semiconductors. Adv. Mater. Interfaces 2019, 6, 1900439. [Google Scholar] [CrossRef] [Green Version]
- Sworakowski, J. How accurate are energies of HOMO and LUMO levels in small-molecule organic semiconductors determined from cyclic voltammetry or optical spectroscopy? Sxnthetic Met. 2018, 235, 125. [Google Scholar] [CrossRef]
- Schauer, F. Electronic structure spectroscopy of organic semiconductors by Energy Resolved–Electrochemical Impedance Spectroscopy (ER-EIS). J. Appl. Phys. 2020, 128, 150902. [Google Scholar] [CrossRef]
- Helmholtz, H. Űber einige Gesetze der Vertheilung elektrischer Ströme in körperlichen Leitern mit Anwendung auf die thierisch-elektrischen Versuche. Ann. Der Phys. Und Chem. 1853, 165, 211. [Google Scholar] [CrossRef] [Green Version]
- Bässler, H.; Kroh, D.; Schauer, F.; Nádaždy, V.; Köhler, A. Mapping the Density of States Distribution of Organic Semiconductors by Employing Energy Resolved–Electrochemical Impedance Spectroscopy. Adv. Funct. Mater. 2020, 31, 2007738. [Google Scholar] [CrossRef]
- Karki, J.; Vollbrecht, J.; Vollbrecht, A.J.; Gillett, F.; Schauer, N.T.Q. Unifying Charge Generation, Recombination, and Extraction in Low-Offset Non-Fullerene Acceptor Organic Solar Cells. Energy Mater. 2020, 10, 2001203. [Google Scholar] [CrossRef]
- Athanasopoulos, S.; Schauer, F.; Nádaždy, V.; Weiß, M.; Kahle, F.-J.; Scherf, U.; Bässler, H.; Köhler, A. What is the Binding Energy of a Charge Transfer State in an Organic Solar Cell? Adv. Energy Mater. 2019, 9, 19008. [Google Scholar] [CrossRef]
- Schauer, F.; Nádaždy, V.; Gmucová, K. Electrochemical impedance spectroscopy for the study of electronic structure in disordered organic semiconductors—Possibilities and limitations. J. Appl. Phys. 2018, 123, 161590. [Google Scholar] [CrossRef]
- Schauer, F.; Tkáč, L.; Ožvoldová, M.; Nádaždy, V.; Gmucová, K.; Jergel, M.; Šiffalovič, P. Effect of crystallinity on UV degradability of poly[methyl(phenyl)silane]by Energy-Resolved Electrochemical Impedance Spectroscopy. AIP Adv. 2017, 7, 055002. [Google Scholar] [CrossRef] [Green Version]
- Schauer, F.; Tkáč, L.; Ožvoldová, M.; Nadáždy, V.; Gmucová, K.; Tkáčová, M.; Chlpík, J. Electronic Structure Mapping of Branching States in Poly[methyl(phenyl)silane] Upon Exposure to UV Radiation. Korean Phys. Soc. 2016, 68, 252. [Google Scholar] [CrossRef]
- Volk, S.; Yazdani, N.; Sanusoglu, E.; Yarema, O.; Yarema, M.; Wood, V. Measuring the Electronic Structure of Nanocrystal Thin Films Using Energy-Resolved Electrochemical Impedance Spectroscopy. J. Phys. Chem. Lett. 2018, 9, 1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisquert, J.; Marcus, R.A. Multiscale modelling of Organic and Hybrid Photovoltaics OS. In Modelling of Organic and Hybrid Photovoltaics; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Wang, Q.; Ito, S.; Graetzel, M.; Fabregat-Santiago, F.; Mora-Sero, I.; Bisquert, J.; Bessho, T.; Imai, H. Characteristics of high efficiency dye-sensitized solar cells. J. Phys. Chem. 2006, B110, 25210. [Google Scholar] [CrossRef]
- Marcus, R.A.; Sutin, N. Electron transfer in chemistry and Biology. Biochim. Biophys. Acta 1985, 811, 265. [Google Scholar] [CrossRef]
- Marcus, R.A. Electron -transfer reactions in chemistry-theory and experiment. Rev. Mod. Phys. 1993, 65, 599. [Google Scholar] [CrossRef] [Green Version]
- Lewis, N.S. Progress in understanding electron-transfer reactions at semiconductor/liquid interfaces. J. Phys. Chem. B 1998, 102B, 4843. [Google Scholar] [CrossRef]
- Fajardo, M.; Lewis, N.S. Free-energy dependence of electron-transfer rate constants at Si/liquid interfaces. J. Phys. Chem. B 1997, 101B, 11136. [Google Scholar] [CrossRef]
- Pomykal, K.E.; Lewis, N.S. Measurement of interfacial charge-transfer rate constants at n-type InP/CH3OH junctions. J. Phys. Chem. B 1997, B101, 2476. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution. Pure Appl. Chem. 2015, 87, 1051. [Google Scholar] [CrossRef] [Green Version]
- Cooper, I. Conjugated Microporous Polymers. Adv. Mater. 2009, 21, 1291. [Google Scholar] [CrossRef]
- Liu, Q.; Li, G.; Tang, Z.; Chen, L.; Liao, B.; Ou, B.; Zhou, Z.; Zhou, H. Design and synthesis of conjugated polymers of tunable pore size distribution. Mater. Chem. Phys. 2017, 186, 11–18. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, H.; Teller, E. Adsorption of Bases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309. [Google Scholar] [CrossRef]
- Ogiegloa, W.; Wormeesterb, H.; Eichhornc, K.-J.; Wesslingd, M.; Benesaa, N.E. In situ ellipsometry studies on swelling of thin polymer films: A review. Prog. Polym. Sci. 2015, 42, 42–78. [Google Scholar] [CrossRef]
- Rose, M.; Böhlmann, W.; Sabo, M.; Kaskel, S. Element–organic frameworks with high permanent porosity. Chem. Commun. 2008, 2462–2464. [Google Scholar] [CrossRef]
- Dawson, R.; Su, F.B.; Niu, H.J.; Wood, C.D.; Jones, J.T.A.; Khimyak, Y.Z.; Cooper, A.I. Mesoporous poly(phenylenevinylene) networks. Macromolecules 2008, 41, 1591. [Google Scholar] [CrossRef]
- Salaneck, R.; Stafstrom, S.; Bredas, J.L. Conjugated Polymer Surfaces and Interfaces; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- de Levie, R. Electrochemical response of porous and rough electrodes. In Advances in Electrochemistry and Electrochemical Engineering; Interscience: New York, NY, USA, 1967; Volume 6, p. 329. [Google Scholar]
- Chidsey, E.D.; Murray, R.W. Electroactive polymer and macromolecular electronics. Science 1986, 231, 25. [Google Scholar] [CrossRef]
- Chidsey, E.D.; Murray, R.W. Redoc capacity and direct–Current electron conductivity in elecroactive materials. J. Phys. Chem. 1986, 90, 1479. [Google Scholar] [CrossRef]
- Careem, M.A.; Velmurugu, Y.; Skaarup, S.; West, K. A voltammetry study on the diffusion of counter ions in polypyrrole films. J. Power Sources 2006, 159, 210. [Google Scholar] [CrossRef]
- Garcia-Belmonte, G.; Pomerantz, Z.; Bisquert, J.; Lellouche, J.-P.; Zaban, A. Analysis of ion diffusion and charging in electronically conducting polydicarbazole films by impedance methods. Electrochim. Acta 2004, 49, 3413. [Google Scholar] [CrossRef]
- Garcia-Belmonte, G. Effect of electrode morphology on the diffusion length of the doping process of electronically conducting polypyrrole films. Electrochem. Commun. 2003, 5, 236. [Google Scholar] [CrossRef]
- Garcia-Belmonte, G.; Bisquert, J. Impedance analysis of galvanostatically synthesized polypyrrole films. Correlation of ionic diffusion and capacitance parameters with the electrode morphology. Electrochim. Acta 2002, 47, 4263. [Google Scholar] [CrossRef]
- Dai, A.; Wan, A.; Magee, C.; Zhang, Y.; Barlow, S.; Marder, S.R.; Kahn, A. Investigation of p-dopant diffusion in polymer films and bulk heterojunctions: Stable spatially-confined doping for all-solution processed solar cells. Org. Electron. 2015, 23, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Ratcliff, L.; Lee, A.; Armstrong, N.R. Work function control of hole-selective polymer/ITO anode contacts: An electrochemical doping study. J. Mater. Chem. 2010, 20, 2672. [Google Scholar] [CrossRef]
- Tang, G.; Ang, M.C.Y.; Choo, K.-K.; Keerthi, V.; Tan, J.-K.; Syafiqah, M.N.; Kugler, T.; Burroughes, J.H.; Png, R.-Q.; Chua, L.-L.; et al. Doped polymer semiconductors with ultrahigh and ultralow work functions for ohmic contacts. Nature 2016, 539, 536. [Google Scholar] [CrossRef]
- Rudolph, M.; Ratcliff, E.L. Normal and inverted regimes of charge transfer controlled by density of states at polymer electrodes. Nat. Commun. 2017, 8, 1048. [Google Scholar] [CrossRef] [Green Version]
- Vorotyntsev, M.A.; Badiali, J.-P.; Inzelt, G. Electrochemical impedance spectroscopy of thin films with two mobile charge carriers: Effects of the interfacial charging. J. Electroanal. Chem. 1999, 472, 7. [Google Scholar] [CrossRef]
- Gonçalves, R.; Pereira, E.C.; Marchesi, L.F. The Overoxidation of poly(3-hexylthiophene) (P3HT) Thin Film: CV and EIS measurements. Int. J. Electrochem. Sci. 2017, 12, 1983. [Google Scholar] [CrossRef]
- Zheng, Z.; Tummala, R.; Wang, T.; Coropceanu, V.; Brédas, J.L. Charge-Transfer States at Organic–Organic Interfaces: Impact of Static and Dynamic Disorders. Adv. Energy Mater. 2019, 9, 1803926. [Google Scholar] [CrossRef]
- Sirringhaus, H.; Brown, P.J.; Friend, R.H.; Nielsen, M.M.; Bechgaard, K.; Langeveld-Voss, B.M.W.; Spiering, A.J.H.; Janssen, R.; Meijer, E.W.; Herwig, P.T.; et al. Two-dimensional charge transport in self-organized, high-mobility conjugated polymers. Nature 1999, 401, 685–688. [Google Scholar] [CrossRef]
- Zen, A.; Saphiannikova, M.; Neher, D.; Grenzer, J.; Grigorian, S.; Pietsch, U.; Asawapirom, U.; Janietz, S.; Scherf, U.; Lieberwirth, I.; et al. Effect of Molecular Weight on the Structure and Crystallinity of Poly(3-hexylthiophene). Macromolecules 2006, 39, 2162–2171. [Google Scholar] [CrossRef]
- Zen, A.; Pflaum, J.; Hirschmann, S.; Zhuang, W.; Jaiser, F.; Asawapirom, U.; Rabe, J.P.; Scherf, U.; Neher, D. Effect of Molecular Weight and Annealing of Poly(3-hexylthiophene)s on the Performance of Organic Field-Effect Transistors. Adv. Funct. Mater. 2004, 14, 757–764. [Google Scholar] [CrossRef]
- DeLongchamp, M.; Kline, R.J.; Fischer, D.A.; Richter, L.J.; Toney, M.F. Molecular Characterization of Organic Electronic Films. Adv. Mater. 2011, 23, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Cook, S.; Tuladhar, S.M.; Choulis, S.; Nelson, J.; Durrant, J.R.; Bradley, D.; Giles, M.; McCulloch, I.; Ha, C.-S.; et al. A strong regioregularity effect in self-organizing conjugated polymer films and high-efficiency polythiophene:fullerene solar cells. Nat. Mater. 2006, 5, 197–203. [Google Scholar] [CrossRef]
- Chen, D.; Nakahara, A.; Wei, D.; Nordlund, D.; Russell, T.P. P3HT/PCBM Bulk Heterojunction Organic Photovoltaics: Correlating Efficiency and Morphology. Nano Lett. 2011, 11, 561–567. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Xie, S.S.; Qian, S.F.; Zhou, T.; Zhao, R.A.; Wang, G.; Qian, L.X.; Li, W.Z. Optical absorption spectra of C70 thin films. J. Appl. Phys. 1996, 80, 459. [Google Scholar] [CrossRef]
- Berson, S.; de Bettignies, R.; Bailly, S.; Guillerez, S. Poly (3-hexylthiophene) fibers for photovoltaic applications. Adv. Funct. Mater. 2007, 17, 1377–1384. [Google Scholar] [CrossRef]
- Dang, M.T.; Hirsch, L.; Wantz, G. P3HT:PCBM, Best Seller in Polymer Photovoltaic Research. Adv. Mater. 2011, 23, 3597. [Google Scholar] [CrossRef]
- Wadsworth, A.; Hamid, Z.; Bidwell, M.; Ashraf, R.S.; Khan, J.I.; Anjum, D.H.; Cendra, C.; Yan, J.; Rezasoltani, E.; Guilbert, A.A.Y.; et al. Progress in Poly (3-Hexylthiophene) Organic Solar Cells and the Influence of Its Molecular Weight on Device Performance. Adv. Energy Mater. 2018, 8, 1801001. [Google Scholar] [CrossRef]
- Schauer, F.; Nádaždy, V.; Gmucová, K.; Váry, T. Electrochemically induced charge injection in disordered organic conductive polymers. J. Appl. Phys. 2018, 124, 165702. [Google Scholar] [CrossRef]
- Shen, X.; Hu, W.; Russell, T.P. Measuring the Degree of Crystallinity in Semicrystalline Regioregular Poly(3-hexylthiophene). Macromolecules 2016, 49, 4501–4509. [Google Scholar] [CrossRef]
- Marcus, R.A. On the Theory of Oxidation-Reduction Reactions Involving Electronic Transfer. J. Chem. Phys. 1956, 24, 966. [Google Scholar] [CrossRef] [Green Version]
- Schmickler, W.; Santos, E. Interfacial Electrochemistry; Springer: Berlin, Germany, 2010. [Google Scholar]
- de Vries, X.; Pascal, F.; Wenzel, W.; Coehoorn, R.; Bobbert, A. Full quantum treatment of charge dynamics in amorphous molecular semiconductors. Phys. Rev. B 2018, 97, 075203. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.Q.; Georgievskii, Y.; Marcus, R.A. On the theory of electronic transfer reactions at semiconductor electrode/liquid interfaces. J. Chem. Phys. 2000, 112, 3358. [Google Scholar] [CrossRef] [Green Version]
- Nadazdy, V.; Schauer, F.; Gmucova, K. Energy resolved electrochemical impedance spectroscopy for electronic structure. Appl. Phys. Lett. 2014, 105, 142109. [Google Scholar] [CrossRef] [Green Version]
- Arkhipov, V.I.; Heremans, P.; Emelianova, E.V.; Bässler, H. Effect of doping on the density-of-states distribution and carrier hopping in disordered organic semiconductors. Phys. Rev. B 2005, 71, 045214. [Google Scholar] [CrossRef]
- Nádaždy, V.; Gmucová, K.; Siffalovic, N.; Vegso, K.; Jergel, M.; Schauer, F.; Majkova, E. Thickness Effect on Structural Defect-Related Density of States and Crystallinity in P3HT Thin Films on ITO Substrates. J. Phys. Chem. C 2018, 122, 5881–5887. [Google Scholar] [CrossRef]
- Marcus, R. Reflections on electron transfer theory. J. Chem. Phys. 2020, 153, 210401. [Google Scholar] [CrossRef]
- Ondersma, J.W.; Hamann, T.W. Measurements and Modeling of Recombination from Nanoparticle TiO2 Electrodes. J. Am. Chem. Soc. 2011, 133, 8264. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schauer, F. Measuring the Pores’ Structure in P3HT Organic Polymeric Semiconductor Films Using Interface Electrolyte/Organic Semiconductor Redox Injection Reactions and Bulk Space-Charge. Polymers 2022, 14, 3456. https://doi.org/10.3390/polym14173456
Schauer F. Measuring the Pores’ Structure in P3HT Organic Polymeric Semiconductor Films Using Interface Electrolyte/Organic Semiconductor Redox Injection Reactions and Bulk Space-Charge. Polymers. 2022; 14(17):3456. https://doi.org/10.3390/polym14173456
Chicago/Turabian StyleSchauer, Franz. 2022. "Measuring the Pores’ Structure in P3HT Organic Polymeric Semiconductor Films Using Interface Electrolyte/Organic Semiconductor Redox Injection Reactions and Bulk Space-Charge" Polymers 14, no. 17: 3456. https://doi.org/10.3390/polym14173456
APA StyleSchauer, F. (2022). Measuring the Pores’ Structure in P3HT Organic Polymeric Semiconductor Films Using Interface Electrolyte/Organic Semiconductor Redox Injection Reactions and Bulk Space-Charge. Polymers, 14(17), 3456. https://doi.org/10.3390/polym14173456






