Adsorptive Behavior of Cu2+ and Benzene in Single and Binary Solutions onto Alginate Composite Hydrogel Beads Containing Pitch Pine-Based Biochar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pitch Pine-Based Biochar and Alginate Hydrogel Beads
2.3. Characterization of Pitch Pine Powder, Biochar, and Alg/BC Beads
2.4. Adsorption of Cu2+ and Benzene onto Adsorbents
2.5. Modeling of Adsorption Kinetics and Isotherms
2.6. Reusability of Alg/BC Hydrogel Beads
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Pitch Pine-Based Biochar
3.2. Characterization of Alg/BC Beads
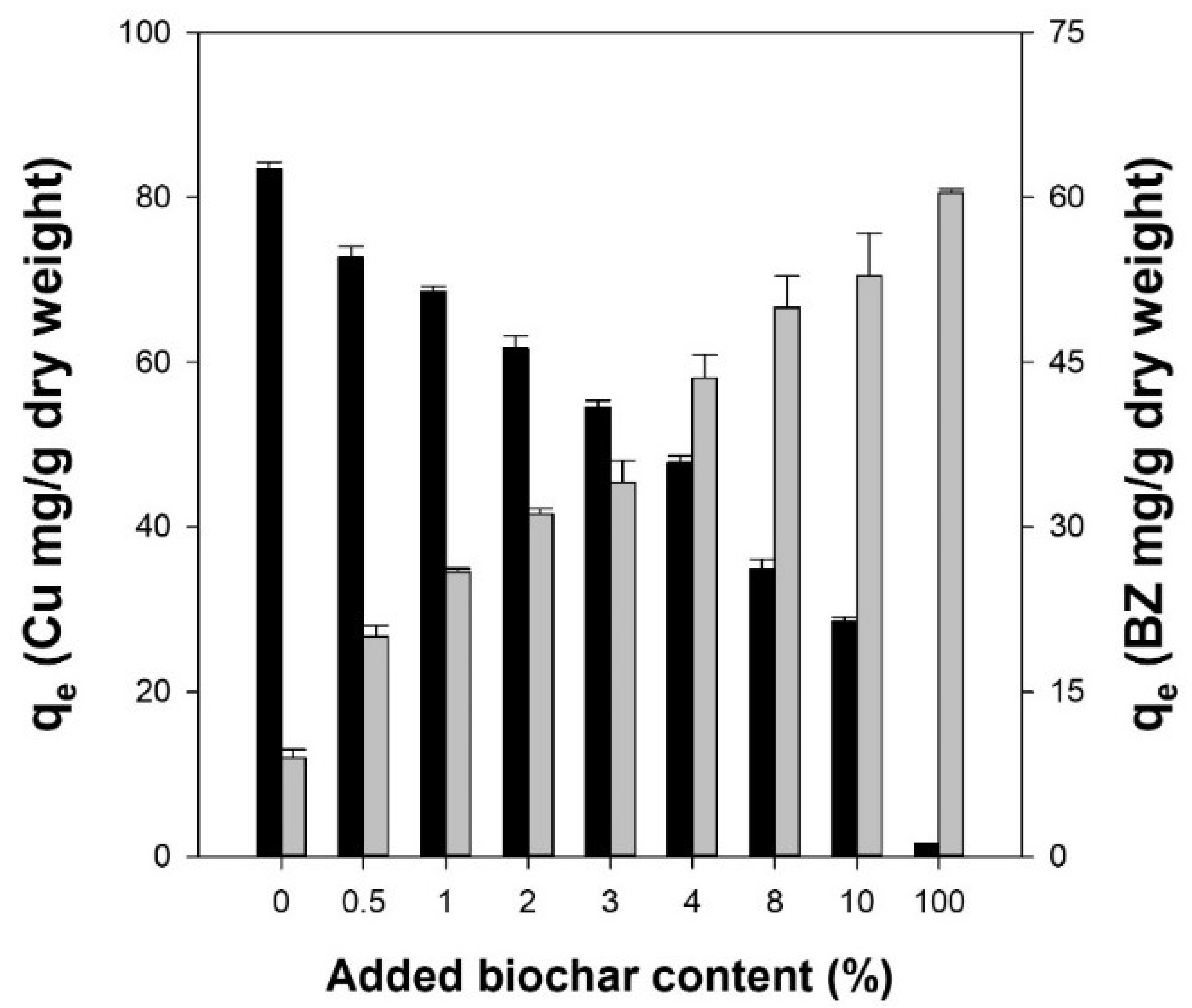
3.3. Adsorption Capacities of Alg/BC Hydrogel Beads for Cu2+ and Benzene
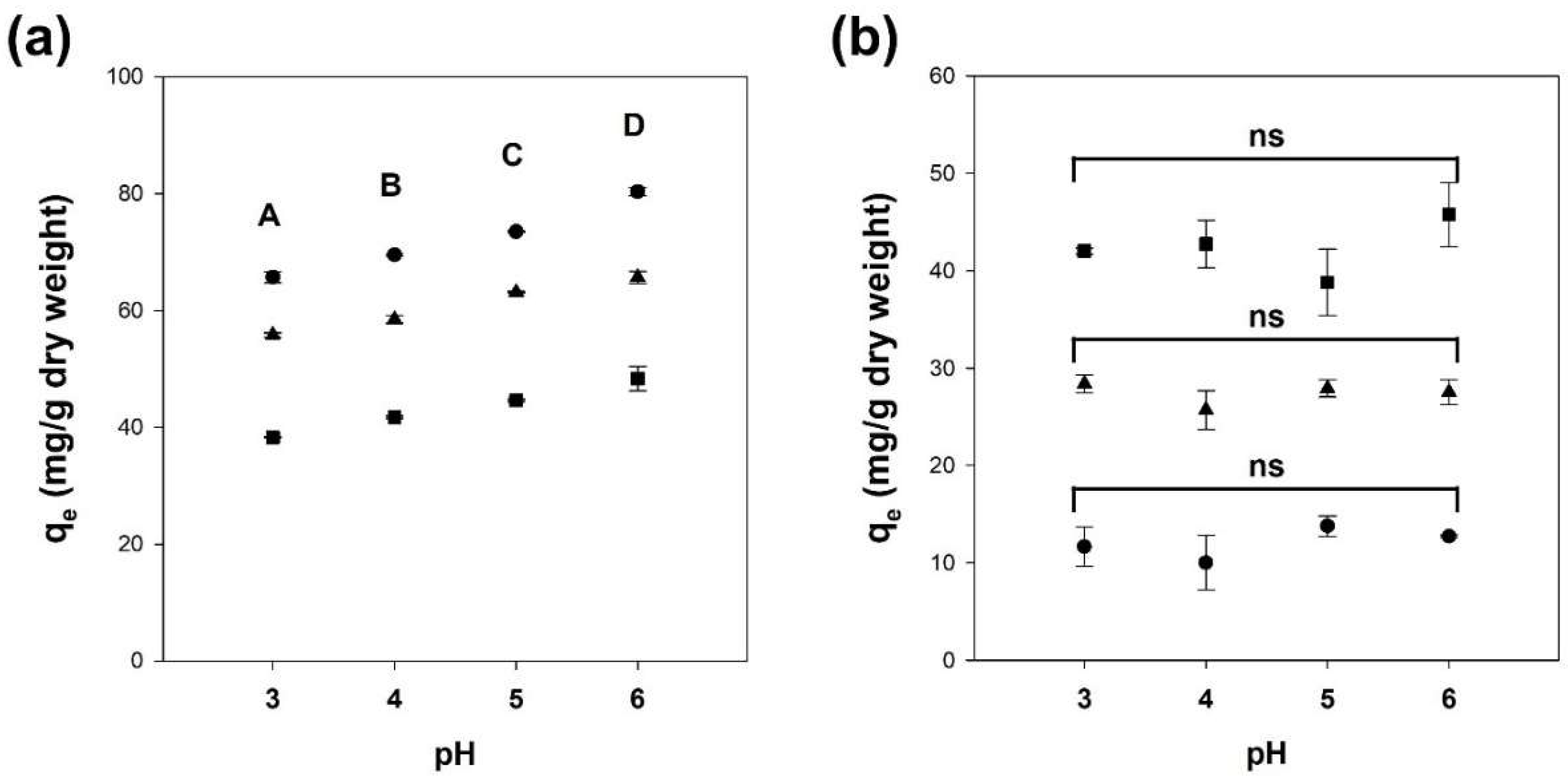
3.4. Effect of Initial Solution pH on the Adsorption of Cu2+ and Benzene
3.5. Adsorption Kinetics Study for Cu2+ and Benzene onto Alg/BC Hydrogel Beads
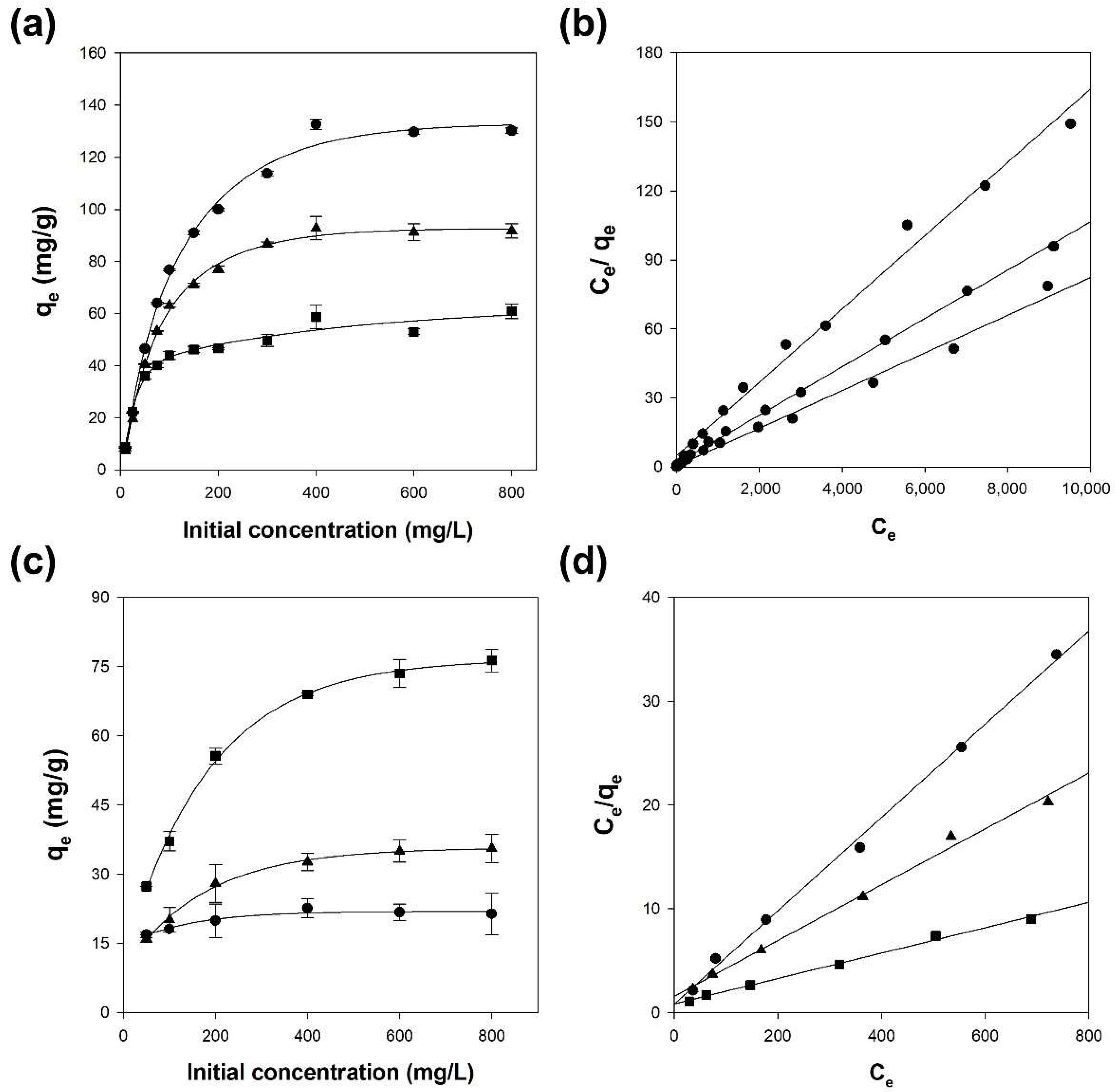
3.6. Adsorption Isotherm Study for Cu2+ and Benzene onto Alg/BC Hydrogel Beads
3.7. Simultaneous Adsorption of Cu2+ and Benzene onto Alg/BC Hydrogel Beads
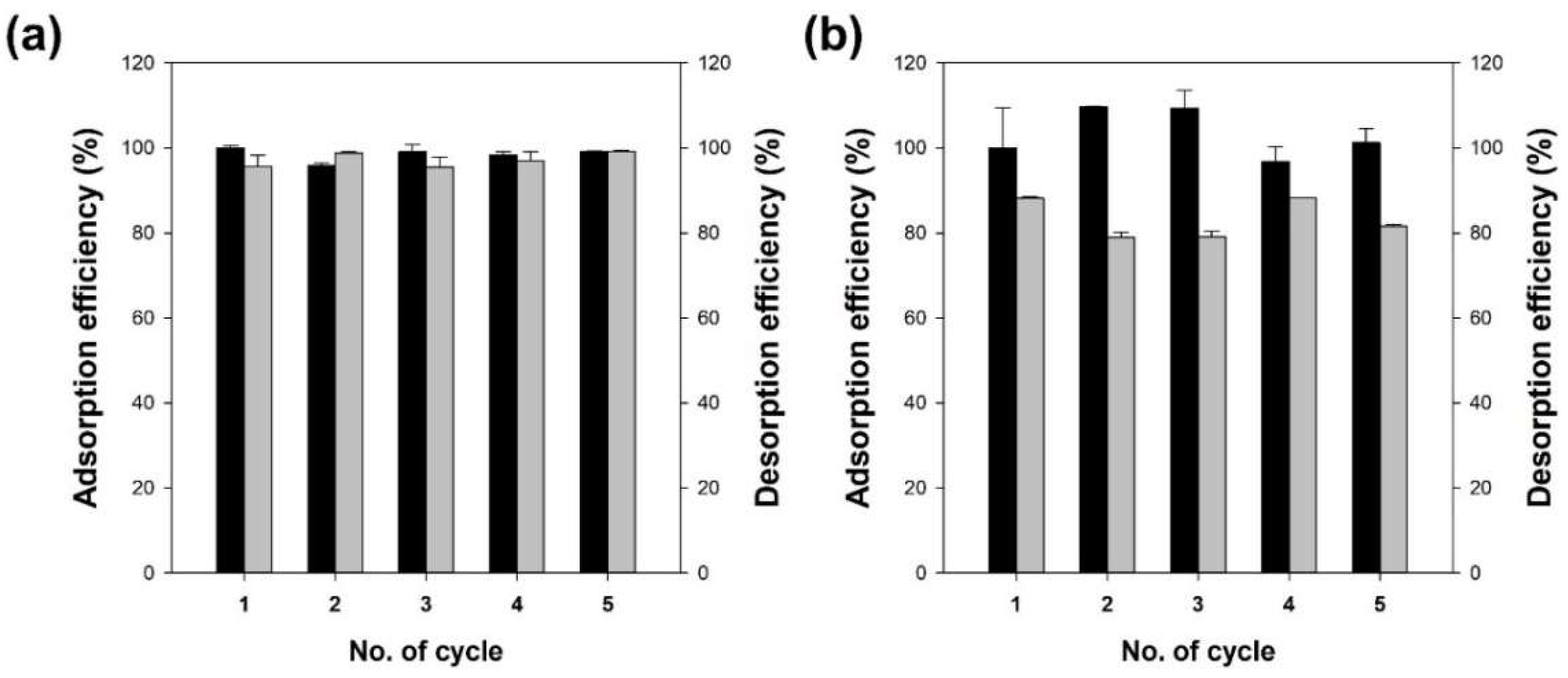
3.8. Reusability of Alg/BC Hydrogel Beads
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gopinath, K.P.; Vo, D.-V.N.; Gnana Prakash, D.; Adithya Joseph, A.; Viswanathan, S.; Arun, J. Environmental applications of carbon-based materials: A review. Environ. Chem. Lett. 2021, 19, 557–582. [Google Scholar] [CrossRef]
- Kim, J.E.; Bhatia, S.K.; Song, H.J.; Yoo, E.; Jeon, H.J.; Yoon, J.-Y.; Yang, Y.; Gurav, R.; Yang, Y.-H.; Kim, H.J. Adsorptive removal of tetracycline from aqueous solution by maple leaf-derived biochar. Bioresour. Technol. 2020, 306, 123092. [Google Scholar] [CrossRef]
- Sutirman, Z.A.; Sanagi, M.M.; Ibrahim, W.A.W. Alginate-based adsorbents for removal of metal ions and radionuclides from aqueous solutions: A review. Int. J. Biol. Macromol. 2021, 174, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, S.; Kouvelos, E.; Katsaros, F. Calcium alginate beads from Laminaria digitata for the removal of Cu+2 and Cd+2 from dilute aqueous metal solutions. Desalination 2008, 224, 293–306. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, S.; Jiang, H.A. Review on conversion of crayfish-shell derivatives to functional materials and their environmental applications. J. Bioresour. Bioprod. 2020, 5, 238–247. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and application of granular activated carbon from biomass waste materials for water treatment: A review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, S.; Tian, Z.; Duan, G.; Pan, H.; Yue, Y.; Li, S.; Jian, S.; Yang, W.; Liu, K. Mofs meet wood: Reusable magnetic hydrophilic composites toward efficient water treatment with super-high dye adsorption capacity at high dye concentration. Chem. Eng. J. 2022, 446, 136851. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Gurav, R.; Kim, H.J.; Yang, Y.-H.; Bhatia, S.K. Evaluation for simultaneous removal of anionic and cationic dyes onto maple leaf-derived biochar using response surface methodology. Appl. Sci. 2020, 10, 2982. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.; Xian, Q.; Shen, F.; Wu, J.; Zhang, Y. Removal of congo red and methylene blue from aqueous solutions by vermicompost-derived biochars. PLoS ONE 2016, 11, e0154562. [Google Scholar] [CrossRef]
- Zhang, H.; Tu, Y.-J.; Duan, Y.-P.; Liu, J.; Zhi, W.; Tang, Y.; Xiao, L.-S.; Meng, L. Production of biochar from waste sludge/leaf for fast and efficient removal of diclofenac. J. Mol. Liq. 2020, 299, 112193. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Choi, T.-R.; Gurav, R.; Bhatia, S.K.; Park, Y.-L.; Kim, H.J.; Kan, E.; Yang, Y.-H. Adsorption behavior of tetracycline onto Spirulina sp.(microalgae)-derived biochars produced at different temperatures. Sci. Total Environ. 2020, 710, 136282. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.; Yu, M.-R.; Yakkala, K.; Koduru, J.R.; Yang, J.-K.; Chang, Y.-Y. Removal studies of Cd (II) and explosive compounds using buffalo weed biochar-alginate beads. J. Ind. Eng. Chem. 2015, 26, 226–233. [Google Scholar] [CrossRef]
- Jung, K.-W.; Jeong, T.-U.; Kang, H.-J.; Ahn, K.-H. Characteristics of biochar derived from marine macroalgae and fabrication of granular biochar by entrapment in calcium-alginate beads for phosphate removal from aqueous solution. Bioresour. Technol. 2016, 211, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Ghani, W.A.W.A.K.; Mohd, A.; da Silva, G.; Bachmann, R.T.; Taufiq-Yap, Y.H.; Rashid, U.; Ala’a, H. Biochar production from waste rubber-wood-sawdust and its potential use in C sequestration: Chemical and physical characterization. Ind. Crops Prod. 2013, 44, 18–24. [Google Scholar] [CrossRef]
- Chen, Z.-l.; Zhang, J.-q.; Huang, L.; Yuan, Z.-h.; Li, Z.-j.; Liu, M.-c. Removal of Cd and Pb with biochar made from dairy manure at low temperature. J. Integr. Agric. 2019, 18, 201–210. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Barquilha, C.E.; Braga, M.C. Adsorption of organic and inorganic pollutants onto biochars: Challenges, operating conditions, and mechanisms. Bioresour. Technol. Rep. 2021, 15, 100728. [Google Scholar] [CrossRef]
- Song, H.J.; Gurav, R.; Bhatia, S.K.; Lee, E.B.; Kim, H.J.; Yang, Y.-H.; Kan, E.; Kim, H.H.; Lee, S.H.; Choi, Y.-K. Treatment of microcystin-LR cyanotoxin contaminated water using Kentucky bluegrass-derived biochar. J. Water Process Eng. 2021, 41, 102054. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Kan, E. Effects of pyrolysis temperature on the physicochemical properties of alfalfa-derived biochar for the adsorption of bisphenol A and sulfamethoxazole in water. Chemosphere 2019, 218, 741–748. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, X.; Chen, K.; Fang, J.; He, F.; Hu, X.; Tsang, D.C.; Ok, Y.S.; Gao, B. Enhanced adsorption performance and governing mechanisms of ball-milled biochar for the removal of volatile organic compounds (VOCs). Chem. Eng. J. 2020, 385, 123842. [Google Scholar] [CrossRef]
- Wang, B.; Gao, B.; Zimmerman, A.R.; Zheng, Y.; Lyu, H. Novel biochar-impregnated calcium alginate beads with improved water holding and nutrient retention properties. J. Environ. Manag. 2018, 209, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wan, Y.; Zheng, Y.; Lee, X.; Liu, T.; Yu, Z.; Huang, J.; Ok, Y.S.; Chen, J.; Gao, B. Alginate-based composites for environmental applications: A critical review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 318–356. [Google Scholar] [CrossRef] [PubMed]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles—A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Gao, X.; Guo, C.; Hao, J.; Zhao, Z.; Long, H.; Li, M. Adsorption of heavy metal ions by sodium alginate based adsorbent-a review and new perspectives. Int. J. Biol. Macromol. 2020, 164, 4423–4434. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Katsaros, F.K.; Kouvelos, E.P.; Nolan, J.W.; Le Deit, H.; Kanellopoulos, N.K. Heavy metal sorption by calcium alginate beads from Laminaria digitata. J. Hazard. Mater. 2006, 137, 1765–1772. [Google Scholar] [CrossRef]
- Peretz, S.; Anghel, D.F.; Vasilescu, E.; Florea-Spiroiu, M.; Stoian, C.; Zgherea, G. Synthesis, characterization and adsorption properties of alginate porous beads. Polym. Bull. 2015, 72, 3169–3182. [Google Scholar] [CrossRef]
- Kaushal, M.; Tiwari, A. Removal of Rhodamine-B from aqueous solution by adsorption onto crosslinked alginate beads. J. Dispers. Sci. Technol. 2010, 31, 438–441. [Google Scholar] [CrossRef]
- Minh, V.X.; Dung, K.T.T.; Lan, P.T.; Hanh, L.T.M.; Dung, N.T. Study on Ni (II) adsorption by calcium alginate beads. Vietnam. J. Chem. 2020, 58, 358–363. [Google Scholar] [CrossRef]
- Isik, Z.; Saleh, M.; Dizge, N. Adsorption studies of ammonia and phosphate ions onto calcium alginate beads. Surf. Interfaces 2021, 26, 101330. [Google Scholar] [CrossRef]
- Yang, J.-S.; Xie, Y.-J.; He, W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Chen, J.; Ouyang, J.; Cai, X.; Xing, X.; Zhou, L.; Liu, Z.; Cai, D. Removal of ciprofloxacin from water by millimeter-sized sodium alginate/H3PO4 activated corncob-based biochar composite beads. Sep. Purif. Technol. 2021, 276, 119371. [Google Scholar] [CrossRef]
- Feng, Q.; Chen, M.; Wu, P.; Zhang, X.; Wang, S.; Yu, Z.; Wang, B. Simultaneous reclaiming phosphate and ammonium from aqueous solutions by calcium alginate-biochar composite: Sorption performance and governing mechanisms. Chem. Eng. J. 2022, 429, 132166. [Google Scholar] [CrossRef]
- Sigdel, A.; Jung, W.; Min, B.; Lee, M.; Choi, U.; Timmes, T.; Kim, S.-J.; Kang, C.-U.; Kumar, R.; Jeon, B.-H. Concurrent removal of cadmium and benzene from aqueous solution by powdered activated carbon impregnated alginate beads. Catena 2017, 148, 101–107. [Google Scholar] [CrossRef]
- Hassan, M.; Liu, Y.; Naidu, R.; Du, J.; Qi, F.; Donne, S.W.; Islam, M.M. Mesoporous biopolymer architecture enhanced the adsorption and selectivity of aqueous heavy-metal ions. ACS Omega 2021, 6, 15316–15331. [Google Scholar] [CrossRef]
- Ohemeng-Boahen, G.; Sewu, D.D.; Woo, S.H. Preparation and characterization of alginate-kelp biochar composite hydrogel bead for dye removal. Environ. Sci. Pollut. Res. 2019, 26, 33030–33042. [Google Scholar] [CrossRef]
- Shaaban, A.; Se, S.-M.; Mitan, N.M.M.; Dimin, M. Characterization of biochar derived from rubber wood sawdust through slow pyrolysis on surface porosities and functional groups. Procedia Eng. 2013, 68, 365–371. [Google Scholar] [CrossRef]
- Kumar, N.S.; Shaikh, H.M.; Asif, M.; Al-Ghurabi, E.H. Engineered biochar from wood apple shell waste for high-efficient removal of toxic phenolic compounds in wastewater. Sci. Rep. 2021, 11, 2586. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Lin, K.-Y.A.; Hong, E.; Kwon, E.E.; Lee, J. The valorization of food waste via pyrolysis. J. Clean. Prod. 2020, 259, 120816. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Badita, C.; Aranghel, D.; Burducea, C.; Mereuta, P. Characterization of sodium alginate based films. Rom. J. Phys. 2020, 65, 1–8. [Google Scholar]
- Mohammed, C.; Mahabir, S.; Mohammed, K.; John, N.; Lee, K.-Y.; Ward, K. Calcium alginate thin films derived from Sargassum natans for the selective adsorption of Cd2+, Cu2+, and Pb2+ ions. Ind. Eng. Chem. Res. 2018, 58, 1417–1425. [Google Scholar] [CrossRef]
- Gürkan, E.H.; İlyas, B.; Tibet, Y. Adsorption performance of heavy metal ions from aqueous solutions by a waste biomass based hydrogel: Comparison of isotherm and kinetic models. Int. J. Environ. Anal. Chem. 2021, 1–18. [Google Scholar] [CrossRef]
- Liu, C.; Ye, J.; Lin, Y.; Wu, J.; Price, G.; Burton, D.; Wang, Y. Removal of Cadmium (II) using water hyacinth (Eichhornia crassipes) biochar alginate beads in aqueous solutions. Environ. Pollut. 2020, 264, 114785. [Google Scholar] [CrossRef]
- Wang, B.; Gao, B.; Wan, Y. Entrapment of ball-milled biochar in Ca-alginate beads for the removal of aqueous Cd (II). J. Ind. Eng. Chem. 2018, 61, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Mohapatra, S.S.; Kumari, U.; Meikap, B.C.; Sen, T.K. Batch and continuous closed circuit semi-fluidized bed operation: Removal of mb dye using sugarcane bagasse biochar and alginate composite adsorbents. J. Environ. Chem. Eng. 2020, 8, 103637. [Google Scholar] [CrossRef]
- Ma, W.; Song, X.; Pan, Y.; Cheng, Z.; Xin, G.; Wang, B.; Wang, X. Adsorption behavior of crystal violet onto opal and reuse feasibility of opal-dye sludge for binding heavy metals from aqueous solutions. Chem. Eng. J. 2012, 193, 381–390. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Swamy, M.M.; Mall, I.D.; Prasad, B.; Mishra, I.M. Adsorptive removal of phenol by bagasse fly ash and activated carbon: Equilibrium, kinetics and thermodynamics. Colloids Surf. A Physicochem. Eng. Asp. 2006, 272, 89–104. [Google Scholar] [CrossRef]
- Wang, B.; Gao, B.; Wan, Y. Comparative study of calcium alginate, ball-milled biochar, and their composites on aqueous methylene blue adsorption. Environ. Sci. Pollut. Res. 2019, 26, 11535–11541. [Google Scholar] [CrossRef]
- Zand, A.D.; Abyaneh, M.R. Adsorption of lead, manganese, and copper onto biochar in landfill leachate: Implication of non-linear regression analysis. Sustain. Environ. Res. 2020, 30, 1–16. [Google Scholar] [CrossRef]
- Mata, Y.; Blázquez, M.; Ballester, A.; González, F.; Munoz, J. Biosorption of cadmium, lead and copper with calcium alginate xerogels and immobilized Fucus vesiculosus. J. Hazard. Mater. 2009, 163, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Sen, T.K.; Yeneneh, A.M.; Meikap, B.C. Synthesis and characterization of a novel Ca-alginate-biochar composite as efficient zinc (Zn2+) adsorbent: Thermodynamics, process design, mass transfer and isotherm modeling. Sep. Sci. Technol. 2019, 54, 1106–1124. [Google Scholar] [CrossRef]
- Oyelude, E.O.; Awudza, J.A.; Twumasi, S.K. Equilibrium, kinetic and thermodynamic study of removal of eosin yellow from aqueous solution using teak leaf litter powder. Sci. Rep. 2017, 7, 12198. [Google Scholar] [CrossRef] [Green Version]




| Adsorbate | Added BC Content (%) | Pseudo-First-Order | Pseudo-Second-Order | Elovich | qe Exp. (mg/g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k1 (10−3/h) | qe1 Cal. (mg/g) | r2 | k2 (10−3g/mg/h) | qe2 Cal. (mg/g) | h (mg/g/h) | r2 | a (mg/g/h) | b (g/mg) | r2 | |||
| Cu2+ | 0 | 291.6 | 43.6 | 0.855 | 11.3 | 84.5 | 80.8 | 0.999 | 764.1 | 0.088 | 0.902 | 80.2 |
| 1 | 378.5 | 53.0 | 0.971 | 13.5 | 69.1 | 64.3 | 0.999 | 340.9 | 0.096 | 0.904 | 65.2 | |
| 4 | 292.5 | 40.1 | 0.957 | 8.8 | 53.4 | 25.1 | 0.998 | 79.8 | 0.105 | 0.946 | 47.4 | |
| Benzene | 0 | 118.0 | 9.7 | 0.972 | 16.1 | 14.2 | 3.3 | 0.989 | 12.4 | 0.417 | 0.915 | 12.4 |
| 1 | 146.0 | 25.9 | 0.981 | 6.4 | 32.7 | 6.8 | 0.992 | 20.0 | 0.166 | 0.944 | 28.1 | |
| 4 | 164.3 | 27.2 | 0.998 | 12.1 | 45.2 | 24.8 | 0.999 | 109.2 | 0.145 | 0.937 | 42.9 | |
| Adsorbate | Added BC Content (%) | Langmuir | Freundlich | |||||
| qm (mg/g) | kL (10−3L/mg) | r2 | 1/n | n | kF (mg/g) | r2 | ||
| Cu2+ | 0 | 122.0 | 17.7 | 0.992 | 0.290 | 3.4 | 12.1 | 0.877 |
| 1 | 93.5 | 7.2 | 0.999 | 0.285 | 3.5 | 9.1 | 0.861 | |
| 4 | 62.5 | 3.5 | 0.990 | 0.223 | 4.5 | 9.2 | 0.854 | |
| Benzene | 0 | 22.2 | 56.9 | 0.997 | 0.113 | 8.8 | 10.6 | 0.740 |
| 1 | 37.1 | 17.4 | 0.994 | 0.260 | 3.9 | 6.6 | 0.950 | |
| 4 | 81.7 | 14.7 | 0.995 | 0.321 | 3.1 | 9.9 | 0.958 | |
| Adsorbate | Added BC Content (%) | Elovich | Dubinin–Radushkevich | |||||
| qm (mg/g) | KE (L/g) | r2 | qm (mg/g) | kDR(10−6 mol2/kJ2) | E (kJ/mol) | r2 | ||
| Cu2+ | 0 | 22.6 | 0.34 | 0.922 | 94.3 | 4.9 | 0.32 | 0.859 |
| 1 | 16.9 | 0.30 | 0.917 | 74.5 | 12.3 | 0.20 | 0.891 | |
| 4 | 9.3 | 0.66 | 0.912 | 46.7 | 18.0 | 0.17 | 0.805 | |
| Benzene | 0 | 3.4 | 9.79 | 0.685 | 20.4 | 52.1 | 0.10 | 0.377 |
| 1 | 9.1 | 0.29 | 0.930 | 30.7 | 156.8 | 0.06 | 0.803 | |
| 4 | 23.7 | 0.13 | 0.943 | 63.5 | 128.1 | 0.06 | 0.791 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Lee, J.W.; Kim, J.E.; Kang, G.; Kim, H.J.; Choi, Y.-K.; Lee, S.H. Adsorptive Behavior of Cu2+ and Benzene in Single and Binary Solutions onto Alginate Composite Hydrogel Beads Containing Pitch Pine-Based Biochar. Polymers 2022, 14, 3468. https://doi.org/10.3390/polym14173468
Park S, Lee JW, Kim JE, Kang G, Kim HJ, Choi Y-K, Lee SH. Adsorptive Behavior of Cu2+ and Benzene in Single and Binary Solutions onto Alginate Composite Hydrogel Beads Containing Pitch Pine-Based Biochar. Polymers. 2022; 14(17):3468. https://doi.org/10.3390/polym14173468
Chicago/Turabian StylePark, Saerom, Jeong Woo Lee, Ji Eun Kim, Gwangnam Kang, Hyung Joo Kim, Yong-Keun Choi, and Sang Hyun Lee. 2022. "Adsorptive Behavior of Cu2+ and Benzene in Single and Binary Solutions onto Alginate Composite Hydrogel Beads Containing Pitch Pine-Based Biochar" Polymers 14, no. 17: 3468. https://doi.org/10.3390/polym14173468
APA StylePark, S., Lee, J. W., Kim, J. E., Kang, G., Kim, H. J., Choi, Y.-K., & Lee, S. H. (2022). Adsorptive Behavior of Cu2+ and Benzene in Single and Binary Solutions onto Alginate Composite Hydrogel Beads Containing Pitch Pine-Based Biochar. Polymers, 14(17), 3468. https://doi.org/10.3390/polym14173468








