In Situ Zymography Analysis of Matrix Metalloproteinases Activity Following Endodontic Irrigation Protocols and Correlation to Root Dentine Bond Strength
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample and Root Canal Preparation
2.2. Root Sectioning
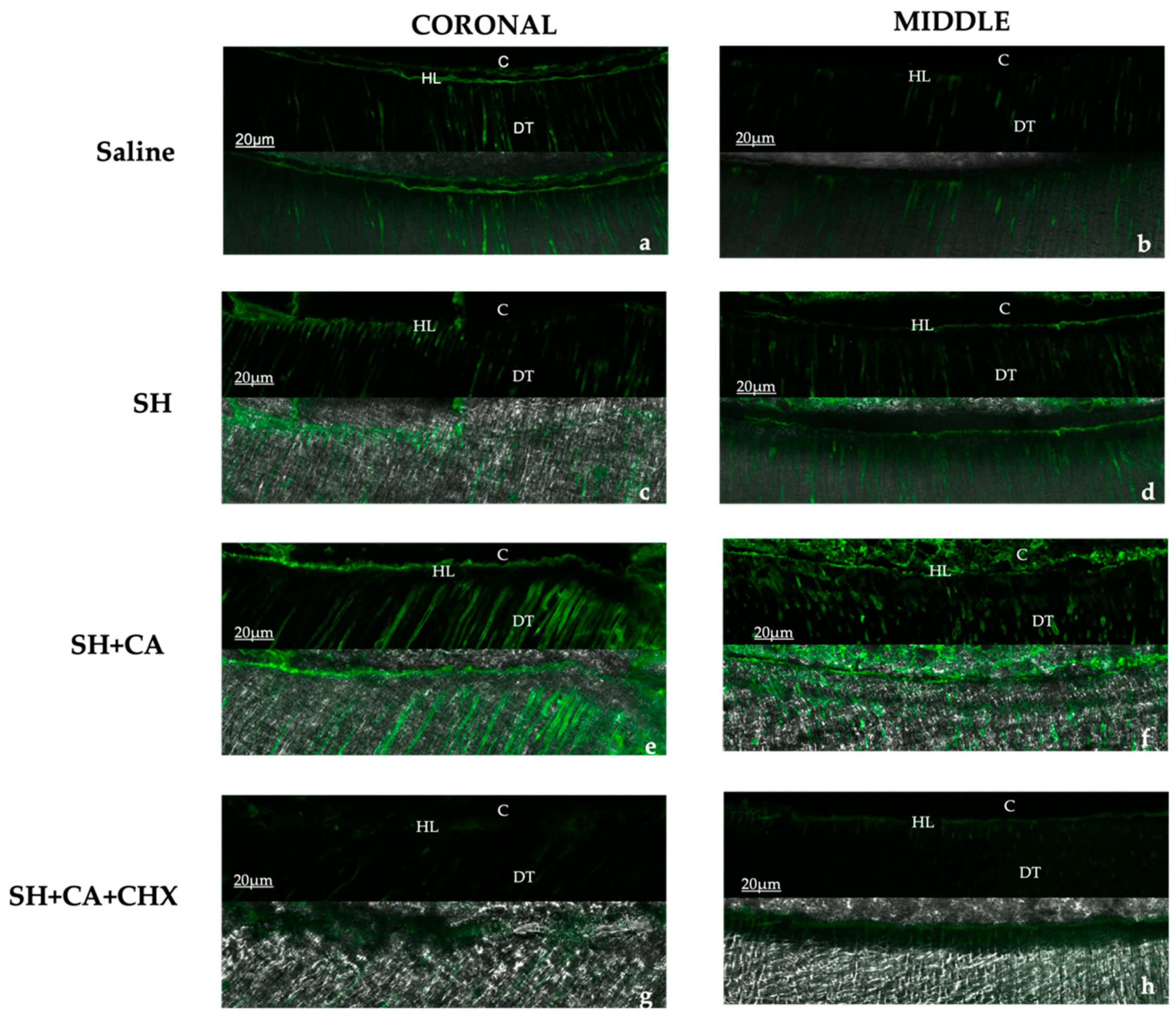
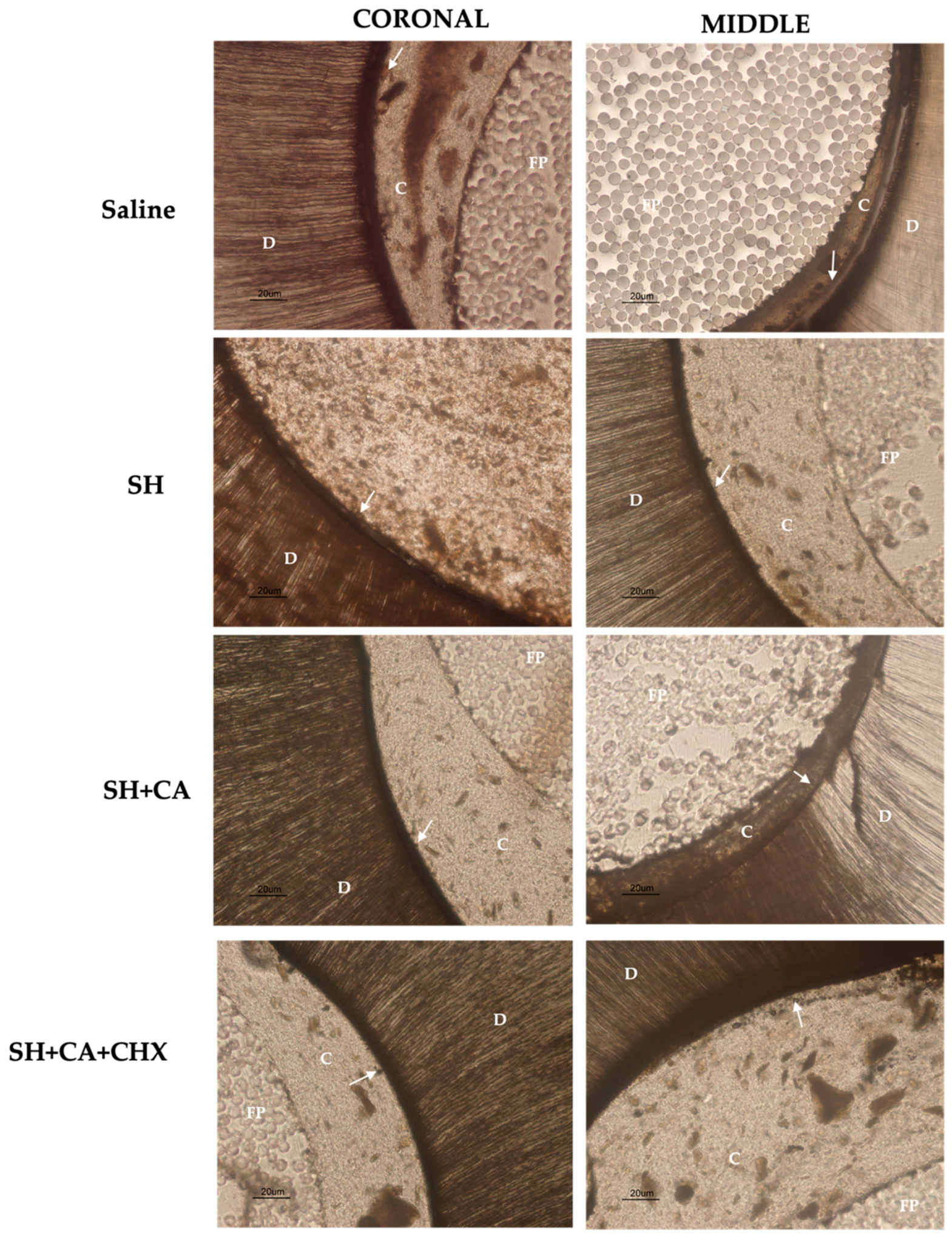
2.3. In Situ Zymography
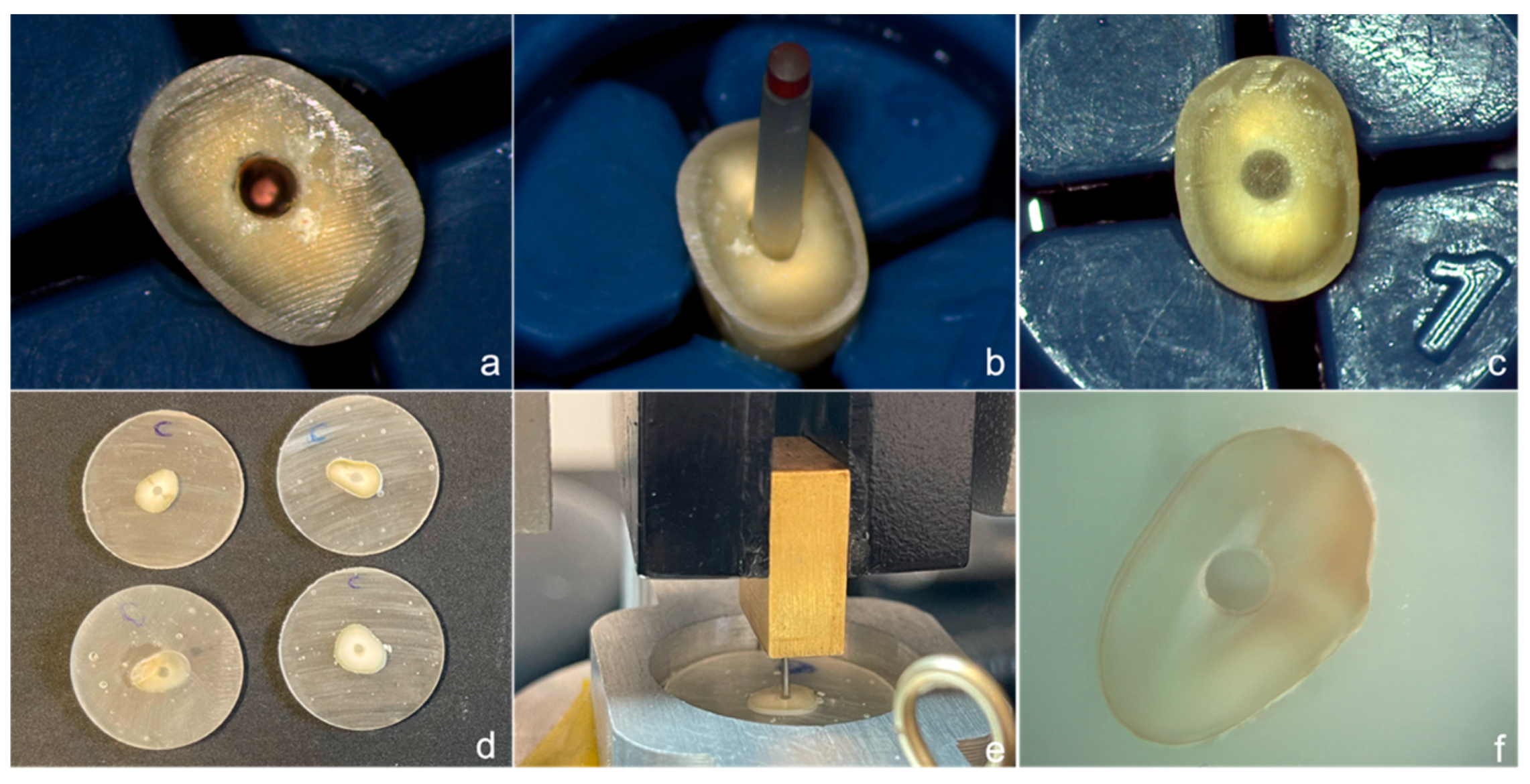
2.4. Push-Out Bond Strength Test
2.5. Scanning Electron Microscopy Analysis
2.6. Nanoleakage Expression
2.7. Statistical Analysis
3. Results
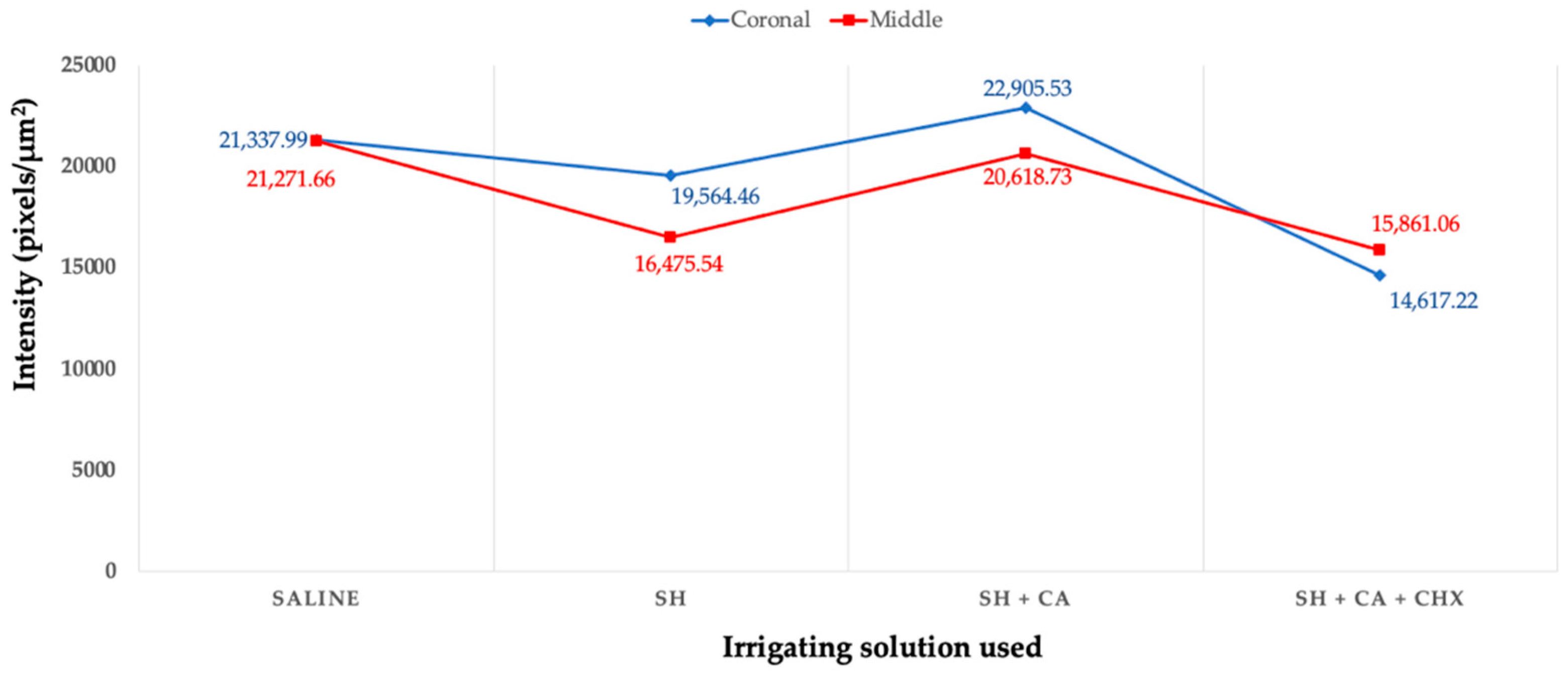
3.1. In Situ Zymography
3.2. Push Out Bond Strength Test
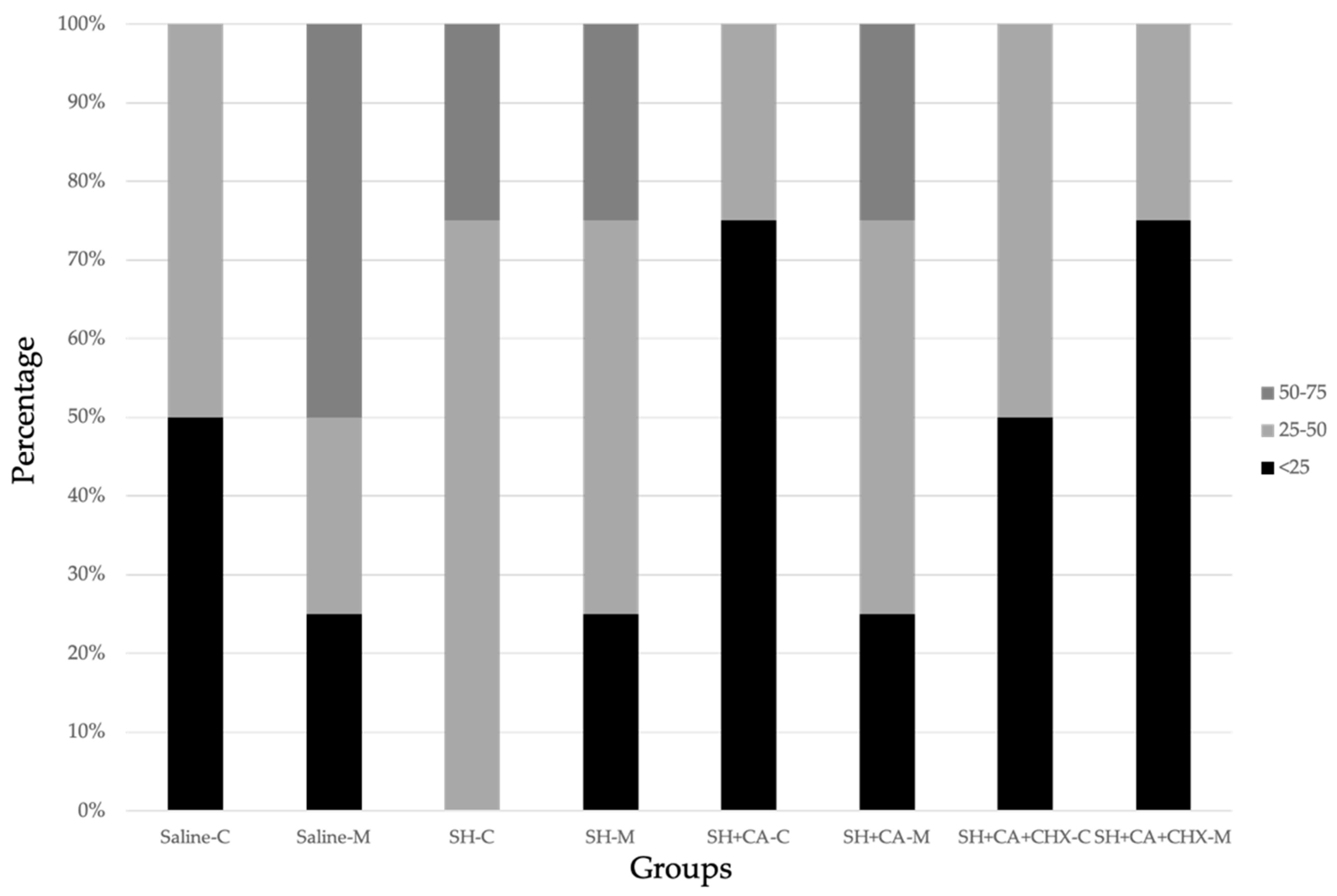
3.3. Nanoleakage Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tosun, S.; Karataslioglu, E.; Tulgar, M.M.; Derindag, G. Retrospective fractal analyses of one-year follow-up data obtained after single-visit nonsurgical endodontic retreatment on periapical radiographs. Clin. Oral. Investig. 2021, 25, 6465–6472. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.L.; Mann, V.; Gulabivala, K. A prospective study of the factors affecting outcomes of nonsurgical root canal treatment: Part 1: Periapical health. Int. Endod. J. 2011, 44, 583–609. [Google Scholar] [CrossRef] [PubMed]
- Azim, A.A.; Griggs, J.A.; Huang, G.J. The Tennessee study: Factors affecting treatment outcome and healing time following nonsurgical root canal treatment. Int. Endod. J. 2016, 49, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Mazzitelli, C.; Papacchini, F.; Monticelli, F.; Toledano, M.; Ferrari, M. Effects of post surface treatments on the bond strength of self-adhesive cements. Am. J. Dent. 2012, 25, 159–164. [Google Scholar]
- Goracci, C.; Ferrari, M. Current perspectives on post systems: A literature review. Aust. Dent. J. 2011, 56, 77–83. [Google Scholar] [CrossRef]
- Vichi, A.; Grandini, S.; Ferrari, M. Comparison between two clinical procedures for bonding fiber posts into a root canal: A microscopic investigation. J. Endod. 2002, 28, 355–360. [Google Scholar] [CrossRef]
- Pirani, C.; Chersoni, S.; Foschi, F.; Piana, G.; Loushine, R.J.; Tay, F.R.; Prati, C. Does hybridization of intraradicular dentin really improve fiber post retention in endodontically treated teeth? J. Endod. 2005, 31, 891–894. [Google Scholar] [CrossRef]
- Carvalho, R.M.; Tjäderhane, L.; Manso, A.P.; Carrilho, M.R.; Carvalho, C.A.R. Dentin as a bonding substrate. Endod. Top. 2009, 21, 62–88. [Google Scholar] [CrossRef]
- Porter, A.E.; Nalla, R.K.; Minor, A.; Jinschek, J.R.; Kisielowski, C.; Radmilovic, V.; Kinney, J.H.; Tomsia, A.P.; Ritchie, R.O. A transmission electron microscopy study of mineralization in age-induced transparent dentin. Biomaterials 2005, 26, 7650–7660. [Google Scholar] [CrossRef]
- Reis, L.C.; Rôças, I.N.; Siqueira, J.F., Jr.; de Uzeda, M.; Lacerda, V.S.; Domingues, R.M.; Moraes, S.R.; Saraiva, R.M. Bacteremia after endodontic procedures in patients with heart disease: Culture and molecular analyses. J. Endod. 2016, 42, 1181–1185. [Google Scholar] [CrossRef]
- Haapasalo, M.; Endal, U.; Zandi, H.; Coil, J.M. Eradication of endodontic infection by instrumentation and irrigation solutions. Endod. Top. 2005, 10, 77–102. [Google Scholar] [CrossRef]
- Bohrer, T.C.; Fontana, P.E.; Rocha, R.O.; Kaizer, O.B. Post-space treatment influences the bond strength in endodontically treated teeth: A systematic review and meta-Analysis of in vitro studies. Oper. Dent. 2021, 46, 132–157. [Google Scholar] [CrossRef]
- Barreto, M.S.; Rosa, R.A.; Seballos, V.G.; Machado, E.; Valandro, L.F.; Kaizer, O.B.; Só, M.V.R.; Bier, C.A.S. Effect of intracanal irrigants on bond strength of fiber posts cemented with a self-adhesive resin cement. Oper. Dent. 2016, 41, 159–167. [Google Scholar] [CrossRef]
- Haapasalo, M.; Shen, Y.; Qian, W.; Gao, Y. Irrigation in endodontics. Dent. Clin. 2010, 54, 291–312. [Google Scholar] [CrossRef]
- Darcey, J.; Jawad, S.; Taylor, C.; Roudsari, R.V.; Hunter, M. Modern endodontic principles part 4: Irrigation. Dent. Update 2016, 43, 20–33. [Google Scholar] [CrossRef]
- Eldeniz, A.U.; Erdemir, A.; Belli, S. Effect of EDTA and citric acid solutions on the microhardness and the roughness of human root canal dentin. J. Endod. 2005, 31, 107–110. [Google Scholar] [CrossRef]
- Marending, M.; Paque, F.; Fischer, J.; Zehnder, M. Impact of irrigant sequence on mechanical properties of human root dentin. J. Endod. 2007, 33, 1325–1328. [Google Scholar] [CrossRef]
- Dotto, L.; Onofre, R.S.; Bacchi, A.; Pereira, G.K. Effect of root canal irrigants on the mechanical properties of endodontically treated teeth: A scoping review. J. Endod. 2020, 46, 596–604. [Google Scholar] [CrossRef]
- Zhang, K.; Kim, Y.K.; Cadenaro, M.; Bryan, T.E.; Sidow, S.J.; Loushine, R.J.; Ling, J.Q.; Pashley, D.H.; Tay, F.R. Effects of different exposure times and concentrations of sodium hypochlorite/ethylenediaminetetraacetic acid on the structural integrity of mineralized dentin. J. Endod. 2010, 36, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.M.; Calixto, A.M.; e Lima, C.N.; Pappen, F.G.; De-Deus, G. Similar influence of stabilized alkaline and neutral sodium hypochlorite solutions on the fracture resistance of root canal–treated bovine teeth. J. Endod. 2014, 40, 1600–1603. [Google Scholar] [CrossRef]
- Sim, T.P.; Knowles, J.C.; Ng, Y.L.; Shelton, J.; Gulabivala, K. Effect of sodium hypochlorite on mechanical properties of dentine and tooth surface strain. Int. Endod. J. 2001, 34, 120–132. [Google Scholar] [CrossRef]
- Şen, B.H.; Ertürk, Ö.; Pişkin, B. The effect of different concentrations of EDTA on instrumented root canal walls. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2009, 108, 622–627. [Google Scholar] [CrossRef]
- Kishen, A.; Sum, C.P.; Mathew, S.; Lim, C.T. Influence of irrigation regimens on the adherence of Enterococcus faecalis to root canal dentin. J. Endod. 2008, 34, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, P.; Varughese, A.A.; Sharma, S.; Subbarao, C.V.; Zehnder, M.; De-Deus, G. Continuous chelation irrigation improves the adhesion of epoxy resin-based root canal sealer to root dentine. Int. Endod. J. 2012, 45, 1097–1102. [Google Scholar] [CrossRef]
- Saleh, A.A.; Ettman, W.M. Effect of endodontic irrigation solutions on microhardness of root canal dentine. J. Dent. 1999, 27, 43–46. [Google Scholar] [CrossRef]
- Dutner, J.; Mines, P.; Anderson, A. Irrigation trends among American Association of Endodontists members: A web-based survey. J. Endod. 2012, 38, 37–40. [Google Scholar] [CrossRef]
- Ferrari, M.; Mason, P.N.; Goracci, C.; Pashley, D.H.; Tay, F.R. Collagen degradation in endodontically treated teeth after clinical function. J. Dent. Res. 2004, 83, 414–419. [Google Scholar] [CrossRef]
- Nishitani, Y.; Yoshiyama, M.; Wadgaonkar, B.; Breschi, L.; Mannello, F.; Mazzoni, A.; Carvalho, R.M.; Tjäderhane, L.; Tay, F.R.; Pashley, D.H. Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur. J. Oral. Sci. 2006, 114, 160–166. [Google Scholar] [CrossRef]
- Perdigão, J.; Reis, A.; Loguercio, A.D. Dentin adhesion and MMPs: A comprehensive review. J. Esthet. Restor. Dent. 2013, 25, 219–241. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H.; Loushine, R.J.; Doyle, M.D.; Gillespie, W.T.; Weller, R.N.; King, N.M. Ultrastructure of smear layer-covered intraradicular dentin after irrigation with BioPure MTAD. J. Endod. 2006, 32, 218–221. [Google Scholar] [CrossRef]
- Santos, J.; Carrilho, M.; Tervahartiala, T.; Sorsa, T.; Breschi, L.; Mazzoni, A.; Pashley, D.; Tay, F.; Ferraz, C.; Tjäderhane, L. Determination of matrix metalloproteinases in human radicular dentin. J. Endod. 2009, 35, 686–689. [Google Scholar] [CrossRef]
- Toledano, M.; Nieto-Aguilar, R.; Osorio, R.; Campos, A.; Osorio, E.; Tay, F.R.; Alaminos, M. Differential expression of matrix metalloproteinase-2 in human coronal and radicular sound and carious dentine. J. Dent. 2010, 38, 635–640. [Google Scholar] [CrossRef]
- Retana-Lobo, C.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M.; de Souza, B.D.; Reyes-Carmona, J. Sodium Hypochlorite and Chlorhexidine Downregulate MMP Expression on Radicular Dentin. Med. Princ. Pract. 2021, 30, 301–307. [Google Scholar] [CrossRef]
- Saber, S.E.; Hashem, A.A. Efficacy of different final irrigation activation techniques on smear layer removal. J. Endod. 2011, 37, 1272–1275. [Google Scholar] [CrossRef]
- Mazzoni, A.; Nascimento, F.D.; Carrilho, M.; Tersariol, I.; Papa, V.; Tjäderhane, L.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; Breschi, L. MMP activity in the hybrid layer detected with in situ zymography. J. Dent. Res. 2012, 91, 467–472. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H.; Suh, B.I.; Carvalho, R.M.; Itthagarun, A. Single-step adhesives are permeable membranes. J. Dent. 2002, 30, 371–382. [Google Scholar] [CrossRef]
- Saboia, V.; Nato, F.; Mazzoni, A.; Orsini, G.; Putignano, A.; Giannini, M.; Breschi, L. Adhesion of a two-step etch-and-rinse adhesive on collagen-depleted dentin. J. Adhes. Dent. 2008, 10, 419–422. [Google Scholar]
- Kalkan, M.; Usumez, A.; Ozturk, A.N.; Belli, S.; Eskitascioglu, G. Bond strength between root dentin and three glass-fiber post systems. J. Prosthet. Dent. 2006, 96, 41–46. [Google Scholar] [CrossRef]
- Kinney, J.H.; Nalla, R.K.; Pople, J.A.; Breunig, T.M.; Ritchie, R.O. Age-related transparent root dentin: Mineral concentration, crystallite size, and mechanical properties. Biomaterials 2005, 26, 3363–3376. [Google Scholar] [CrossRef] [PubMed]
- Breschi, L.; Maravic, T.; Cunha, S.R.; Comba, A.; Cadenaro, M.; Tjäderhane, L.; Pashley, D.H.; Tay, F.R.; Mazzoni, A. Dentin bonding systems: From dentin collagen structure to bond preservation and clinical applications. Dent. Mater. 2018, 34, 78–96. [Google Scholar] [CrossRef]
- Elnaghy, A.M. Effect of QMix irrigant on bond strength of glass fibre posts to root dentine. Int. Endod. J. 2014, 47, 280–289. [Google Scholar] [CrossRef]
- Ari, H.; Erdemir, A.; Belli, S. Evaluation of the effect of endodontic irrigation solutions on the microhardness and the roughness of root canal dentin. J. Endod. 2004, 30, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Basrani, B.; Haapasalo, M. Update on endodontic irrigating solutions. Endod. Top. 2012, 27, 74–102. [Google Scholar] [CrossRef]
- Carvalho, M.P.M.; Morari, V.H.C.; Susin, A.H.; Rocha, R.D.O.; Valandro, L.F.; Soares, F.Z.M. Endodontic irrigation protocols: Effects on bonding of adhesive systems to coronal enamel and dentin. J. Esthet. Restor. Dent. 2017, 29, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Carrilho, M.R.; Geraldeli, S.; Tay, F.; De Goes, M.F.; Carvalho, R.M.; Tjäderhane, L.; Reis, A.F.; Hebling, J.; Mazzoni, A.; Breschi, L.; et al. In vivo preservation of the hybrid layer by chlorhexidine. J. Dent. Res. 2007, 86, 529–533. [Google Scholar] [CrossRef]
- Cecchin, D.; de Almeida, J.F.; Gomes, B.P.; Zaia, A.A.; Ferraz, C.C. Influence of chlorhexidine and ethanol on the bond strength and durability of the adhesion of the fiber posts to root dentin using a total etching adhesive system. J. Endod. 2011, 37, 1310–1315. [Google Scholar] [CrossRef]
- Zheng, P.; Zaruba, M.; Attin, T.; Wiegand, A. Effect of different matrix metalloproteinase inhibitors on microtensile bond strength of an etch-and-rinse and a self-etching adhesive to dentin. Oper. Dent. 2015, 40, 80–86. [Google Scholar] [CrossRef]
- Maravić, T.; Comba, A.; Cunha, S.R.; Angeloni, V.; Cadenaro, M.; Visinitini, E.; Navarra, C.O.; Salgarello, S.; Breschi, L.; Mazzoni, A. Long-term bond strength and endogenous enzymatic activity of a chlorhexidine-containing commercially available adhesive. J. Dent. 2019, 84, 60–66. [Google Scholar] [CrossRef]
- Breschi, L.; Maravic, T.; Comba, A.; Cunha, S.R.; Loguercio, A.D.; Reis, A.; Hass, V.; Cadenaro, M.; Mancuso, E.; Mayer-Santos, E.; et al. Chlorhexidine preserves the hybrid layer in vitro after 10-years aging. Dent. Mater. 2020, 36, 672–680. [Google Scholar] [CrossRef]
- Kouvas, V.; Liolios, E.; Vassiliadis, I.; Parissis-Messimeris, S.; Boutsioukis, A. Influence of smear layer on depth of penetration of three endodontic sealers: An SEM study. Dent. Traumatol. 1998, 14, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Neto, M.D.; Passarinho-Neto, J.G.; Carvalho-Júnior, J.R.; Cruz-Filho, A.M.; Pécora, J.D.; Saquy, P.C. Evaluation of the effect of EDTA, EGTA and CDTA on dentin adhesiveness and microleakage with different root canal sealers. Braz. Dent. J. 2002, 13, 123–128. [Google Scholar] [CrossRef]
- Doğan, H.; Çalt, S. Effects of chelating agents and sodium hypochlorite on mineral content of root dentin. J. Endod. 2001, 27, 578–580. [Google Scholar] [CrossRef]
- Ari, H.; Erdemir, A. Effects of endodontic irrigation solutions on mineral content of root canal dentin using ICP-AES technique. J. Endod. 2005, 31, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Cobankara, F.K.; Erdogan, H.; Hamurcu, M. Effects of chelating agents on the mineral content of root canal dentin. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2011, 112, e149–e154. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.H.; Mao, C.Y.; Kern, M. Effect of different irrigation on smear layer removal after post space preparation. J. Endod. 2009, 35, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Perdigão, J.; Lopes, M.; Geraldeli, S.; Lopes, G.C.; Garcıa-Godoy, F. Effect of a sodium hypochlorite gel on dentin bonding. Dent. Mater. 2000, 16, 311–323. [Google Scholar] [CrossRef]
- Kupai, K.; Szucs, G.; Cseh, S.; Hajdu, I.; Csonka, C.; Csont, T.; Ferdinandy, P. Matrix metalloproteinase activity assays: Importance of zymography. J. Pharmacol. Toxicol. Methods 2010, 61, 205–209. [Google Scholar] [CrossRef]
- Mungall, B.A.; Pollitt, C.C. In situ zymography: Topographical considerations. J. Biochem. Biophys. Methods 2001, 47, 169–176. [Google Scholar] [CrossRef]

| Groups | Mean (SD) | Failure Mode (%) (AD/AP/C/M) | |
|---|---|---|---|
| Coronal | Middle | ||
| I (saline) | 5.19 (1.52) | 2.12 (0.67) | 40/20/10/30 |
| II (SH) | 8.64 (3.18) | 3.16 (0.49) | 20/10/20/50 |
| III (SH + CA) | 6.78 (3.46) | 3.43 (0.82) | 30/20/10/40 |
| IV (SH + CA + CHX) | 14.71 (3.22) | 4.93 (0.67) | 10/10/10/70 |
| Saline | SH | SH + CA | SH + CA + CHX | |||||
|---|---|---|---|---|---|---|---|---|
| Coronal | Middle | Coronal | Middle | Coronal | Middle | Coronal | Middle | |
| >75 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 50–75 | 0 | 50 | 25 | 25 | 0 | 25 | 0 | 0 |
| 25–50 | 50 | 25 | 75 | 50 | 25 | 50 | 50 | 25 |
| <25 | 50 | 25 | 0 | 25 | 75 | 25 | 50 | 75 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baruwa, A.O.; Mazzitelli, C.; Maravic, T.; Martins, J.N.R.; Mazzoni, A.; Ginjeira, A. In Situ Zymography Analysis of Matrix Metalloproteinases Activity Following Endodontic Irrigation Protocols and Correlation to Root Dentine Bond Strength. Polymers 2022, 14, 3567. https://doi.org/10.3390/polym14173567
Baruwa AO, Mazzitelli C, Maravic T, Martins JNR, Mazzoni A, Ginjeira A. In Situ Zymography Analysis of Matrix Metalloproteinases Activity Following Endodontic Irrigation Protocols and Correlation to Root Dentine Bond Strength. Polymers. 2022; 14(17):3567. https://doi.org/10.3390/polym14173567
Chicago/Turabian StyleBaruwa, Abayomi Omokeji, Claudia Mazzitelli, Tatjana Maravic, Jorge N. R. Martins, Annalisa Mazzoni, and António Ginjeira. 2022. "In Situ Zymography Analysis of Matrix Metalloproteinases Activity Following Endodontic Irrigation Protocols and Correlation to Root Dentine Bond Strength" Polymers 14, no. 17: 3567. https://doi.org/10.3390/polym14173567
APA StyleBaruwa, A. O., Mazzitelli, C., Maravic, T., Martins, J. N. R., Mazzoni, A., & Ginjeira, A. (2022). In Situ Zymography Analysis of Matrix Metalloproteinases Activity Following Endodontic Irrigation Protocols and Correlation to Root Dentine Bond Strength. Polymers, 14(17), 3567. https://doi.org/10.3390/polym14173567












