Optimize PLA/EVA Polymers Blend Compositional Coating for Next Generation Biodegradable Drug-Eluting Stents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PLA/EVA Blends
2.3. Preparation of Sheets
2.4. Characterization of Blends
3. Results and Discussion
3.1. Scanning Electron Microscopy (SEM)
3.2. Fourier Transform Infrared (FTIR-ATR) Spectroscopy
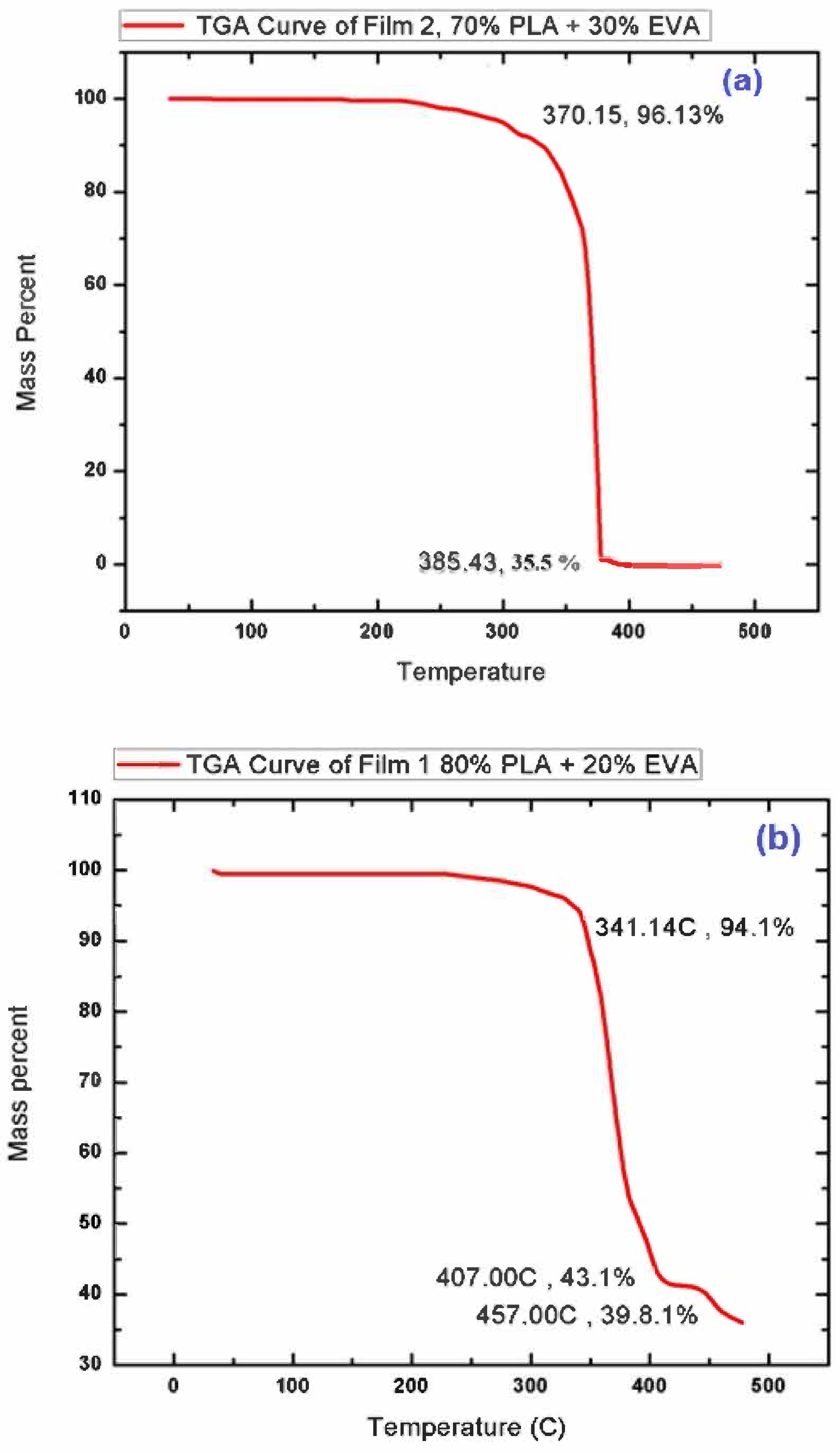
3.3. Thermo-Gravimetric Analysis (TGA)
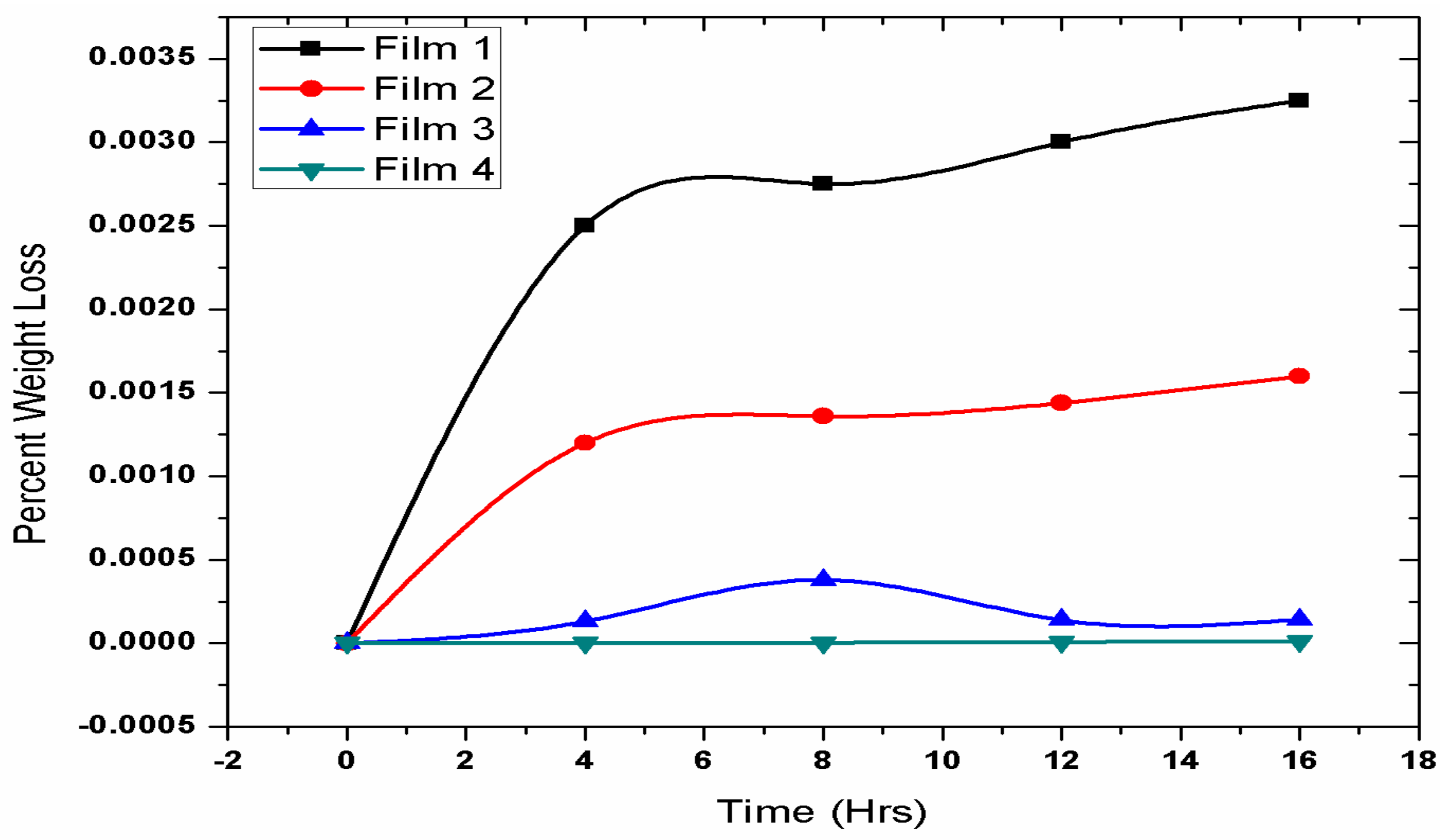
3.4. Biodegradation Test
3.5. Mechanical Testing
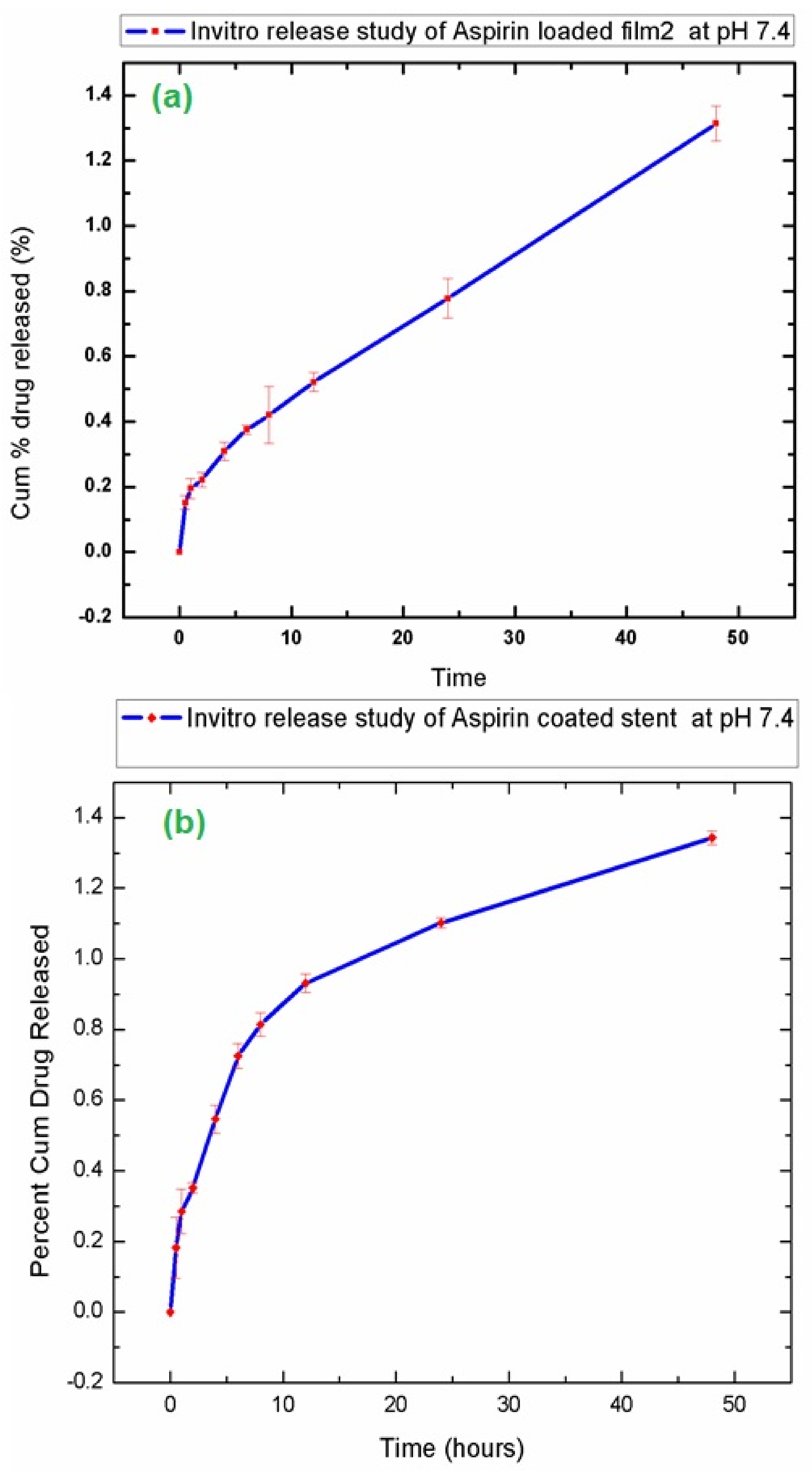
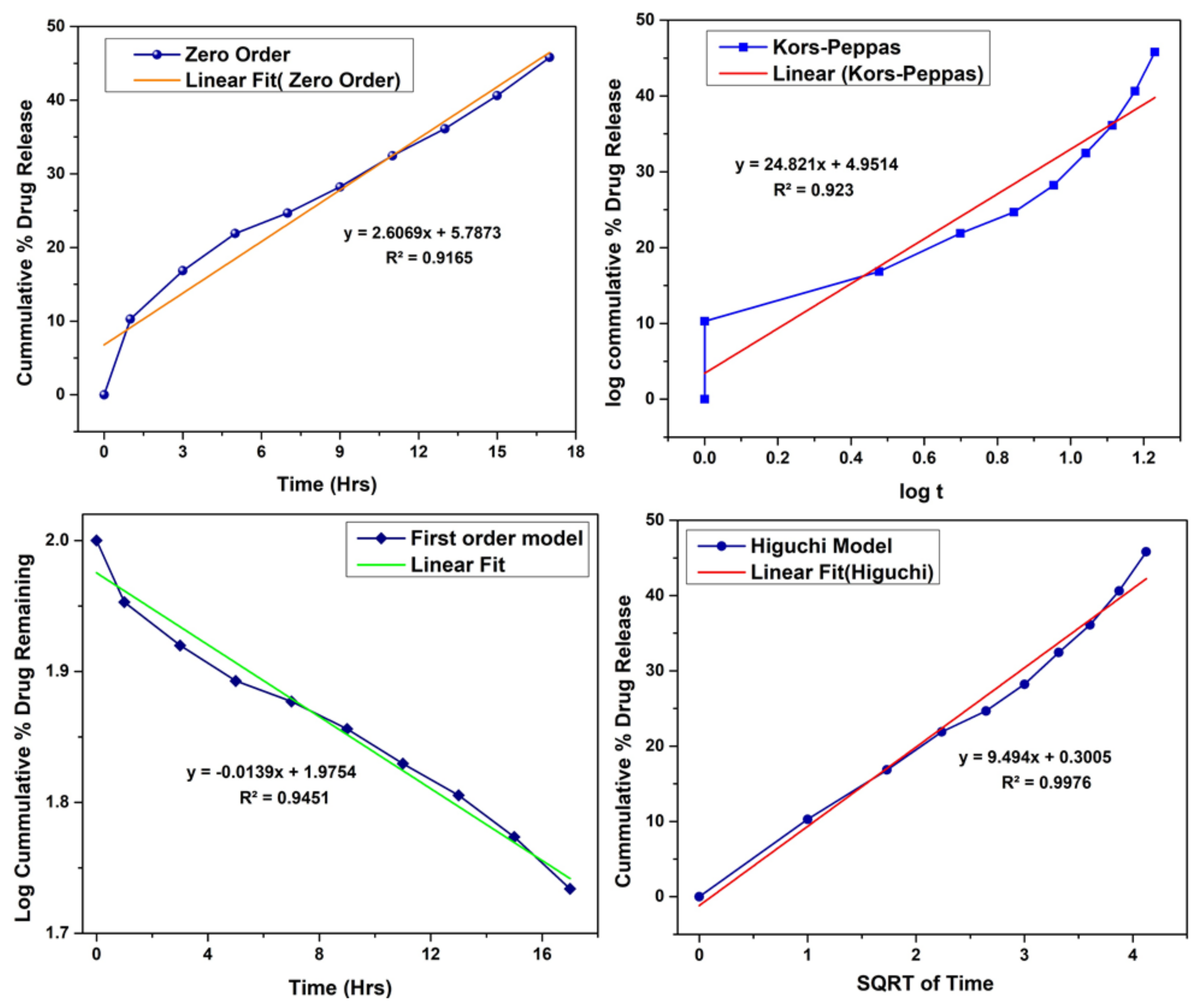
3.6. In-Vitro Drug Release Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durant, T.M. Cardio-Vascular Disease. Phila. Med. 1947, 43, 625–627. [Google Scholar] [PubMed]
- Sigwart, U.; Puel, J.; Mirkovitch, V.; Joffre, F.; Kappenberger, L. Intravascular Stents To Prevent Occlusion And Re-Stenosis After Transluminal Angioplasty. N. Engl. J. Med. 1987, 316, 701–706. [Google Scholar] [CrossRef]
- Palmaz, J.C.; Kopp, D.T.; Hayashi, H.; Schatz, R.A.; Hunter, G.; Tio, F.O.; Garcia, O.; Alvarado, R.; Rees, C.; Thomas, S.C. Normal And Stenotic Renal Arteries: Experimental Balloon-Expandable Intraluminal Stenting. Radiology 1987, 164, 705–708. [Google Scholar] [CrossRef]
- Chhabra, L.; Zain, M.A.; Siddiqui, W.J. Coronary Stents; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Borhani, S.; Hassanajili, S.; Hossein, S.; Tafti, A.; Rabbani, S. Cardiovascular Stents: Overview, Evolution, And Next Generation; Springer: Berlin/Heidelberg, Germany, 2018; Volume 7, ISBN 0123456789. [Google Scholar]
- Ni, L.; Chen, H.; Luo, Z.; Yu, Y. Bioresorbable vascular stents and drug-eluting stents in treatment of coronary heart disease: A meta-analysis. J. Cardiothorac. Surg. 2020, 15, 26. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, P.; Nyffenegger, T.; Sabti, Z.; Buset, E.; Toggweiler, S.; Kobza, R.; Cuculi, F. A Novel Approach To Treat In-Stent Restenosis: 6-And 12-Month Results Using The Everolimus-Eluting Bioresorbable Vascular Scaffold. Eurointervention 2016, 11, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Approaches, D.; Hypertension, S. 8. Cardiovascular Disease And Risk Management. Diabetes Care 2015, 38, S49–S57. [Google Scholar]
- Pawar, R.P.; Tekale, S.U.; Shisodia, S.U.; Totre, J.T.; Domb, A.J. Biomedical Applications of Poly(Lactic Acid). Rec. Pat. Regen. Med. 2014, 4, 40–51. [Google Scholar]
- Hamid, H.; Coltart, J. “Miracle Stents”—A Future Without Restenosis. Mcgill J. Med. 2007, 10, 105–111. [Google Scholar] [PubMed]
- Sheiban, I.; Villata, G.; Bollati, M.; Sillano, D.; Lotrionte, M.; Biondi-Zoccai, G. Next-Generation Drug-Eluting Stents In Coronary Artery Disease: Focus on Everolimus-Eluting Stent (Xience V®). Vasc. Health Risk Manag. 2008, 4, 31–38. [Google Scholar] [CrossRef]
- Otsuka, F.; Nakano, M.; Ladich, E.; Kolodgie, F.D.; Virmani, R. Pathologic Etiologies Of Late And Very Late Stent Thrombosis Following First-Generation Drug-Eluting Stent Placement. Thrombosis 2012, 2012, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Wu, L.; Wang, J.; Yang, Z.; Tu, Q.; Zhang, X.; Huang, N. Surface-Degradable Drug-Eluting Stent With Anticoagulation, Antiproliferation, and Endothelialization Functions. Biomolecules 2019, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.S.; Moore, J.E. Biomechanical Challenges To Polymeric Biodegradable Stents. Ann. Biomed. Eng. 2016, 44, 560–579. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Zhou, S.; Liang, X.; Li, Q.; Han, W. In Vitro Study Of Poly(Ethylene Carbonate) As A Drug-Eluting Stent Coating. Prog. Nat. Sci. Mater. Int. 2012, 22, 295–302. [Google Scholar] [CrossRef]
- Lukman, S.K.; Hussien Al-Ashwal, R.; Md. Khudzari, A.Z.; Saidin, S. Emerging Of Cardiovascular Metal Stent: A Review On Drug-Eluting Stent Towards The Utilisation Of Herbal Coating. Malaysian J. Fundam. Appl. Sci. 2019, 15, 225–231. [Google Scholar] [CrossRef]
- Genina, N.; Holländer, J.; Jukarainen, H.; Mäkilä, E.; Salonen, J.; Sandler, N. Ethylene Vinyl Acetate (EVA) As A New Drug Carrier For 3D Printed Medical Drug Delivery Devices. Eur. J. Pharm. Sci. 2016, 90, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Cardea, S.; Baldino, L.; Scognamiglio, M.; Reverchon, E. 3D PLLA/Ibuprofen Composite Scaffolds Obtained By A Supercritical Fluids Assisted Process. J. Mater. Sci. Mater. Med. 2014, 25, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Loke, Y.K. Critical Overview On The Benefits And Harms Of Aspirin. Pharmaceuticals 2010, 3, 1491–1506. [Google Scholar] [CrossRef] [PubMed]
- Weisman, S.M.; Graham, D.Y. Evaluation Of The Benefits and Risks of Low-Dose Aspirin in the Secondary Prevention Of Cardiovascular And Cerebrovascular Events. Arch. Intern. Med. 2002, 162, 2197–2202. [Google Scholar] [CrossRef]
- Ittaman, S.V.; Vanwormer, J.J.; Rezkalla, S.H. The Role of Aspirin in the Prevention of Cardiovascular Disease. Clin. Med. Res. 2014, 12, 147–154. [Google Scholar] [CrossRef]
- Dai, Y.; Ge, J. Clinical Use Of Aspirin in Treatment and Prevention of Cardiovascular Disease. Thrombosis 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Hou, D.; Huibregtse, B.; Dawkins, K.; Donnelly, J.; Roy, K.; Chen, J.P.; Akinapelli, A. Current State of Bioabsorbable Polymer-Coated Drug-Eluting Stents. Curr. Cardiol. Rev. 2017, 13, 139–154. [Google Scholar] [CrossRef]
- Wilson, S.J.; Newby, D.E.; Dawson, D.; Irving, J.; Berry, C. Duration Of Dual Antiplatelet Therapy In Acute Coronary Syndrome. Heart 2017, 103, 573–580. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Teng, L.; Sun, F.; Li, Y. Synthesis and Biological Application of Polylactic Acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic Acid: Synthesis And Biomedical Applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [PubMed]
- Abd Alsaheb, R.A.; Aladdin, A.; Othman, N.Z.; Abd Malek, R.; Leng, O.M.; Aziz, R.; El Enshasy, H.A. Recent Applications Of Polylactic Acid In Pharmaceutical And Medical Industries. J. Chem. Pharm. Res. 2015, 7, 51–63. [Google Scholar]
- Thavornyutikarn, B.; Chantarapanich, N.; Sitthiseripratip, K.; Thouas, G.A.; Chen, Q. Bone tissue engineering scaffolding: Computer-aided scaffolding techniques. Prog. Biomater. 2014, 3, 61–102. [Google Scholar] [CrossRef] [PubMed]
- Alojaly, H.; Benyounis, K.Y. Packaging With Plastics And Polymeric Materials. Ref. Modul. Mater. Sci. Mater. Eng. 2020, 1–17. [Google Scholar]
- Schneider, C.; Langer, R.; Loveday, D.; Hair, D. Applications Of Ethylene Vinyl Acetate Copolymers (EVA) In Drug Delivery Systems. J. Control. Release 2017, 262, 284–295. [Google Scholar] [CrossRef] [PubMed]
- A Akl, M.; Elrazek HM, A.; Bary EM, A. Poly (Ethylene-Co-Vinyl Acetate) Blends For Controlled Drug Release. Am. J. Adv. Drug Deliv. 2018, 6, 52–60. [Google Scholar] [CrossRef]
- Likittanaprasong, N.; Seadan, M.; Suttiruengwong, S. Impact Property Enhancement Of Poly (Lactic Acid) With Different Flexible Copolymers. IOP Conf. Ser. Mater. Sci. Eng. 2015, 87, 012069. [Google Scholar] [CrossRef]
- Moura, I.; Botelho, G.; Machado, A.V. Characterization Of EVA/PLA Blends When Exposed To Different Environments. J. Polym. Environ. 2014, 22, 148–157. [Google Scholar] [CrossRef]
- Číková, E.; Kuliček, J.; Janigová, I.; Omastová, M. Electrospinning of Ethylene Vinyl Acetate/Poly(Lactic Acid) Blends On A Water Surface. Materials 2018, 11, 1737. [Google Scholar] [CrossRef]
- Ma, P. Tailoring the Properties of Bio-Based and Biocompostable Polymer Blends. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2011. [Google Scholar]
- Sangeetha, V.H.; Valapa, R.B.; Nayak, S.K.; Varghese, T.O. Investigation on The Influence of EVA Content on The Mechanical and Thermal Characteristics of Poly(Lactic Acid) Blends. J. Polym. Environ. 2018, 26, 1–14. [Google Scholar] [CrossRef]
- Jiménez, A.; Ruseckaite, R.A. Polymer Degradation And Stability: Editorial. Polym. Degrad. Stab. 2010, 95, 2125. [Google Scholar] [CrossRef]
- ShenXiang, g.; Feng, L.; Bian, X.; Li, G.; Chen, X. Evaluation of PLA content in PLA/PBAT blends using TGA. Polym. Test. 2020, 81, 106211. [Google Scholar] [CrossRef]
- Hu, T.; Yang, J.; Cui, K.; Rao, Q.; Yin, T.; Tan, L.; Zhang, Y.; Li, Z.; Wang, G. Controlled Slow-Release Drug-Eluting Stents For The Prevention Of Coronary Restenosis: Recent Progress And Future Prospects. ACS Appl. Mater. Interfaces 2015, 7, 11695–11712. [Google Scholar] [CrossRef]
- Van Hengel, C. Stress-Strain Curve. In Fibre Metal Laminates; Vlot, A., Gunnink, J.W., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 101–109. [Google Scholar]
- Abdullah, N.A.S.; Mohamad, Z. The Effect of Dynamic Vulcanization on The Morphological And Mechanical Properties of The Toughened Poly (Lactic Acid)/Epoxidized Natural Rubber. Malaysian J. Fundam. Appl. Sci. 2018, 14, 348–352. [Google Scholar] [CrossRef]
- Abdullah, N.A.S.; Mohamad, Z.; Man, S.H.C.; Baharulrazi, N.; Majid, R.A.; Jusoh, M.; Ngadi, N. Thermal and Toughness Enhancement of Poly (Lactic Acid) Bio-Nanocomposites. Chem. Eng. Trans. 2019, 72, 427–432. [Google Scholar]
- Ferri, J.M.; Garcia-Garcia, D.; Rayón, E.; Samper, M.D.; Balart, R. Compatibilization And Characterization Of Polylactide And Biopolyethylene Binary Blends By Non-Reactive And Reactive Compatibilization Approaches. Polymers 2020, 12, 1344. [Google Scholar] [CrossRef] [PubMed]
- Bazhenov, S. Mechanical Behavior of Filled Thermoplastic Polymers. Met. Ceram. Polym. Compos. Var. Uses 2011, 171–195. [Google Scholar]
- Haward, R.N. The Derivation Of A Strain Hardening Modulus From True Stress-Strain Curves For Thermoplastics. Polymer 1994, 35, 3858–3862. [Google Scholar] [CrossRef]
- Han, D.H.; Choi, M.C.; Nagappan, S.; Kim, Y.M.; Kim, H.S. Ethylene vinyl acetate (EVA)/poly(lactic acid) (PLA) blends and their foams. Mol. Cryst. Liq. Cryst. 2020, 707, 38–45. [Google Scholar] [CrossRef]



| Sample | PLA % | EVA % | Polymer | Drug % | Rotational Speed (rpm) | Temperature (T) | Time (t) |
|---|---|---|---|---|---|---|---|
| Film1 (F-1) | 80 | 20 | 70 | 30 | 80 | 180 | 25 min |
| Film 2 (F-2) | 70 | 30 | 70 | 30 | 75 | 165 | 25 |
| Film 3 (F-3) | 50 | 50 | 70 | 30 | 65 | 160 | 20 |
| Film 4 (F-4) | 30 | 70 | 70 | 30 | 50 | 150 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishaque, N.; Naseer, N.; Abbas, M.A.; Javed, F.; Mushtaq, S.; Ahmad, N.M.; Khan, M.F.A.; Ahmed, N.; Elaissari, A. Optimize PLA/EVA Polymers Blend Compositional Coating for Next Generation Biodegradable Drug-Eluting Stents. Polymers 2022, 14, 3547. https://doi.org/10.3390/polym14173547
Ishaque N, Naseer N, Abbas MA, Javed F, Mushtaq S, Ahmad NM, Khan MFA, Ahmed N, Elaissari A. Optimize PLA/EVA Polymers Blend Compositional Coating for Next Generation Biodegradable Drug-Eluting Stents. Polymers. 2022; 14(17):3547. https://doi.org/10.3390/polym14173547
Chicago/Turabian StyleIshaque, Naila, Nauman Naseer, Muhammad Asad Abbas, Fatima Javed, Shehla Mushtaq, Nasir M. Ahmad, Muhammad Farhan Ali Khan, Naveed Ahmed, and Abdelhamid Elaissari. 2022. "Optimize PLA/EVA Polymers Blend Compositional Coating for Next Generation Biodegradable Drug-Eluting Stents" Polymers 14, no. 17: 3547. https://doi.org/10.3390/polym14173547
APA StyleIshaque, N., Naseer, N., Abbas, M. A., Javed, F., Mushtaq, S., Ahmad, N. M., Khan, M. F. A., Ahmed, N., & Elaissari, A. (2022). Optimize PLA/EVA Polymers Blend Compositional Coating for Next Generation Biodegradable Drug-Eluting Stents. Polymers, 14(17), 3547. https://doi.org/10.3390/polym14173547