Fabrication and Characterization of a Novel Smart-Polymer Actuator with Nanodispersed CNT/Pd Composite Interfacial Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
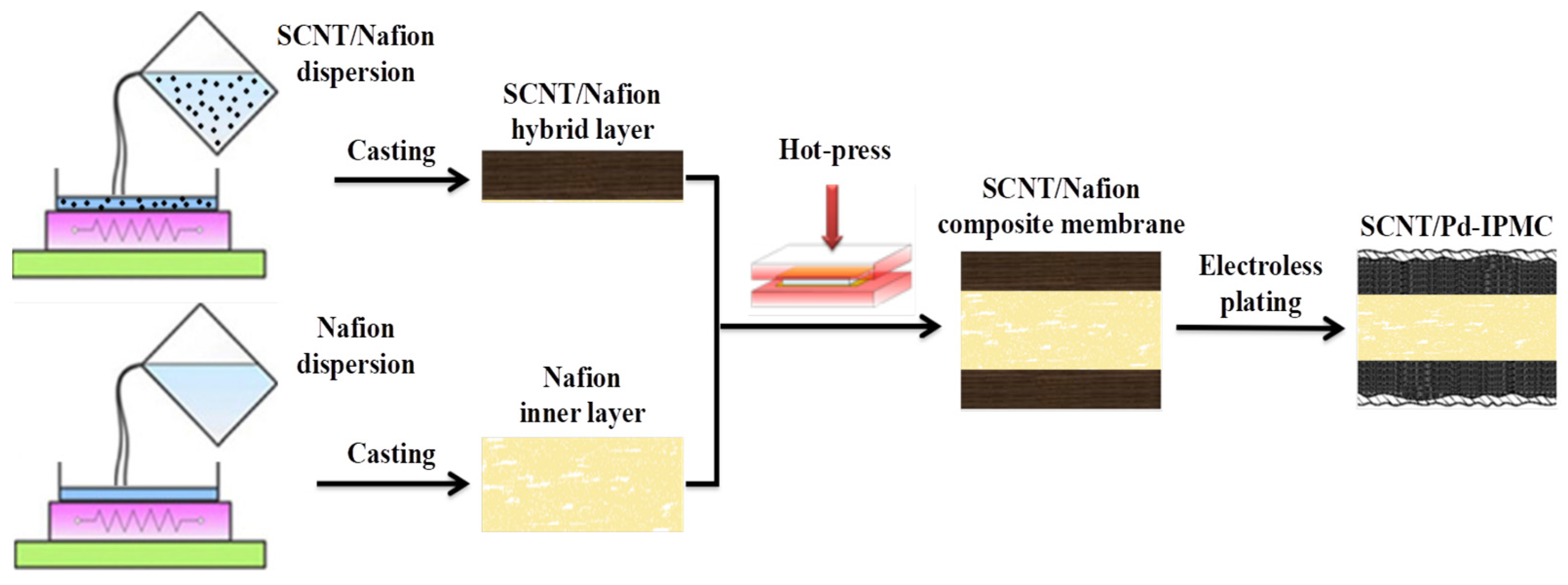
2.2. Preparation of SCNT/Nafion Composite Matrix Membrane
2.3. Fabrication of IPMCs
2.4. Characterizations
3. Results and Discussion
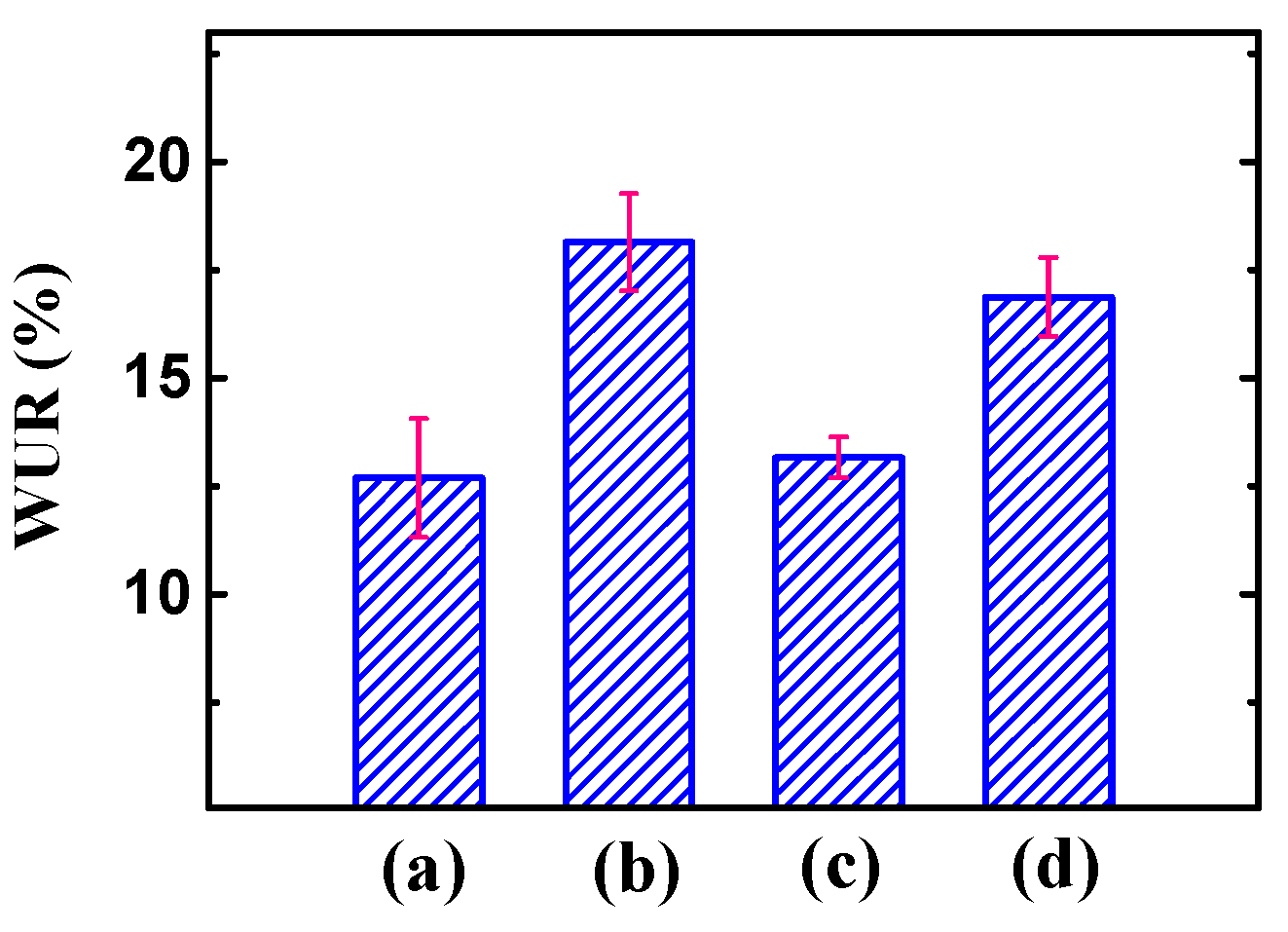
3.1. WUR and IEC of the Membranes
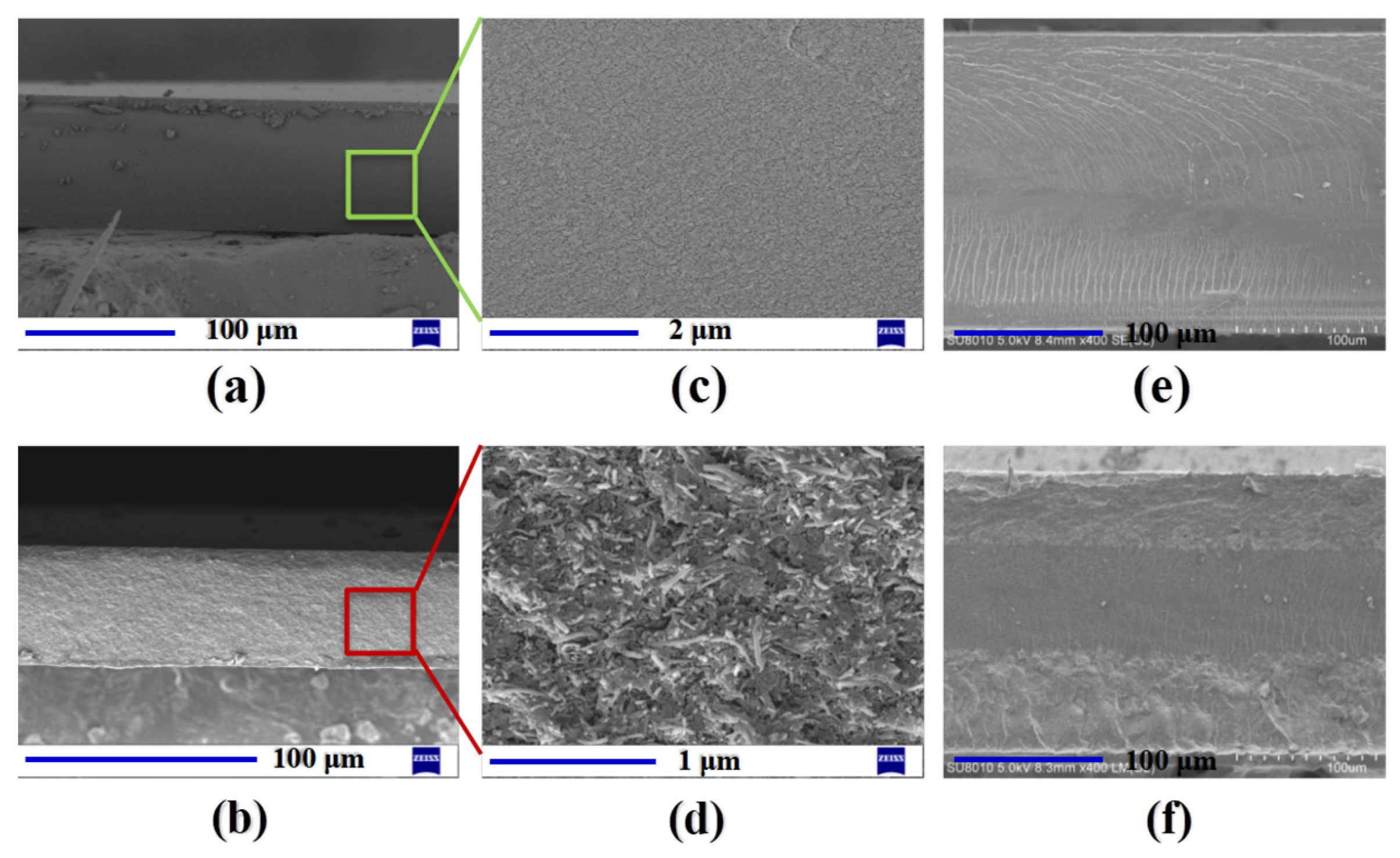
3.2. Cross-Section SEM Images of the Membranes and IPMCs
3.3. Electrochemical Properties of the IPMCs
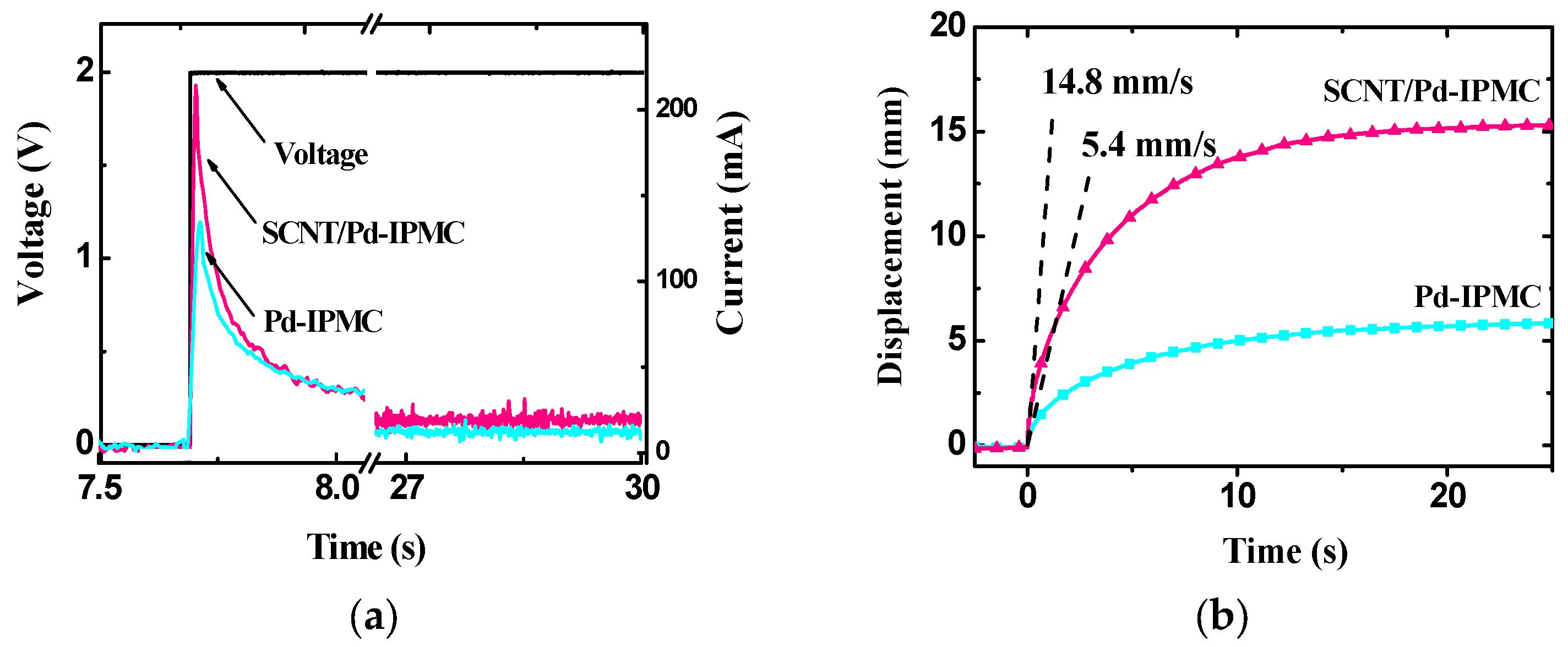
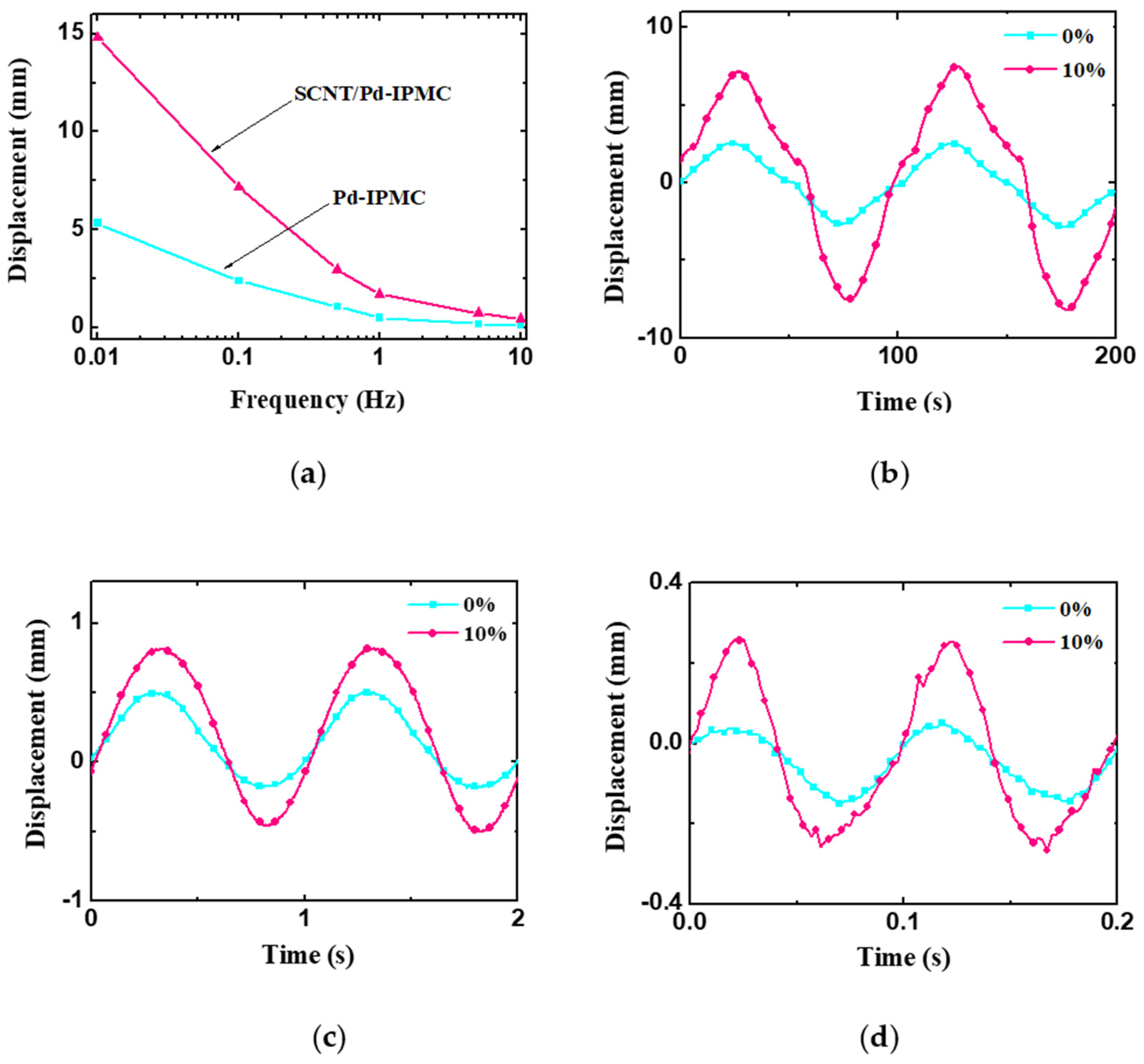
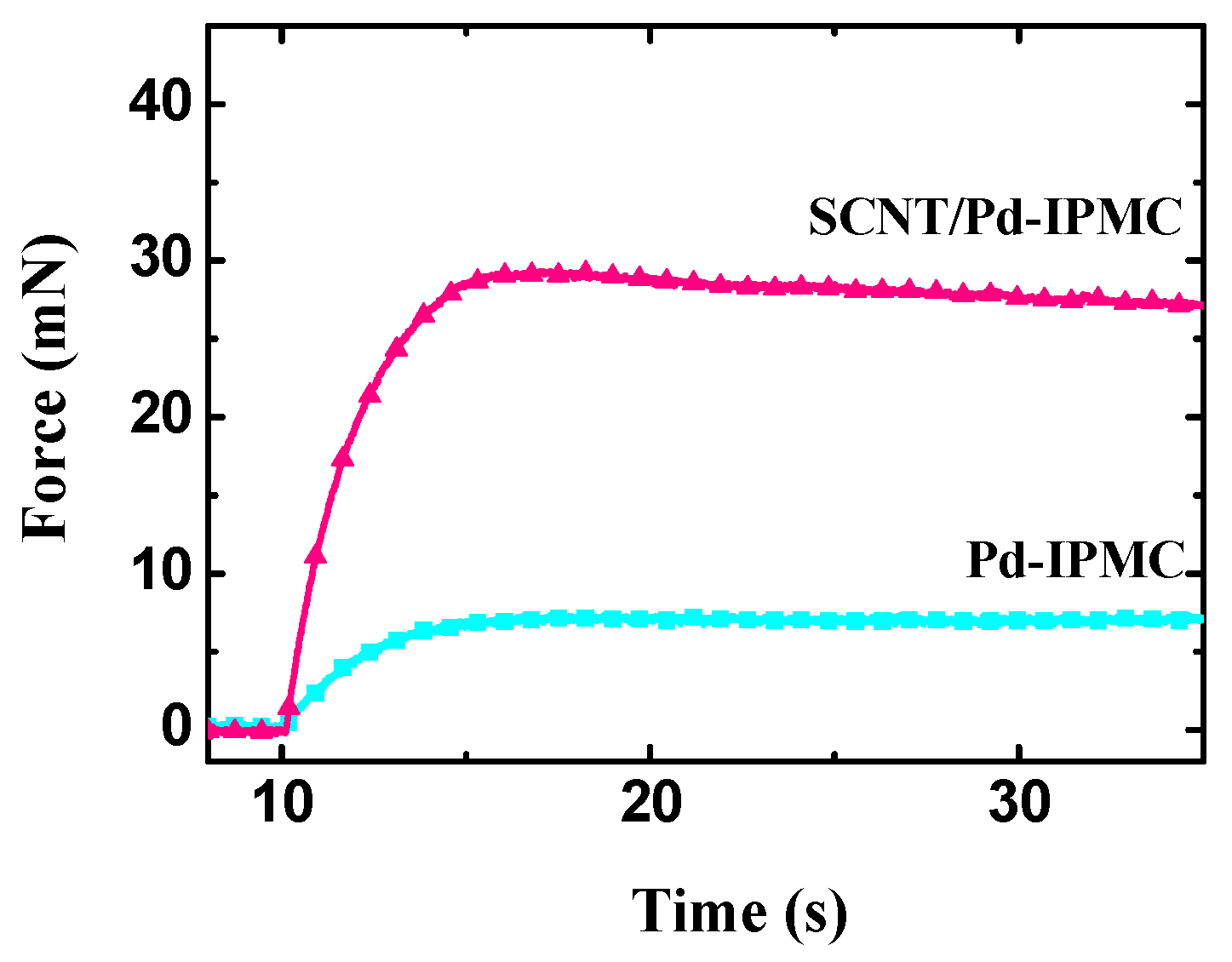
3.4. Electromechanical Behaviors of the IPMCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jo, C.; Pugal, D.; Oh, I.; Kim, K.J.; Asaka, K. Recent advances in ionic polymer-metal composite actuators and their modeling and applications. Prog. Polym. Sci. 2013, 38, 1037–1066. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Y.; Tang, G.; Ru, J.; Zhu, Z.; Li, B.; Guo, C.; Li, L.; Zhu, D. Ionic Flexible Sensors: Mechanisms, Materials, Structures, and Applications. Adv. Funct. Mater. 2022, 32, 2110417. [Google Scholar] [CrossRef]
- Shahinpoor, M.; Kim, K.J. Ionic polymer-metal composites: I. Fundamental. Smart Mater. Struct. 2001, 10, 819–833. [Google Scholar] [CrossRef]
- Ru, J.; Zhao, D.; Wang, J.; Chang, L.; Wang, T. Effect of Doping Polyethylene Oxide on the Properties of Nafion-IPMC Actuators. Funct. Mater. Lett. 2022, 15, 2251021. [Google Scholar] [CrossRef]
- Zhu, Z.; Chang, L.; Asaka, K.; Wang, Y.; Chen, H.; Zhao, H.; Li, D. Comparative experimental investigation on the actuation mechanisms of ionic polymer-metal composites with different backbones and water contents. J. Appl. Phys. 2014, 115, 124903. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, D.S.; Zhang, X.; Tian, A.; Wang, X. Models of displacement and blocking force of ionic-polymer metal composites based on actuation mechanism. Appl. Phys. A 2020, 126, 365. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, T.; Zhao, C.; Wang, K.; Wang, J.; Ru, J.; Sheng, J.; Chang, L.; Li, L. Experimental investigation on the physical parameters of ionic polymer metal composites sensors for humidity perception. Sens. Actuators B Chem. 2021, 345, 130421. [Google Scholar] [CrossRef]
- Tang, G.; Wang, Y.; Hao, M.; Zhang, L.; Ru, J.; Long, F.; Li, L. A novel strategy to enhance the generating power of ionic polymer metal composites through magnetoelectricity. Smart Mater. Struct. 2021, 30, 065013. [Google Scholar] [CrossRef]
- Shen, Q.; Olsen, Z.; Stalbaum, T.; Trabia, S.; Oh, I. Basic design of a biomimetic underwater soft robot with switchable swimming modes and programmable artificial muscles. Smart Mater. Struct. 2020, 29, 035038. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H. Voltage-induced beating vibration of a dielectric elastomer membrane. Nonlinear Dynam. 2020, 100, 2225–2239. [Google Scholar] [CrossRef]
- Zhao, D.; Ru, J.; Wang, T.; Wang, Y.; Chang, C. Enhancement of Ionic Polymer-Metal Composite Actuators with Polyethylene Oxide in Air Operating Authors. Polymers 2021, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yang, L.; Zhang, D.; Song, W. High performance, flexible and renewable nano-biocomposite artificial muscle based on mesoporous cellulose/ionic liquid electrolyte membrane. Sens. Actuat. B Chem. 2019, 283, 579–589. [Google Scholar] [CrossRef]
- Park, J.; Han, M.; Song, D.; Jho, J. Ionic Polymer-Metal Composite Actuators Obtained from Radiation-Grafted Cation- and Anion-Exchange Membranes. ACS Appl. Mater. Interfaces 2014, 6, 22847–22854. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Chen, W. Carbon Nanotube and Graphene-based Bioinspired Electrochemical Actuators. Adv. Mater. 2014, 26, 1025–1043. [Google Scholar] [CrossRef]
- Bian, K.; Liu, H.; Tai, G.; Zhu, K.; Xiong, K. Enhanced Actuation Response of Nafion-Based Ionic Polymer Metal Composites by Doping BaTiO3 Nanoparticles. J. Phys. Chem. C 2016, 120, 12377–12384. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Zhu, Y.; Zhu, D.; Chen, H. Formation and characterization of dendritic interfacial electrodes inside an ionomer. ACS Appl. Mater. Interfaces 2017, 9, 30258–30262. [Google Scholar] [CrossRef]
- Wang, H.; Cho, J.; Song, D.; Jang, J.; Jho, J.; Park, J. High-Performance Electroactive Polymer Actuators Based on Ultra-Thick Ionic Polymer-Metal Composites with Nanodispersed Metal Electrodes. ACS Appl. Mater. Interfaces 2017, 9, 21998–22005. [Google Scholar] [CrossRef]
- Chang, L.; Yang, Q.; Niu, Q.; Wang, Y.; Liu, Y.; Lu, P.; He, Q.; Wu, Y.; Hu, Y. High-performance ionic polymer-metal composite actuators fabricated with microneedle roughening. Smart Mater. Struct. 2019, 28, 015007. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.; Song, D.; Jho, J. Highly Enhanced Force Generation of Ionic Polymer-Metal Composite Actuators via Thickness Manipulation. ACS Appl. Mater. Interfaces 2015, 7, 16659–16667. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Chee, P.; Lim, E.; Tan, R. A novel crenellated ionic polymer-metal composite (IPMC) actuator with enhanced electromechanical performances. Smart Mater. Struct. 2019, 28, 115011. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, Y.; Liang, Y.; Ren, L.; Ren, L. High-performance ionic-polymer-metal composite: Toward large-deformation fast-response artificial muscles. Adv. Funct. Mater. 2020, 30, 1908508. [Google Scholar] [CrossRef]
- Ru, J.; Bian, C.; Zhu, Z.; Wang, Y.; Zhang, J.; Horiuchi, T.; Sugino, T.; Liu, X.; Chen, H.; Asaka, K. Controllable and durable Ionic electroactive polymer actuator based on nanoporous carbon nanotube film electrode. Smart Mater. Struct. 2019, 28, 085032. [Google Scholar] [CrossRef]
- Wu, G.; Wu, X.; Xu, Y.; Cheng, H.; Meng, J.; Yu, Q.; Shi, X.; Zhang, K.; Chen, W.; Chen, S. High-Performance Hierarchical Black-Phosphorous-Based Soft Electrochemical Actuators in Bioinspired Applications. Adv. Mater. 2019, 31, 1806492. [Google Scholar] [CrossRef]
- Guo, D.; Wang, L.; Wang, X.; Xiao, Y.; Ding, Y. Pedot coating enhanced electromechanical performances and prolonged stable working time of ipmc actuator. Sens. Actuat. B-Chem. 2020, 305, 127488. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Wang, Y.; Zhu, Z.; Li, D. Effect of dehydration on the mechanical and physicochemical properties of gold- and palladiumionomeric polymer-metal composite (IPMC) actuators. Electrochim. Acta 2014, 129, 450–458. [Google Scholar] [CrossRef]
- Asaka, K.; Fujiwara, N.; Oguro, K.; Onishi, K.; Sewa, S. State of water and ionic conductivity of solid polymer electrolyte membranes in relation to polymer actuators. J. Electroanal. Chem. 2001, 505, 24–32. [Google Scholar] [CrossRef]
- Wu, G.; Hu, Y.; Liu, Y.; Zhao, J.; Chen, X.; Whoehling, V.; Plesse, C.; Nguyen, G.; Vidal, F.; Chen, W. Graphitic carbon nitride nanosheet electrode-based high-performance ionic actuator. Nat. Commun. 2015, 6, 7258. [Google Scholar] [CrossRef]
- Lee, P.; Hyun, J.; Jeoung, S.; Nam, J.; Hwang, T.; Kim, K.; Solasa, K. Ionic polymer metal composites for use as an organic electrolyte supercapacitor. Smart Mater. Struct. 2019, 28, 5. [Google Scholar] [CrossRef]
- Palmre, V.; Kim, S.; Pugal, D.; Kim, K. Improving electromechanical output of IPMC by high surface area Pd-Pt electrodes and tailor ionomer membrane thickness. Int. J. Smart Nano Mater. 2011, 5, 99–113. [Google Scholar] [CrossRef]
- He, Q.; Song, L.; Yu, M.; Dai, Z. Fabrication, characteristics and electrical model of an ionic polymer metal-carbon nanotube composite. Smart Mater. Struct. 2015, 24, 075001. [Google Scholar] [CrossRef]
- Tiwari, R.; Garcia, E. The State of Understanding of Ionic Polymer Metal Composite Architecture: A Review. Smart Mater. Struct. 2011, 20, 083001. [Google Scholar] [CrossRef]
- Chang, L.; Chen, H.; Zhu, Z.; Li, B. Manufacturing process and electrode properties of palladium-electroded ionic polymer-metal composite. Smart Mater. Struct. 2012, 21, 065018. [Google Scholar] [CrossRef]
- Cellini, F.; Grillo, A.; Porfiri, M. Ionic Polymer Metal Composites with Polypyrrole-silver Electrodes. Appl. Phys. Lett. 2015, 106, 131902. [Google Scholar] [CrossRef]
- Naji, L.; Safari, M.; Moaven, S. Fabrication of SGO/Nafion-based IPMC Soft Actuators with Sea Anemone-like Pt Electrodes and Enhanced Actuation Performance. Carbon 2016, 100, 243–257. [Google Scholar] [CrossRef]
- Kim, S.; Kim, K. Palladium Buffer-Layered High Performance Ionic Polymer-Metal Composites. Smart Mater. Struct. 2008, 17, 035011. [Google Scholar] [CrossRef]
- Lee, J.; Yoo, Y. Preparation and performance of IPMC actuators with electrospun Nafion®-MWNT composite electrodes. Sens. Actuat. B-Chem. 2011, 159, 103–111. [Google Scholar] [CrossRef]
- Tamagawa, H.; Lin, W.; Kikuchi, K.; Sasaki, M. Bending control of nafion-based electroactive polymer actuator coated with multi-walled carbon nanotubes. Sens. Actuat. B-Chem. 2011, 156, 375–382. [Google Scholar] [CrossRef]
- Ru, J.; Wang, Y.; Chang, L.; Chen, H.; Li, D. Preparation and characterization of water-soluble carbon nanotube reinforced Nafion membranes and so-based ionic polymer metal composite actuators. Smart Mater. Struct. 2016, 25, 095006. [Google Scholar] [CrossRef]
- Ru, J.; Zhu, Z.; Wang, Y.; Chen, H.; Li, D. Tunable actuation behavior of ionic polymer metal composite utilizing carboxylated carbon nanotube-doped Nafion matrix. RSC Adv. 2018, 8, 3090–3094. [Google Scholar] [CrossRef]
- Du, C.; Zhao, T.; Liang, Z. Sulfonation of carbon-nanotube supported platinum catalysts for polymer electrolyte fuel cells. J. Power Sources 2008, 176, 9–15. [Google Scholar] [CrossRef]
- Hudson, J.; Casavant, M.; Tour, J. Water-Soluble, Exfoliated, Nonroping Single-Wall Carbon Nanotubes. J. Am. Chem. Soc. 2004, 126, 11158. [Google Scholar] [CrossRef] [PubMed]
- Akle, B.; Leo, D.; Hickner, M.; McGrath, J. Correlation of Capacitance and Actuation in Ionomeric Polymer Transducers. J. Mater. Sci. 2010, 40, 3715–3724. [Google Scholar] [CrossRef]
- Tiwari, R.; Kim, K. Effect of Metal Diffusion on Mechanoelectric Property of Ionic Polymer-Metal Composite. Appl. Phys. Lett. 2010, 97, 244104. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Z.; Chen, H.; Luo, B.; Chang, L.; Wang, Y.; Li, D. Effects of preparation steps on the physical parameters and electromechanical properties of IPMC actuators. Smart Mater. Struct. 2014, 23, 125015. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Wang, Y.; Luo, B.; Chang, L.; Zhu, Z.; Li, B. Influence of additives on the properties of casting nafion membranes and so-based ionic polymer-metal composite actuators. Polym. Eng. Sci. 2014, 54, 818–830. [Google Scholar] [CrossRef]
- Ru, J.; Zhu, Z.; Wang, Y.; Chen, H.; Bian, C.; Li, D. A moisture and electric coupling stimulated ionic polymer-metal composite actuator with controllable deformation behavior. Smart Mater. Struct. 2018, 27, 02LT01. [Google Scholar] [CrossRef]
- Park, J.; Palmre, V.; Hwang, T.; Kim, K.; Yim, W.; Bae, C. Electromechanical performance and other characteristics of IPMCs fabricated with various commercially available ion exchange membranes. Smart Mater. Struct. 2014, 23, 074001. [Google Scholar] [CrossRef]
- Thomassin, J.; Kollar, J.; Caldarella, G.; Germain, A.; Jerome, R.; Detrembleur, C. Beneficial effect of carbon nanotubes on the performances of Nafion membranes in fuel cell applications. J. Membr. Sci. 2007, 303, 252–257. [Google Scholar] [CrossRef]
- Jung, J.; Jeon, J.; Sridhar, V.; Oh, I. Electro-active graphene-Nafion actuators. Carbon 2011, 49, 1279–1289. [Google Scholar] [CrossRef]
- Bauer, F.; Pollard, M. Microstructural characterization of Zr-phosphate-Nafion-membranes for direct methanol fuel cell (DMFC) applications. J. Membr. Sci. 2004, 233, 141–149. [Google Scholar] [CrossRef]
- Gupta, V.; Miura, N. Influence of the microstructure on the supercapacitive behavior of polyaniline/single-wall carbon nanotube composites. J. Power Sources 2006, 157, 616–620. [Google Scholar] [CrossRef]
- Mukai, K.; Asaka, K.; Kiyohara, K.; Sugino, T.; Takeuchi, I.; Fukushima, T.; Aida, T. High performance fully plastic actuator based on ionic-liquid-based bucky gel. Electrochim. Acta 2008, 53, 5555–5562. [Google Scholar] [CrossRef]
- Landi, B.; Raffaelle, R.; Heben, M.; Alleman, J.; Vanderveer, W.; Gennett, T. Single wall carbon nanotube-Nafion composite actuators. Nano Lett. 2002, 2, 1329–1332. [Google Scholar] [CrossRef]
- Paquette, J.; Kim, K. Ionomeric electroactive polymer artificial muscle for naval applications. IEEE J. Ocean. Eng. 2004, 29, 729–737. [Google Scholar] [CrossRef]
- Jung, J.; Vadahanambi, S.; Oh, I. Electro-Active Nano-Composite Actuator Based on Fullerene-Reinforced Nafion. Compos. Sci. Technol. 2010, 70, 584–592. [Google Scholar] [CrossRef]
- Dibakar, B.; Bishakh, B.; Ashish, D. Pseudo-rigid body modeling of IPMC for a partially compliant four-bar mechanism for work volume generation. J. Intell. Mater. Syst. Struct. 2009, 20, 51–61. [Google Scholar]



| IPMC | WD (KJ/m3) |
|---|---|
| Pure Nafion (0 wt% SCNT) | 0.60 |
| 10 wt% SCNT in SCNT/Pd composite electrode | 5.83 |
| 0.25 wt% SCNT in SCNT/Nafion composite electrolyte | 0.79 a |
| 0.5 wt% SCNT in SCNT/Nafion composite electrolyte | 1.67 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ru, J.; Zhao, D.; Zhu, Z.; Wang, Y. Fabrication and Characterization of a Novel Smart-Polymer Actuator with Nanodispersed CNT/Pd Composite Interfacial Electrodes. Polymers 2022, 14, 3494. https://doi.org/10.3390/polym14173494
Ru J, Zhao D, Zhu Z, Wang Y. Fabrication and Characterization of a Novel Smart-Polymer Actuator with Nanodispersed CNT/Pd Composite Interfacial Electrodes. Polymers. 2022; 14(17):3494. https://doi.org/10.3390/polym14173494
Chicago/Turabian StyleRu, Jie, Dongxu Zhao, Zicai Zhu, and Yanjie Wang. 2022. "Fabrication and Characterization of a Novel Smart-Polymer Actuator with Nanodispersed CNT/Pd Composite Interfacial Electrodes" Polymers 14, no. 17: 3494. https://doi.org/10.3390/polym14173494
APA StyleRu, J., Zhao, D., Zhu, Z., & Wang, Y. (2022). Fabrication and Characterization of a Novel Smart-Polymer Actuator with Nanodispersed CNT/Pd Composite Interfacial Electrodes. Polymers, 14(17), 3494. https://doi.org/10.3390/polym14173494








