Semiconducting Soft Submicron Particles from the Microwave-Driven Polymerization of Diaminomaleonitrile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the DAMN Polymers
2.2. Characterization of the DAMN Polymers
2.2.1. Elemental Analysis
2.2.2. FTIR Spectroscopy
2.2.3. C Solid-State Cross Polarization/Magic Angle Spinning Nuclear Magnetic Resonance (CP/MAS) NMR
2.2.4. Powder XRD
2.2.5. UV-Vis Spectroscopy
2.2.6. Thermal Analysis
2.2.7. Scanning Electron Microscopy (SEM)
2.2.8. Particle Size Analysis
2.2.9. Surface Area
2.2.10. Density
2.2.11. Gel-Permeation Chromatography (GPC)
2.3. Electrochemical Measurements
3. Results and Discussion
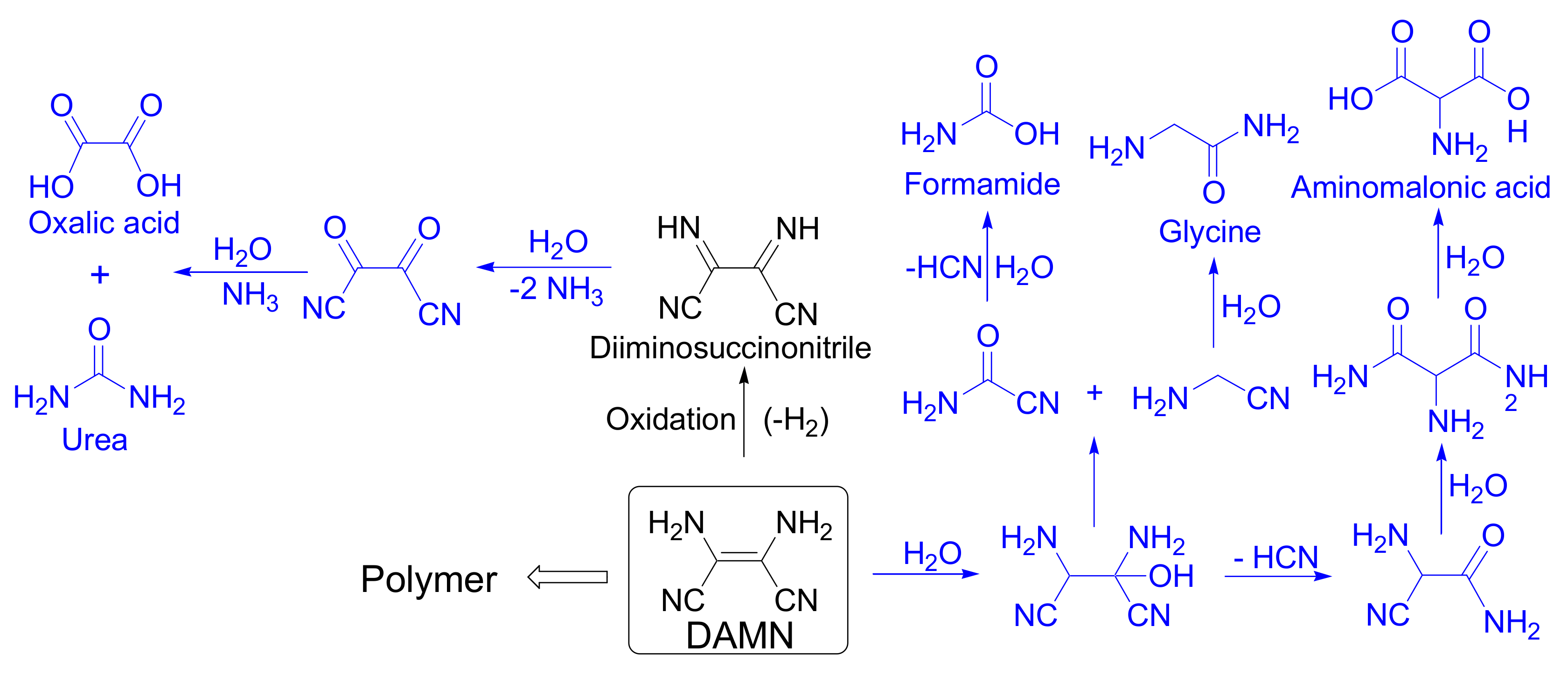
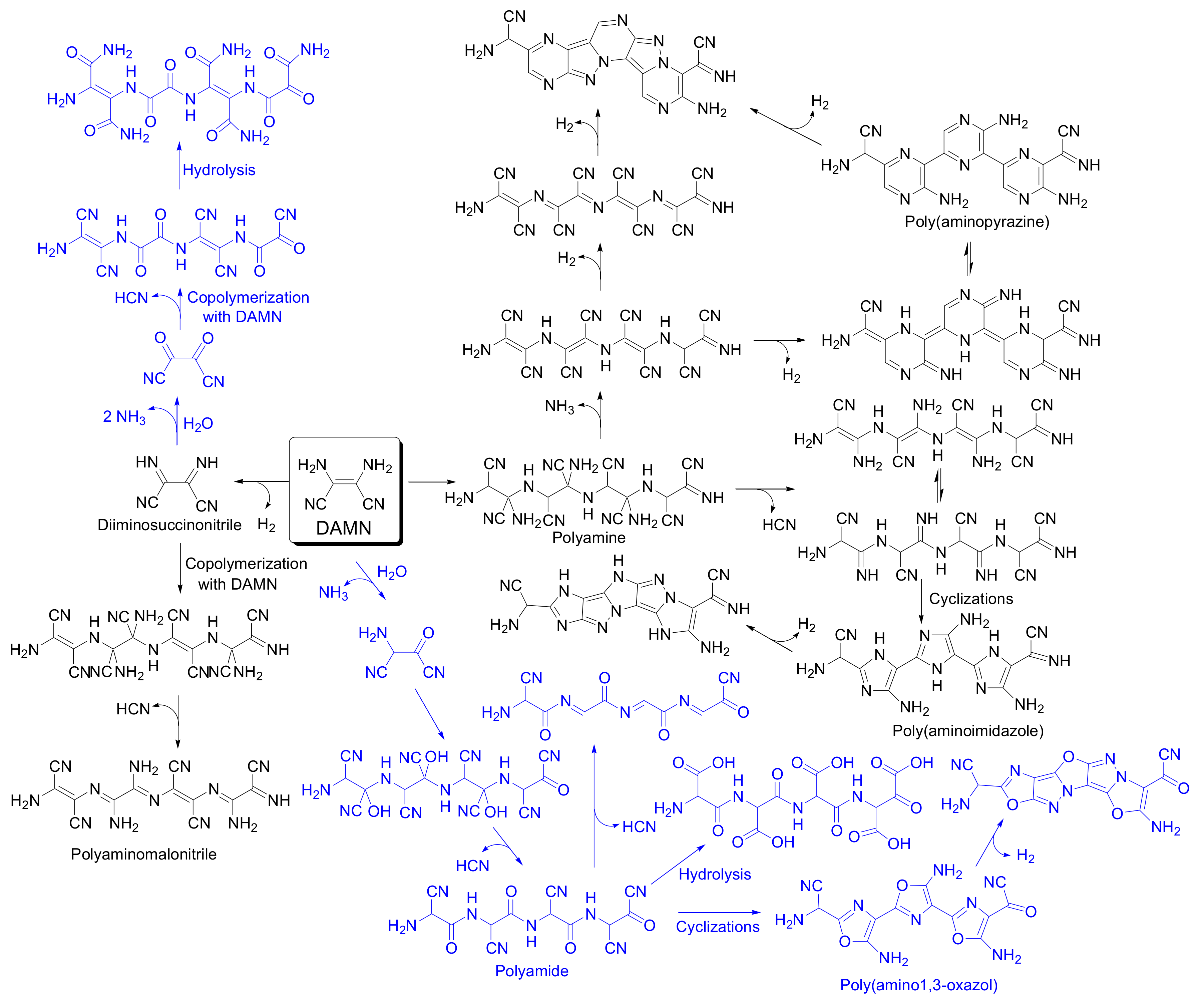
3.1. Synthetic Conditions and Chemical Composition of the DAMN Polymeric Submicron Particles
3.2. Structural Characterization
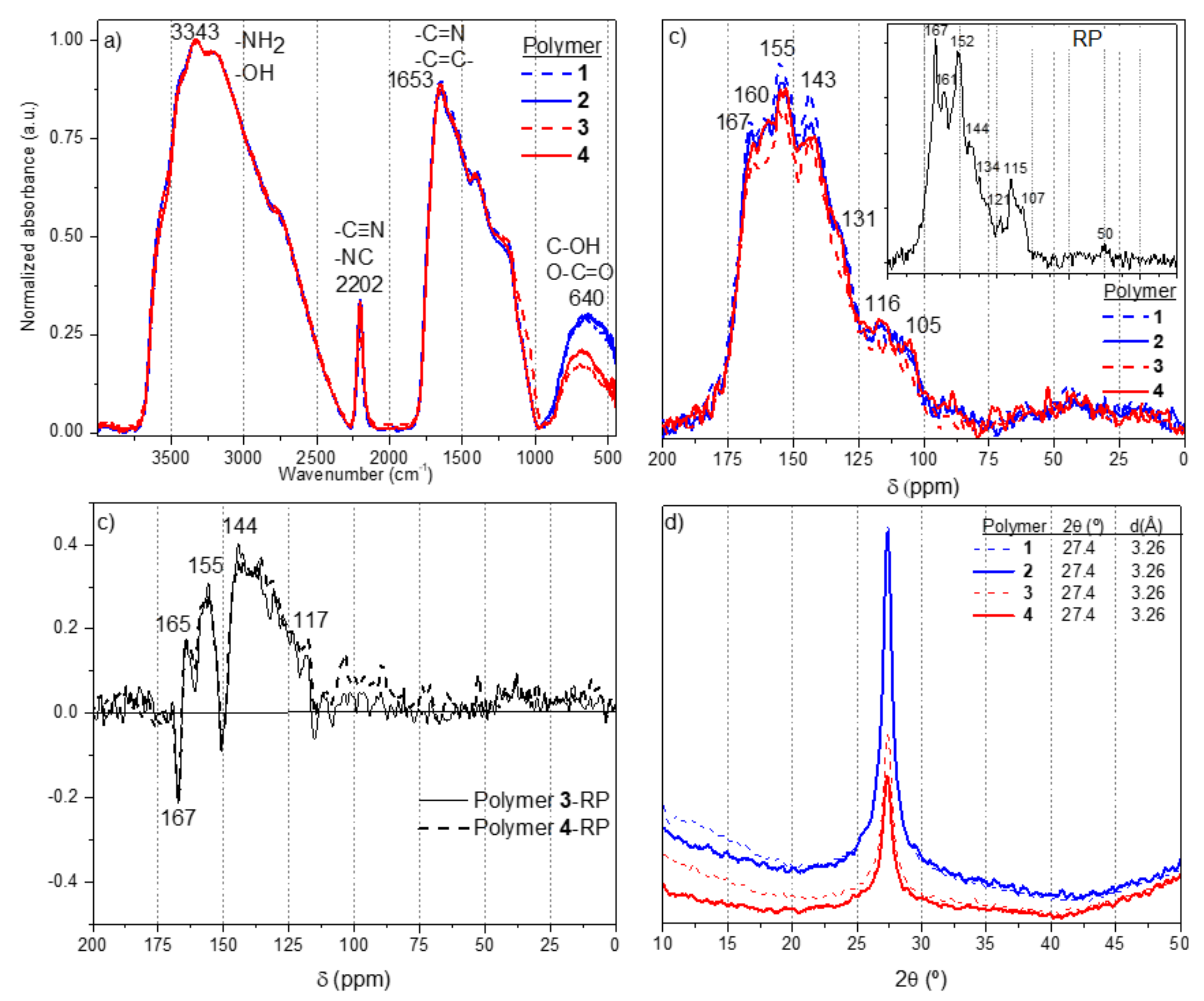
3.3. Thermal Stability
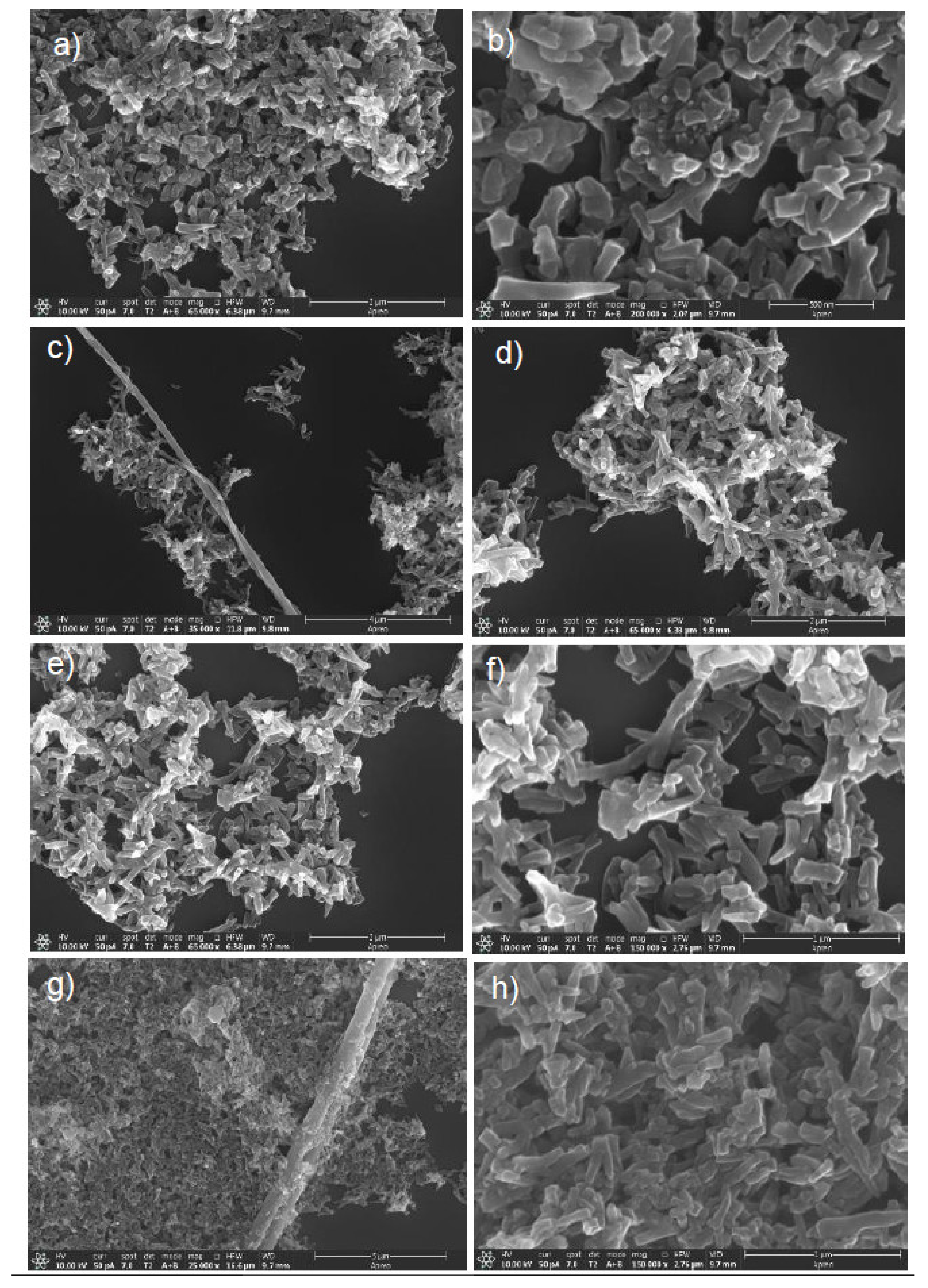
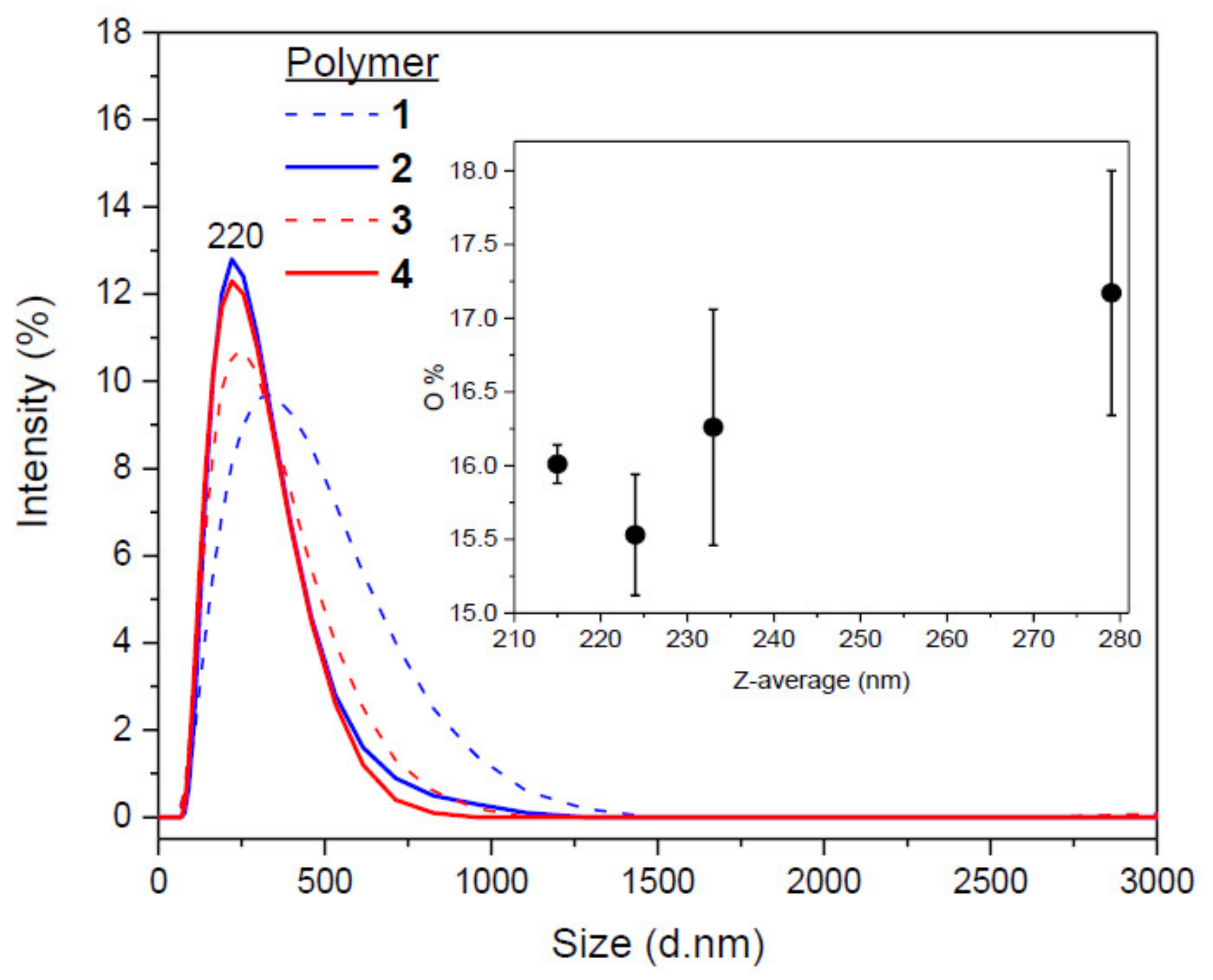
3.4. Morphological and Textural Properties
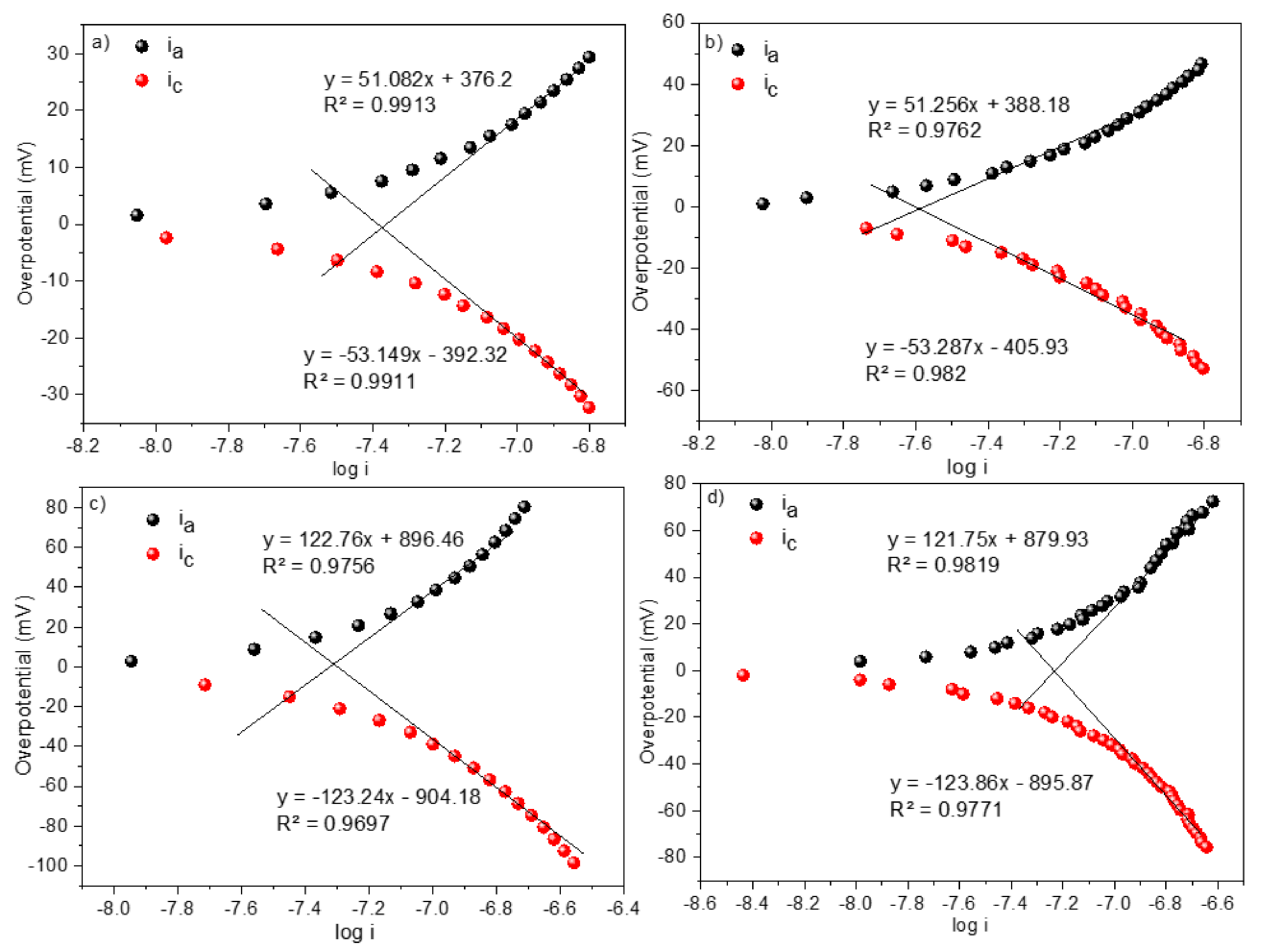
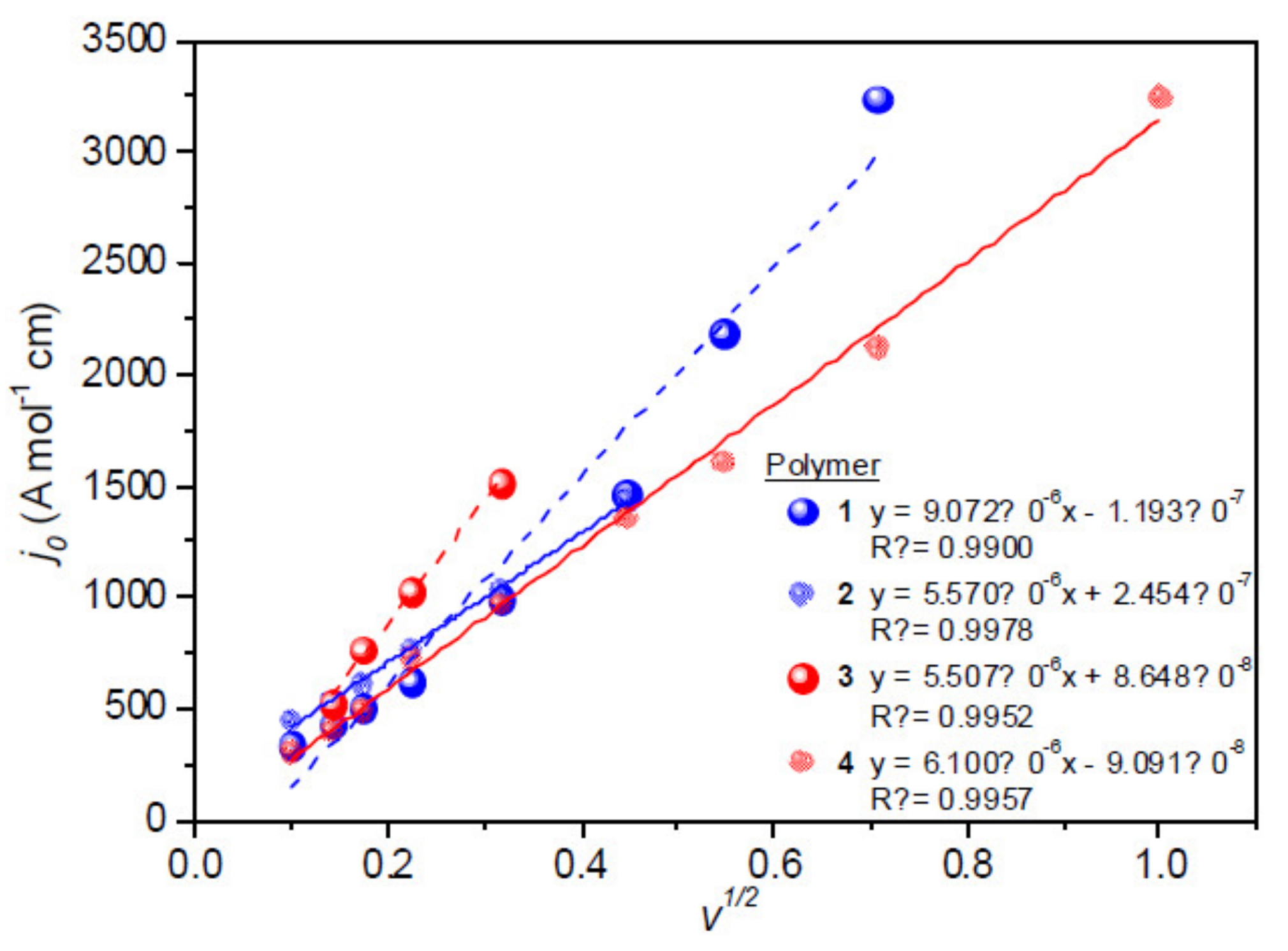
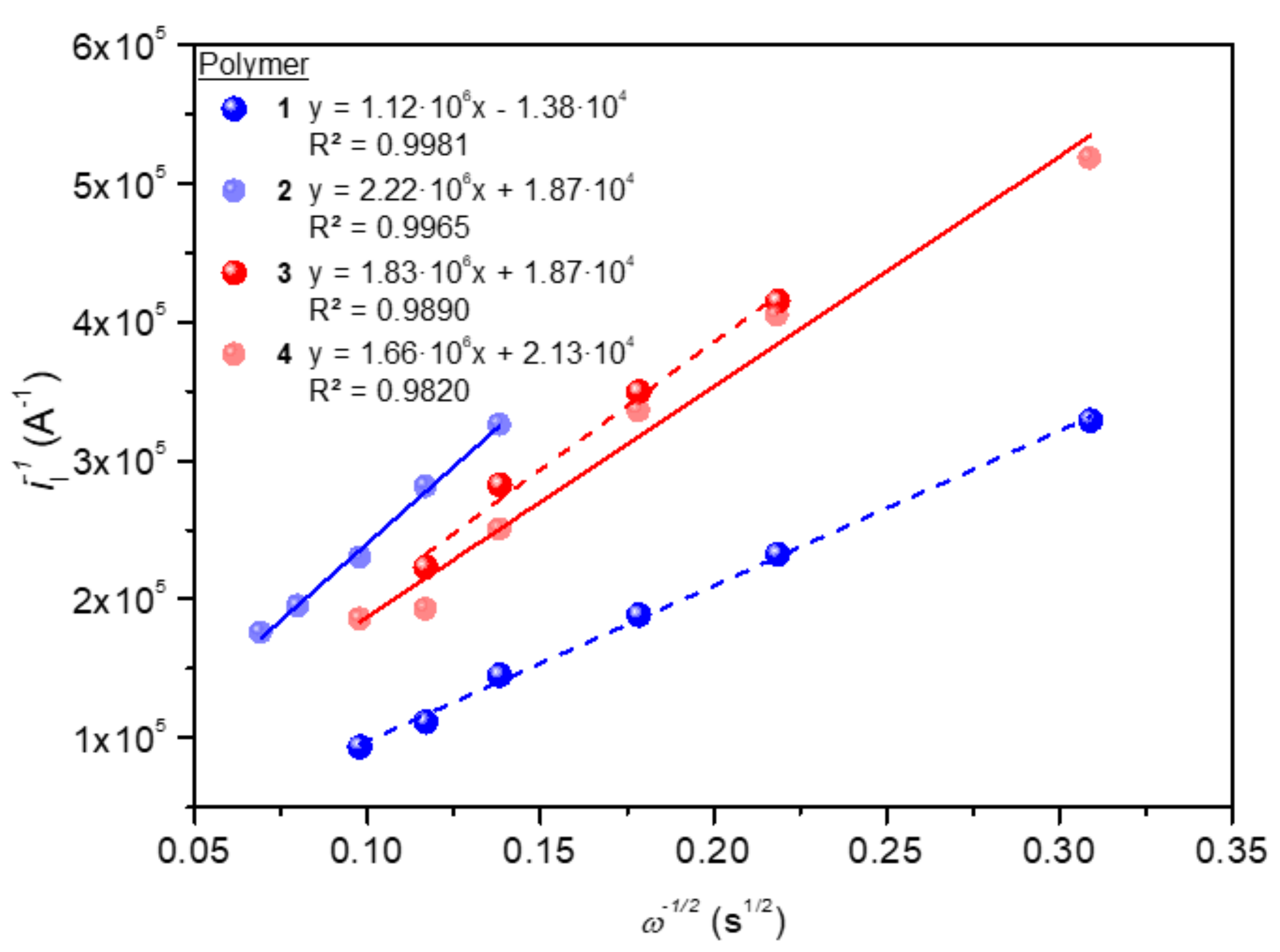
3.5. Electrochemistry
3.6. New Insights in Prebiotic Chemistry and Materials Science
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szady-Chełmieniecka, A.; Kołodziej, B.; Morawiak, M.; Kamieński, B.; Schilf, W. Spectroscopic studies of the intramolecular hydrogen bonding in o-hydroxy Schiff bases, derived from diaminomaleonitrile, and their deprotonation reaction products. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 189, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Aruna; Rani, B.; Swami, S.; Agarwala, A.; Behera, D.; Shrivastava, R. Recent progress in development of 2,3-diaminomaleonitrile (DAMN) based chemosensors for sensing of ionic and reactive oxygen species. RSC Adv. 2019, 9, 30599–30614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Bermejo, M.; de la Fuente, J.L.; Pérez-Fernández, C.; Mateo-Martí, E. A Comprehensive Review of HCN-Derived Polymers. Processes 2021, 9, 597. [Google Scholar] [CrossRef]
- d’Ischia, M.; Manini, P.; Moracci, M.; Saladino, R.; Ball, V.; Thissen, H.; Evans, R.A.; Puzzarini, C.; Barone, V. Astrochemistry and Astrobiology: Materials Science in Wonderland? Int. J. Mol. Sci. 2019, 20, 4079. [Google Scholar] [CrossRef] [Green Version]
- Thissen, H.; Evans, R.A.; Ball, V. Films and Materials Derived from Aminomalononitrile. Processes 2021, 9, 82. [Google Scholar] [CrossRef]
- Zhou, X.; Fang, Y.; Su, Y.; Ge, C.; Jin, B.; Li, Z.; Wu, S. Preparation and Characterization of Poly-Hydrogen Cyanide Nanofibers with High Visible Light Photocatalytic Activity. Catal. Commun. 2014, 46, 197–200. [Google Scholar] [CrossRef]
- Rahm, M.; Lunine, J.I.; Usher, D.A.; Shalloway, D. Polymorphism and Electronic Structure of Polyimine and Its Potential Significance for Prebiotic Chemistry on Titan. Proc. Natl. Acad. Sci. USA 2016, 113, 8121–8126. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Bermejo, M.; de la Fuente, J.L.; Carretero-González, J.; García-Fernández, L.; Aguilar, M.R. A Comparative Study on HCN Polymers Synthesized by Polymerization of NH4CN or Diaminomaleonitrile in Aqueous Media: New Perspectives for Prebiotic Chemistry and Materials Science. Chem. A Eur. J. 2019, 25, 11437–11455. [Google Scholar] [CrossRef]
- Thissen, H.; Koegler, A.; Salwiczek, M.; Easton, C.D.; Qu, Y.; Lithgow, T.; Evans, R.A. Prebiotic-Chemistry Inspired Polymer Coatings for Biomedical and Material Science Applications. NPG Asia Mater. 2015, 7, e225. [Google Scholar] [CrossRef] [Green Version]
- Menzies, D.J.; Ang, A.; Thissen, H.; Evans, R.A. Adhesive Prebiotic Chemistry Inspired Coatings for Bone Contacting Applications. ACS Biomater. Sci. Eng. 2017, 3, 793–806. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Chiang, Y.-L.; Chang, Y.-H.; Thissen, H.; Tsai, S.-W. An Aqueous-Based Process to Bioactivate Poly(ε-Caprolactone)/Mesoporous Bioglass Composite Surfaces by Prebiotic Chemistry-Inspired Polymer Coatings for Biomedical Applications. Colloids Surf. B Biointerfaces 2021, 205, 111913. [Google Scholar] [CrossRef]
- Ball, V. Antioxidant Activity of Films Inspired by Prebiotic Chemistry. Mater. Lett. 2021, 285, 129050. [Google Scholar] [CrossRef]
- Pérez-Fernández, C.; Ruiz-Bermejo, M.; Gálvez-Martínez, S.; Mateo-Martí, E. An XPS Study of HCN-Derived Films on Pyrite Surfaces: A Prebiotic Chemistry Standpoint towards the Development of Protective Coatings. RSC Adv. 2021, 11, 20109–20117. [Google Scholar] [CrossRef]
- Liao, T.-Y.; Easton, C.D.; Thissen, H.; Tsai, W.-B. Aminomalononitrile-Assisted Multifunctional Antibacterial Coatings. ACS Biomater. Sci. Eng. 2020, 6, 3349–3360. [Google Scholar] [CrossRef]
- Jung, J.; Menzies, D.J.; Thissen, H.; Easton, C.D.; Evans, R.A.; Henry, R.; Deletic, A.; McCarthy, D.T. New Prebiotic Chemistry Inspired Filter Media for Stormwater/Greywater Disinfection. J. Hazard. Mater. 2019, 378, 120749. [Google Scholar] [CrossRef]
- Ruiz-Bermejo, M.; García-Armada, P.; Mateo-Martí, E.; de la Fuente, J.L. HCN-derived polymers from thermally induced polymerization of diaminomaleonitrile: A non-enzymatic peroxide sensor based on prebiotic chemistry. Eur. Polym. J. 2022, 162, 110897. [Google Scholar] [CrossRef]
- Matthews, C.N.; Moser, R.E. Peptide Synthesis from Hydrogen Cyanide and Water. Nature 1967, 215, 1230–1234. [Google Scholar] [CrossRef]
- Budil, D.E.; Roebber, J.L.; Liebman, S.A.; Matthews, C.N. Multifrequency Electron Spin Resonance Detection of Solid-State Organic Free Radicals in HCN Polymer and a Titan Tholin. Astrobiology 2003, 3, 323–329. [Google Scholar] [CrossRef]
- Vujošević, S.I.; Negrón-Mendoza, A.; Draganić, Z.D. Radiation-Induced Polymerization in Dilute Aqueous Solutions of Cyanides. Orig. Life Evol. Biosph. 1990, 20, 49–54. [Google Scholar] [CrossRef]
- Negrón-Mendoza, A.; Draganić, Z.D.; Navarro-González, R.; Draganić, I.G. Aldehydes, Ketones, and Carboxylic Acids Formed Radiolytically in Aqueous Solutions of Cyanides and Simple Nitriles. Radiat. Res. 1983, 95, 248–261. [Google Scholar] [CrossRef]
- Mizutani, H.; Mikuni, H.; Takahasi, M.; Noda, H. Study on the Photochemical Reaction of HCN and Its Polymer Products Relating to Primary Chemical Evolution. Orig. Life Evol. Biosph. 1975, 6, 513–525. [Google Scholar] [CrossRef]
- Villafañe-Barajas, S.A.; Ruiz-Bermejo, M.; Rayo Pizarroso, P.; Galvez-Martinez, S.; Mateo-Martí, E.; Colín-García, M. A serpentinite lizardite-HCN interaction leading the increasing of molecular complexity in an alkaline hydrothermal scenario: Implications for the origin of life studies. Life 2021, 11, 661. [Google Scholar] [CrossRef]
- Ferris, J.P.; Joshi, P.C.; Edelson, E.H.; Lawless, J.G. HCN: A Plausible Source of Purines, Pyrimidines and Amino Acids on the Primitive Earth. J. Mol. Evol. 1978, 11, 293–311. [Google Scholar] [CrossRef]
- Ferris, J.P.; Hagan Jr, W.J. HCN and Chemical Evolution: The Possible Role of Cyano Compounds in Prebiotic Synthesis. Tetrahedron 1984, 40, 1093–1120. [Google Scholar] [CrossRef]
- Schwartz, A.W.; Voet, A.B.; Van der Veen, M. Recent Progress in the Prebiotic Chemistry of HCN. Orig. Life 1984, 14, 91–98. [Google Scholar] [CrossRef]
- Eastman, M.P.; Helfrich, F.S.E.; Umantsev, A.; Porter, T.L.; Weber, R. Exploring the Structure of a Hydrogen Cyanide Polymer by Electron Spin Resonance and Scanning Force Microscopy. Scanning 2003, 25, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Fernández, A.; Ruiz-Bermejo, M.; de la Fuente, J.L. Modelling the Kinetics and Structural Property Evolution of a Versatile Reaction: Aqueous HCN Polymerization. Phys. Chem. Chem. Phys. 2018, 20, 17353–17366. [Google Scholar] [CrossRef] [PubMed]
- Mas, I.; de la Fuente, J.L.; Ruiz-Bermejo, M. Temperature Effect on Aqueous NH4CN Polymerization: Relationship between Kinetic Behaviour and Structural Properties. Eur. Polym. J. 2020, 132, 109719. [Google Scholar] [CrossRef]
- Toh, R.J.; Evans, R.; Thissen, H.; Voelcker, N.H.; d’Ischia, M.; Ball, V. Deposition of Aminomalononitrile-Based Films: Kinetics, Chemistry, and Morphology. Langmuir 2019, 35, 9896–9903. [Google Scholar] [CrossRef] [PubMed]
- Mas, I.; Hortelano, C.; Ruiz-Bermejo, M.; de la Fuente, J.L. Highly Efficient Melt Polymerization of Diaminomaleonitrile. Eur. Polym. J. 2021, 143, 110185. [Google Scholar] [CrossRef]
- Hortelano, C.; Ruiz-Bermejo, M.; de la Fuente, J.L. Solid-state polymerization of diaminomaleonitrile: Toward a new generation of conjugated functional materials. Polymer 2021, 223, 123696. [Google Scholar] [CrossRef]
- Mamajanov, I.; Herzfeld, J. HCN Polymers Characterized by SSNMR: Solid State Reaction of Crystalline Tetramer (Diaminomaleonitrile). J. Chem. Phys. 2009, 130, 134504. [Google Scholar] [CrossRef] [Green Version]
- Cataldo, F.; Lilla, E.; Ursini, O.; Angelini, G. TGA–FT-IR Study of Pyrolysis of Poly(Hydrogen Cyanide) Synthesized from Thermal Decomposition of Formamide. Implications in Cometary Emissions. J. Anal. Appl. Pyrolysis 2010, 87, 34–44. [Google Scholar] [CrossRef]
- Enchev, V.; Angelov, I.; Dincheva, I.; Stoyanova, N.; Slavova, S.; Rangelov, M.; Markova, N. Chemical Evolution: From Formamide to Nucleobases and Amino Acids without the Presence of Catalyst. J. Biomol. Struct. Dyn. 2020, 39, 5563–5578. [Google Scholar] [CrossRef]
- Hortal, L.; Pérez-Fernández, C.; de la Fuente, J.L.; Valles, P.; Mateo-Martí, E.; Ruiz-Bermejo, M. A Dual Perspective on the Microwave-Assisted Synthesis of HCN Polymers towards the Chemical Evolution and Design of Functional Materials. Sci. Rep. 2020, 10, 22350. [Google Scholar] [CrossRef]
- Pérez-Fernández, C.; Valles, P.; González-Toril, E.; Mateo-Martí, E.; de la Fuente, J.L.; Ruiz-Bermejo, M. Tuning the morphology in the nanoscale of NH4CN polymers synthesized by microwave radiation: A comparative study. Polymers 2022, 14, 57. [Google Scholar] [CrossRef]
- Yang, C.; Wang, B.; Zhang, L.; Yin, L.; Wang, X. Synthesis of Layered Carbonitrides from Biotic Molecules for Photoredox Transformations. Angew. Chem. Int. Ed. 2017, 56, 6627–6631. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Bermejo, M.; de la Fuente, J.L.; Rogero, C.; Menor-Salván, C.; Osuna-Esteban, S.; Martín-Gago, J.A. New Insights into the Characterization of ‘Insoluble Black HCN Polymers. Chem. Biodiver. 2012, 9, 25–40. [Google Scholar] [CrossRef]
- Ferris, J.P.; Edelson, E.H. Chemical Evolution. 31. Mechanism of the Condensation of Cyanide to Hydrogen Cyanide Oligomers. J. Org. Chem. 1978, 43, 3989–3995. [Google Scholar] [CrossRef]
- De la Fuente, J.L.; Ruiz-Bermejo, M.; Nna-Mvondo, D.; Minard, R. Further progress into the thermal characterization of HCN polymers. Polym. Degrad. Stab. 2014, 110, 241–251. [Google Scholar] [CrossRef]
- Khare, B.N.; Sagan, C.; Thompson, W.R.; Arakawa, E.T.; Meisse, C.; Tuminello, P.S. Optical Properties of Poly-HCN and Their Astronomical Applications. Can. J. Chem. 1994, 72, 678–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalton, S.; Heatley, F.; Budd, P.M. Thermal Stabilization of Polyacrylonitrile Fibres. Polymer 1999, 40, 5531–5543. [Google Scholar] [CrossRef]
- Miller, T.S.; Jorge, A.B.; Suter, T.M.; Sella, A.; Corà, F.; McMillan, P.F. Carbon nitrides: Synthesis and characterization of a new class of functional materials. Phys. Chem. Chem. Phys. 2017, 19, 15613–15638. [Google Scholar] [CrossRef] [Green Version]
- Ciria-Ramos, I.; Navascués, N.; Diaw, F.; Furgeaud, C.; Arenal, R.; Ansón-Casaos, A.; Juarez-Perez, E.J. Formamidinium halide salts as precursors of carbon nitrides. Carbon 2022, 196, 1035–1046. [Google Scholar] [CrossRef]
- Yamamoto, T.; Lee, B.-L. New Soluble, Coplanar Poly(naphthalene-2,6-diyl)-Type π-Conjugated Polymer, Poly(pyrimido[5,4-d]pyrimidine-2,6-diyl), with Nitrogen Atoms at All of the o-Positions. Synthesis, Solid Structure, Optical Properties, Self-Assembling Phenomena, and Redox Behavior. Macromolecules 2002, 35, 2993–2999. [Google Scholar] [CrossRef]
- Wait, S.C.; Wesley, J.W. Azanaphthalenes: Part I. Huckel orbital calculations. J. Mol. Spectrosc. 1966, 19, 25–33. [Google Scholar] [CrossRef]
- Ruiz-Bermejo, M.; de la Fuente, J.L.; Marín-Yaseli, M.R. The Influence of Reaction Conditions in Aqueous HCN Polymerization on the Polymer Thermal Degradation Properties. J. Anal. Appl. Pyrolysis 2017, 124, 103–112. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods. In Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2001; ISBN 0-471-04372-9. [Google Scholar]
- Wang, J. Analytical Electrochemistry, 3rd ed.; Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; p. 32. ISBN 13 978-0-471-67879-3. [Google Scholar]
- Admassie, S.; Inganäs, O.; Mammo, W.; Perzon, E.; Andersson, M.R. Electrochemical and optical studies of the band gaps of alternating polyfluorene copolymers. Synth. Met. 2006, 156, 614–623. [Google Scholar] [CrossRef]
- Johansson, T.; Mammo, W.; Svensson, M.; Andersson, M.R.; Inganäs, O. Electrochemical bandgaps of substituted polythiophenes. J. Mater. Chem. 2003, 13, 1316–1323. [Google Scholar] [CrossRef]
- Ruiz-Bermejo, M.; Zorzano, M.-P.; Osuna-Esteban, S. Simple organics and biomonomers identified in HCN polymers: An overview. Life 2013, 3, 421–448. [Google Scholar] [CrossRef] [Green Version]
- Rimmer, P.B.; Shorttle, O. Origin of Life’s Building Blocks in Carbon- and Nitrogen-Rich Surface Hydrothermal Vents. Life 2019, 9, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deamer, D. Where did Life Begin? Testing Ideas in Prebiotic Analogue Conditions. Life 2021, 11, 134. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rao, J.; Pu, K. Recent Progress on Semiconducting Polymer Nanoparticles for Molecular Imaging and Cancer Phototherapy. Biomaterials 2018, 155, 217–235. [Google Scholar] [CrossRef] [PubMed]
- Masilamani, G.; Batchu, H.; Amsallem, D.; Bedi, A. Novel Series of Diaminoanthraquinone-Based π-Extendable Building Blocks with Tunable Optoelectronic Properties. ACS Omega 2022, 7, 25874–25880. [Google Scholar] [CrossRef]
- Takahashi, T.; Matsuoka, K.; Takimiya, K.; Otsubo, T.; Aso, Y. Extensive Quinoidal Oligothiophenes with Dicyanomethylene Groups at Terminal Positions as Highly Amphoteric Redox Molecules. J. Am. Chem. Soc. 2005, 127, 8928–8929. [Google Scholar] [CrossRef]
- Bedi, A.; Senanayak, S.P.; Das, S.; Narayan, K.S.; Zade, S.S. Cyclopenta[c]thiophene oligomers based solution processable D–A copolymers and their application as FET materials. Polym. Chem. 2012, 3, 1453–1460. [Google Scholar] [CrossRef]





| Polymer | T (°C) | t (min) | Air | P (bar) (a) | α (%) | C/H | C/N | N/O (b) |
|---|---|---|---|---|---|---|---|---|
| 1 | 170 | 16 | - | 12 | 36 ± 3 | 1.00 ± 0.17 | 1.28 ± 0.02 | 2.47 ± 0.18 |
| 2 | 190 | 3.2 | - | 17 | 33 ± 3 | 1.02 ± 0.09 | 1.25 ± 0.02 | 2.83 ± 0.11 |
| 3 | 170 | 16 | + | 12 | 38 ± 2 | 1.08 ± 0.16 | 1.28 ± 0.01 | 2.66 ± 0.20 |
| 4 | 190 | 3.2 | + | 17 | 33 ± 2 | 1.02 ± 0.02 | 1.26 ± 0.01 | 2.70 ± 0.04 |
| EOR (%) | EOC (%) | |||
|---|---|---|---|---|
| Conditions | 170 °C | 190 °C | 170 °C | 190 °C |
| N2/MWR | 76 ± 2 | 74 ± 2 | 47 ± 1 | 48 ± 1 |
| Air/MWR | 77 ± 3 | 73 ± 1 | 48 ± 2 | 48 ± 1 |
| EOR (%) | EOC (%) | |||
| Air/CTS | 86 ± 1 | 45 ± 1 | ||
| Polymer | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Z-average (nm) | 279 | 224 | 233 | 215 | |
| PdI (a) | 0.24 | 0.16 | 0.24 | 0.18 | |
| BET Surface area (m2/g) | LP (b) | 33.2 | 27.8 | 29.8 | 32.4 |
| HP (c) | 34.6 | 29.2 | 31.3 | 33.5 | |
| Density (g/cm3) | 2.04 | 2.42 | 2.29 | 2.09 | |
| Mn (d) | 5300 | 7000 | 5900 | 6000 | |
| Polymer | E0′ (V) | n | α | D (cm2 s−1) | kf (cm s−1) | k0 (cm s−1) | j0 (A cm−2) |
|---|---|---|---|---|---|---|---|
| 1 | −0.77 | 0.97 | 0.50 | 1.52 × 10−4 | 0.27 | 3.58 × 10−4 | 1.35 × 10−7 |
| 2 | −0.77 | 0.97 | 0.49 | 1.05 × 10−4 | 0.29 | 3.19 × 10−4 | 8.44 × 10−7 |
| 3 | −0.77 | 0.96 | 0.50 | 1.47 × 10−4 | 0.29 | 2.91 × 10−4 | 6.84 × 10−7 |
| 4 | −0.77 | 0.96 | 0.50 | 1.43 × 10−4 | 0.26 | 3.19 × 10−4 | 8.41 × 10−7 |
| E0ox (V) | E0red (V) | HOMO (eV) | LUMO (eV) | Eg (eV) |
|---|---|---|---|---|
| 0.34 | −0.77 | 4.99 | 3.88 | 1.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Bermejo, M.; García-Armada, P.; Valles, P.; de la Fuente, J.L. Semiconducting Soft Submicron Particles from the Microwave-Driven Polymerization of Diaminomaleonitrile. Polymers 2022, 14, 3460. https://doi.org/10.3390/polym14173460
Ruiz-Bermejo M, García-Armada P, Valles P, de la Fuente JL. Semiconducting Soft Submicron Particles from the Microwave-Driven Polymerization of Diaminomaleonitrile. Polymers. 2022; 14(17):3460. https://doi.org/10.3390/polym14173460
Chicago/Turabian StyleRuiz-Bermejo, Marta, Pilar García-Armada, Pilar Valles, and José L. de la Fuente. 2022. "Semiconducting Soft Submicron Particles from the Microwave-Driven Polymerization of Diaminomaleonitrile" Polymers 14, no. 17: 3460. https://doi.org/10.3390/polym14173460
APA StyleRuiz-Bermejo, M., García-Armada, P., Valles, P., & de la Fuente, J. L. (2022). Semiconducting Soft Submicron Particles from the Microwave-Driven Polymerization of Diaminomaleonitrile. Polymers, 14(17), 3460. https://doi.org/10.3390/polym14173460








