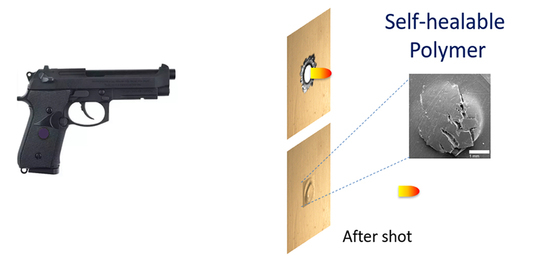
Self-Healability of Poly(Ethylene-co-Methacrylic Acid): Effect of Ionic Content and Neutralization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
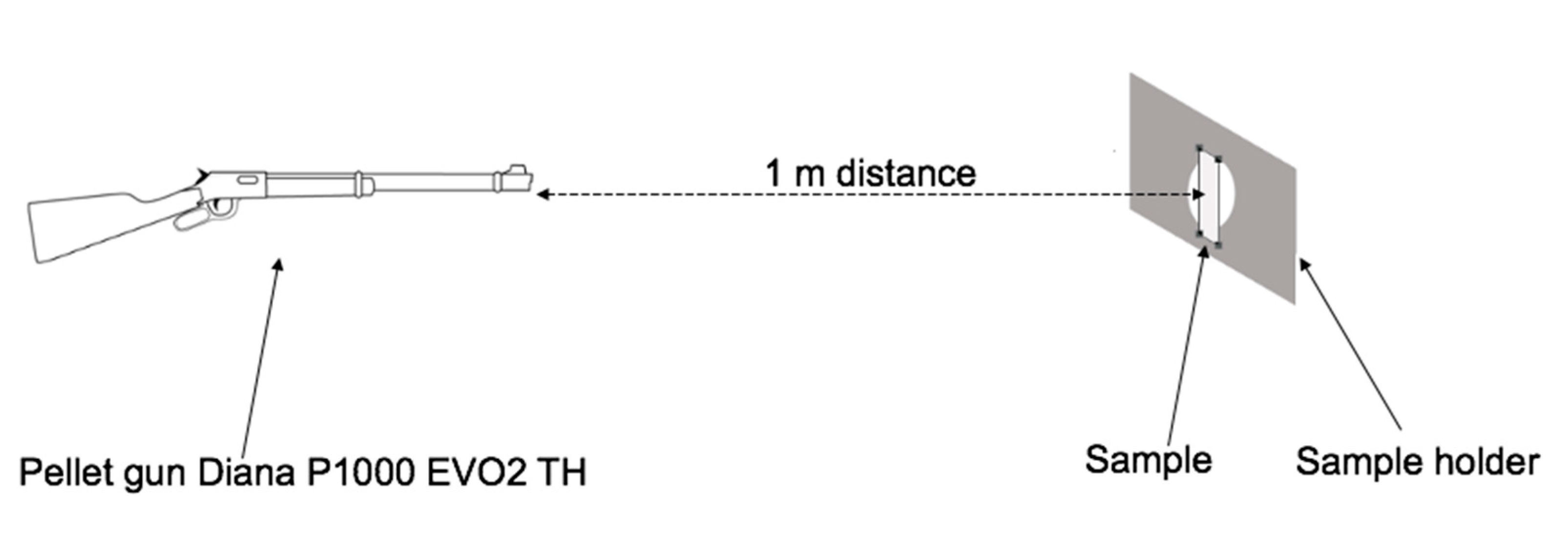
2.3. Methods
3. Results and Discussion
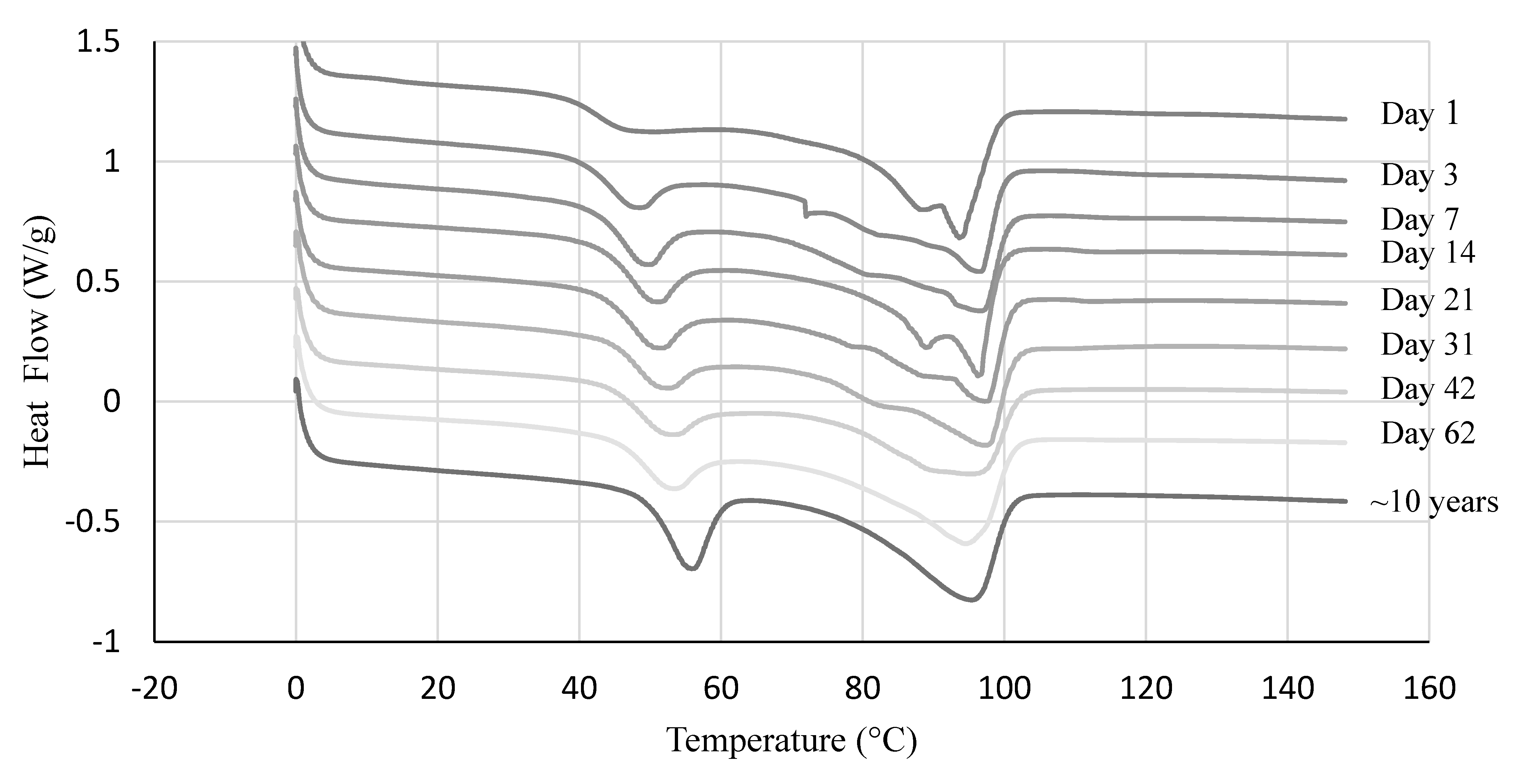
3.1. Effect of Aging on Thermal and Mechanical Properties
3.2. Thermal Properties
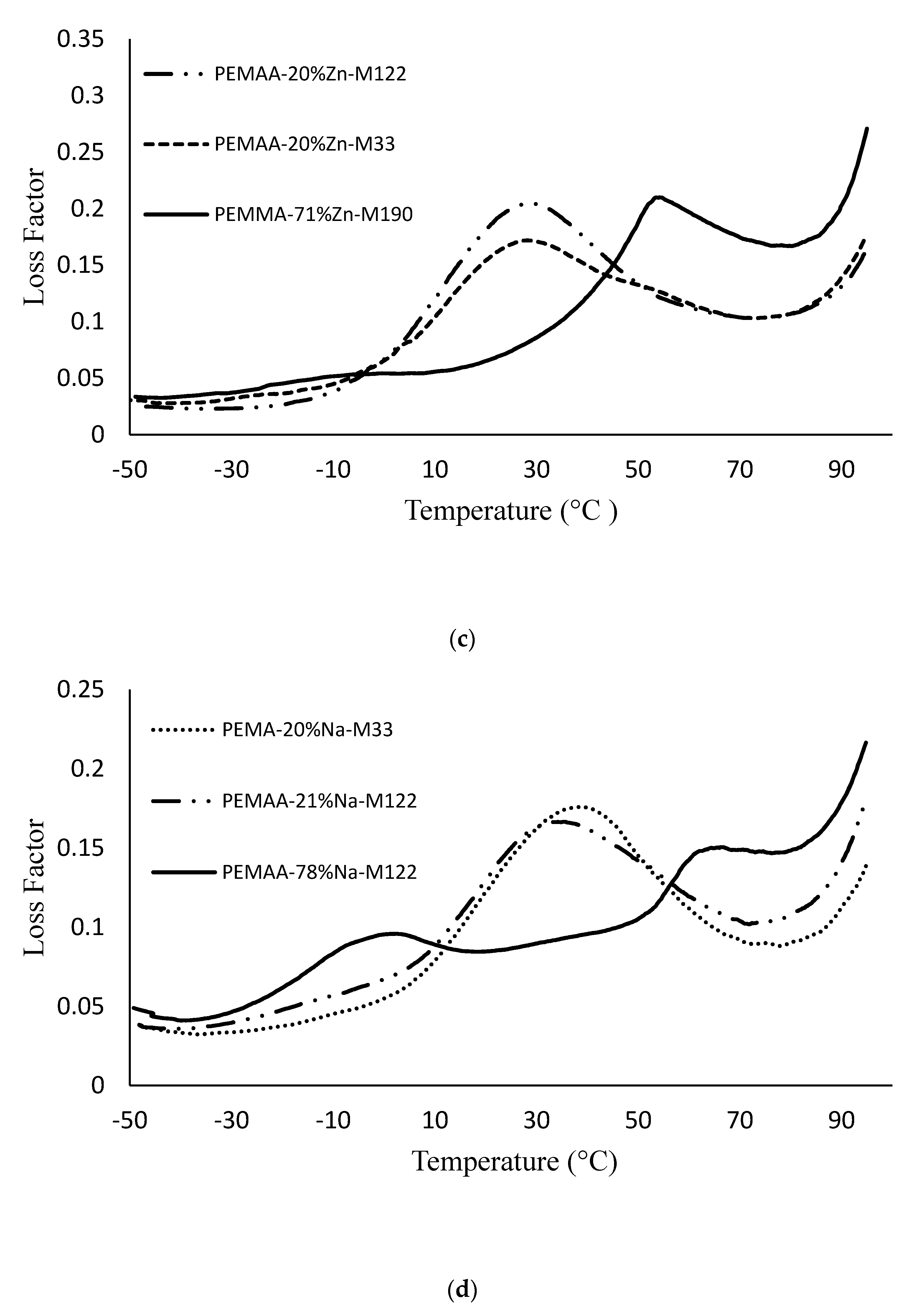
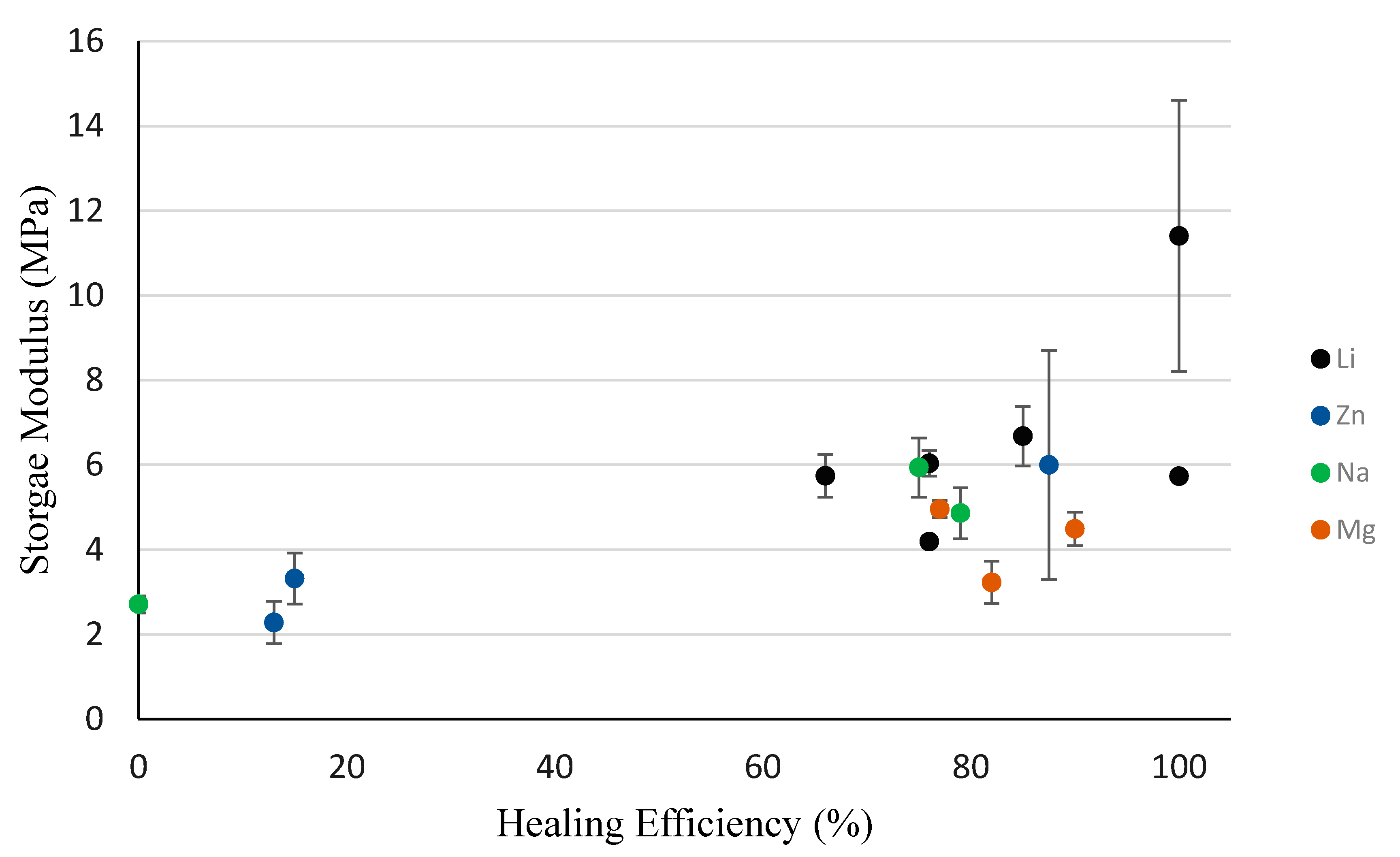
3.3. DMA Results
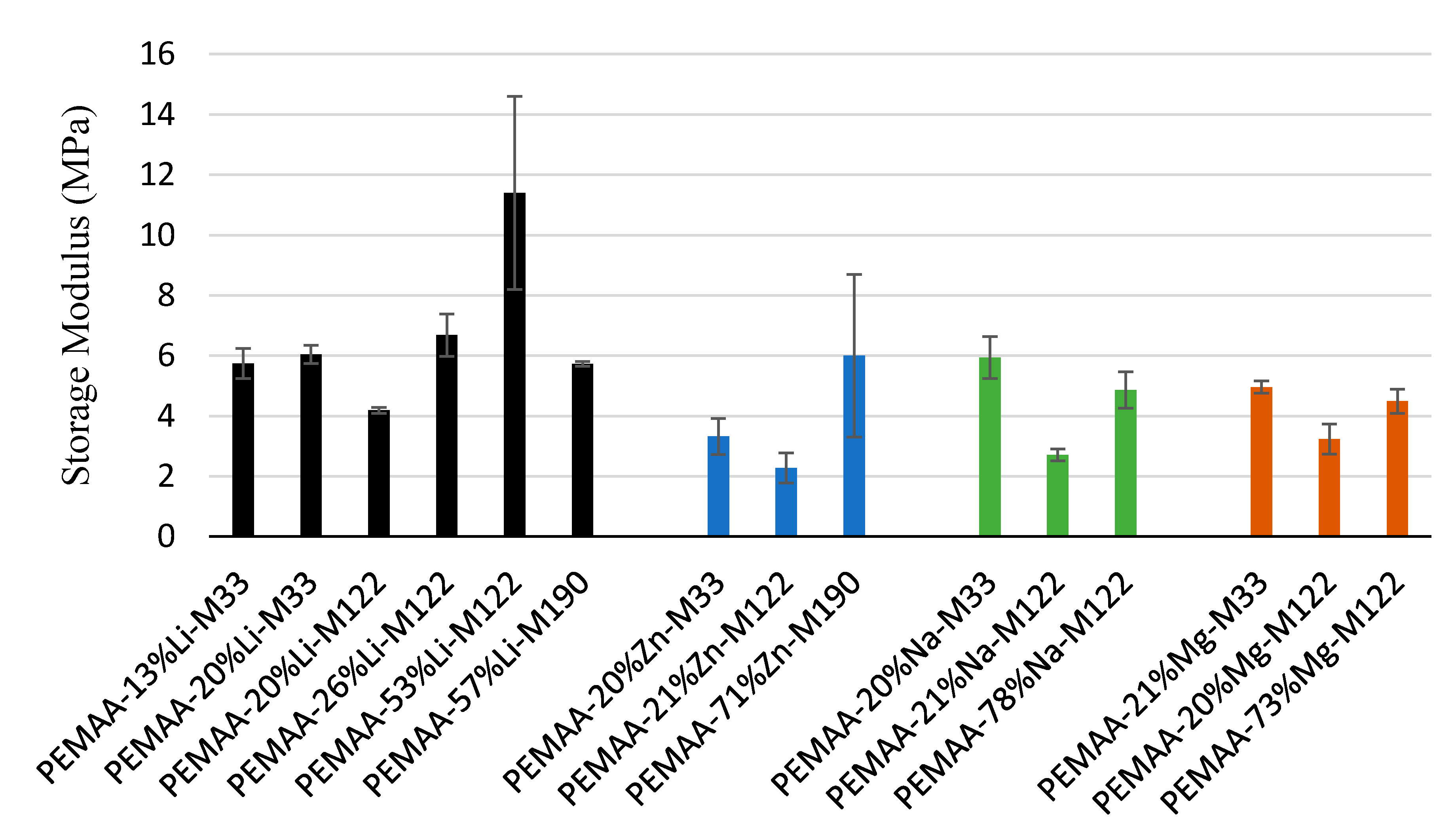
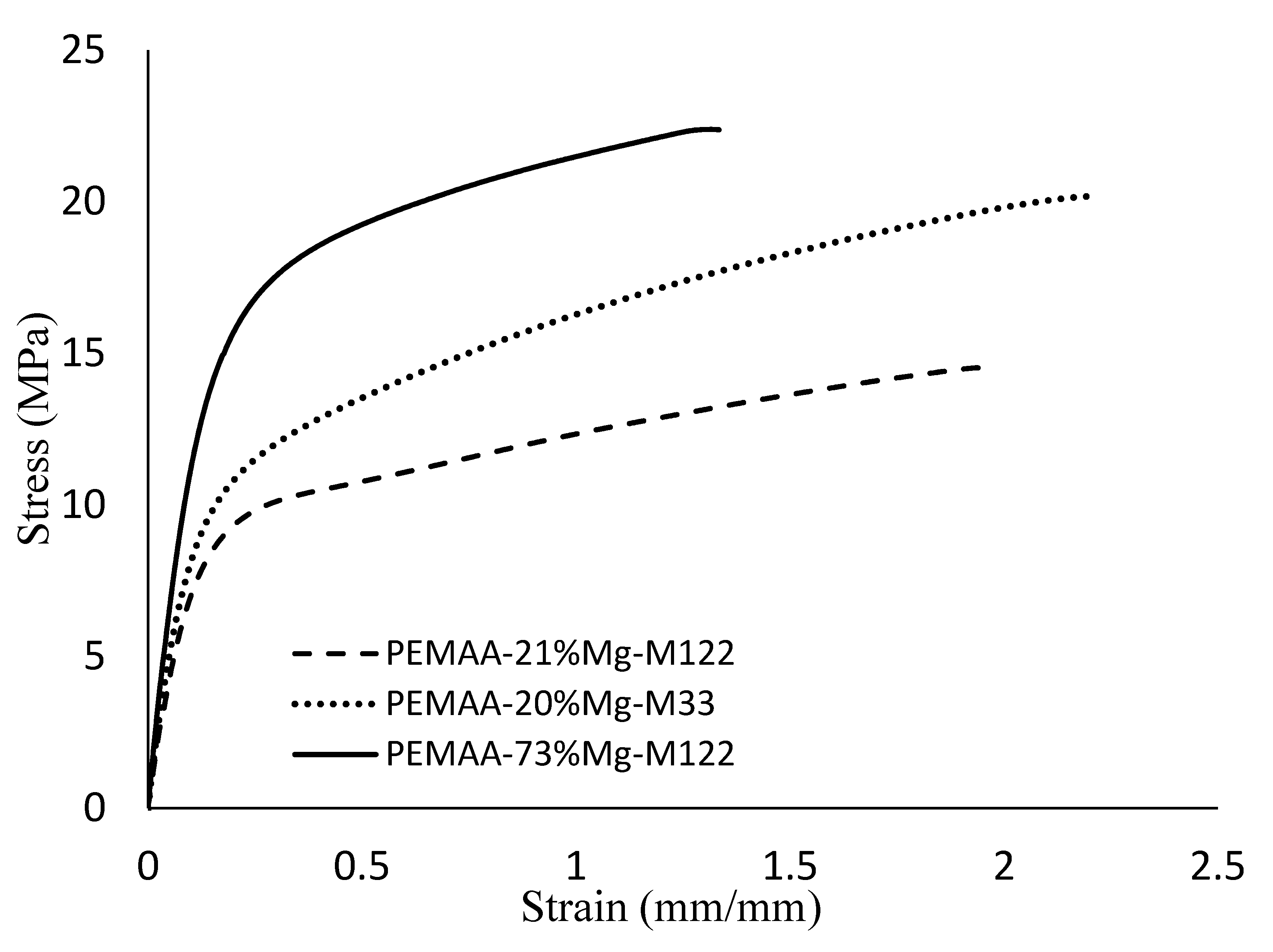
3.4. Mechanical Properties
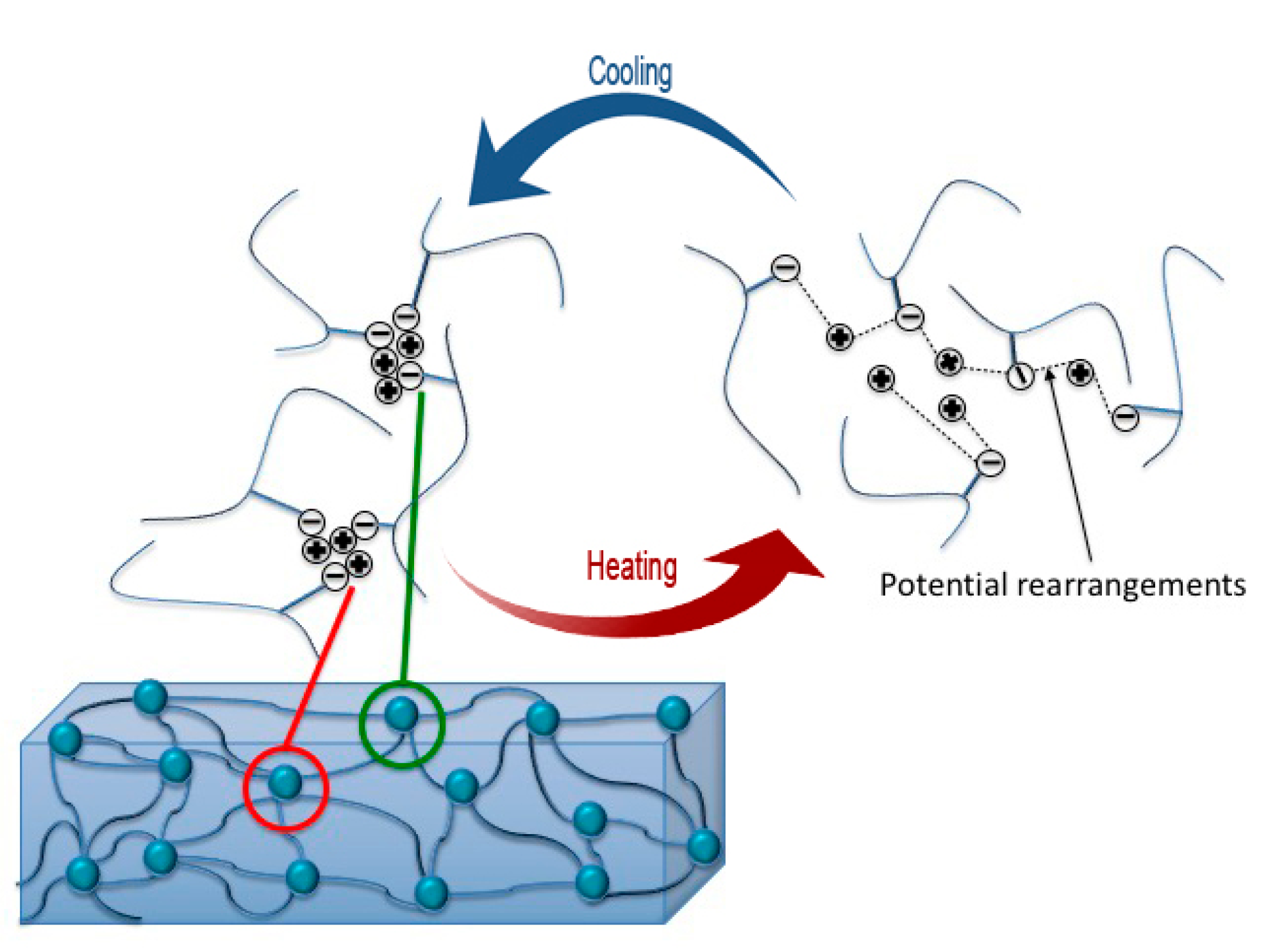
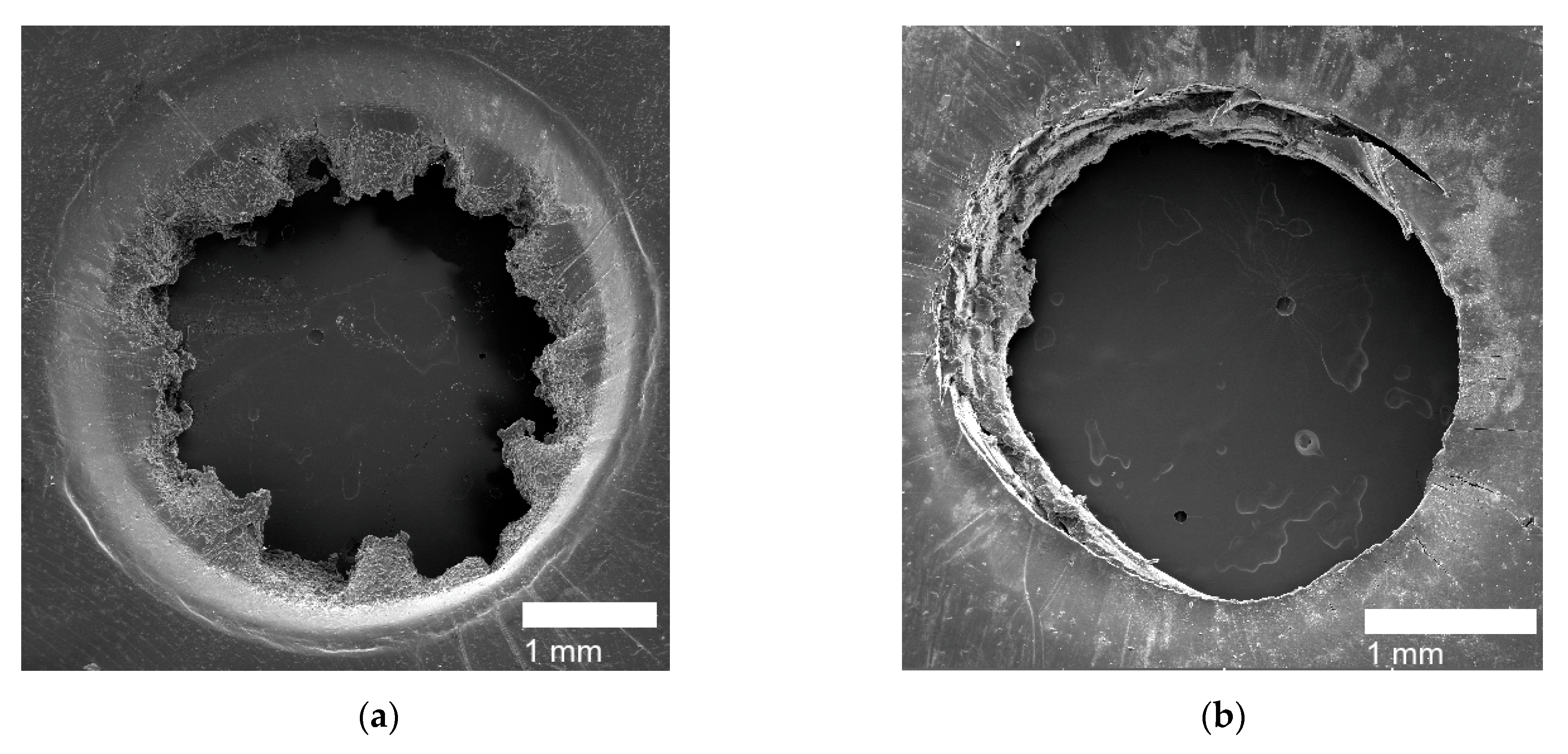
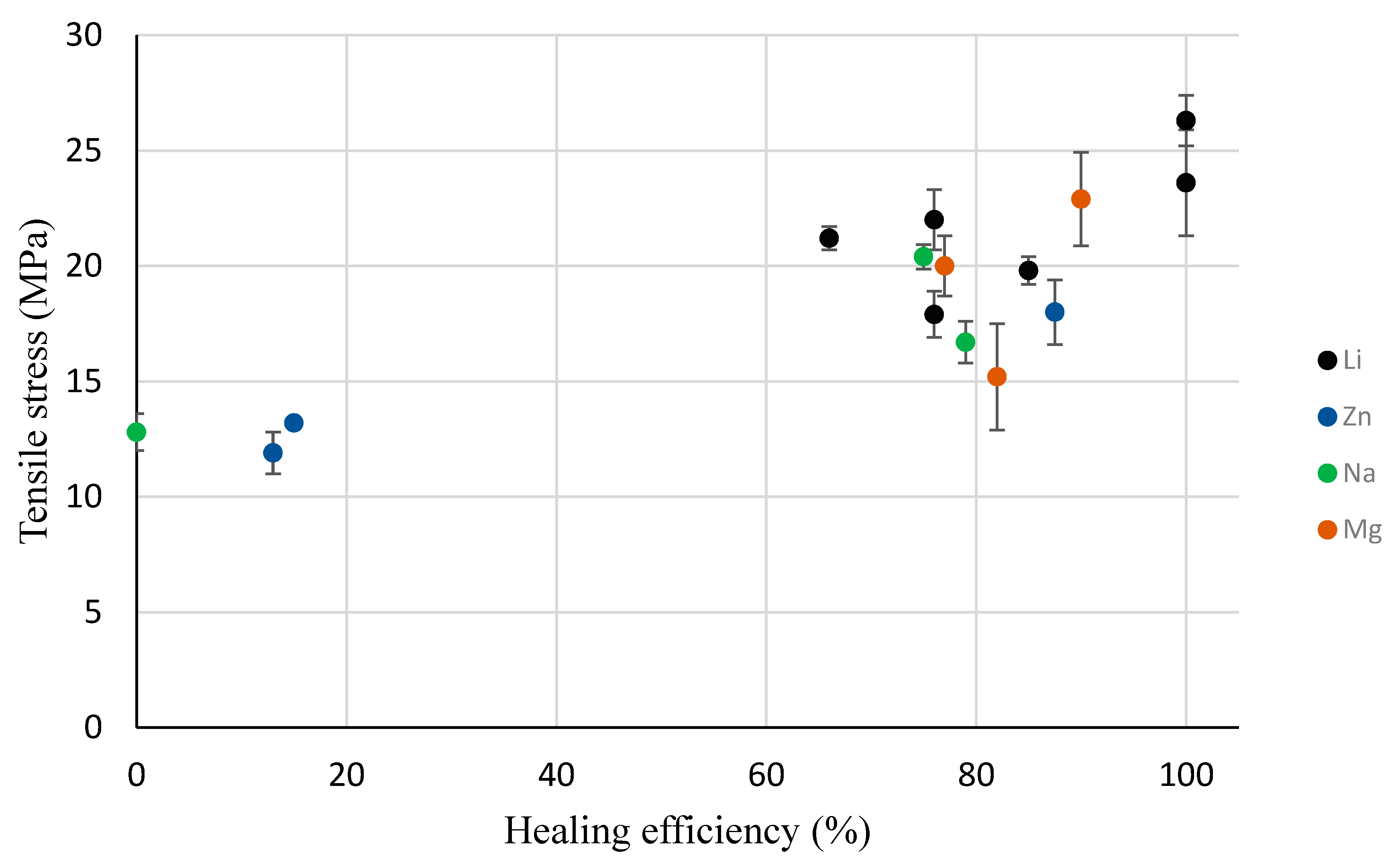
3.5. Self-healing Response and Analysis
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blaiszik, B.J.; Kramer, S.L.B.; Olugebefola, S.C.; Moore, J.S.; Sottos, N.R.; White, S.R. Self-Healing Polymers and Composites. Annu. Rev. Mater. Res. 2010, 40, 179–211. [Google Scholar] [CrossRef]
- Kumar Banshiwal, J.; Nath Tripathi, D. Self-Healing Polymer Composites for Structural Application. In Functional Materials; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Jackson, A.C.; Bartelt, J.A.; Marczewski, K.; Sottos, N.R.; Braun, P.V. Silica-Protected Micron and Sub-Micron Capsules and Particles for Self-Healing at the Microscale. Macromol. Rapid Commun. 2011, 32, 82. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; El-Zein, A.; Airey, D.W.; Alonso-Marroquin, F.; Schubel, P.; Manalo, A. Self-healing polymers: Synthesis methods and applications. Nano-Struct. Nano-Objects 2020, 23, 100500. [Google Scholar] [CrossRef]
- Wu, K.; Chen, Y.; Luo, J.; Liu, R.; Sun, G.; Liu, X.J. Preparation of dual-chamber microcapsule by Pickering emulsion for self-healing application with ultra-high healing efficiency. Colloid Interface Sci. 2021, 600, 660. [Google Scholar] [CrossRef] [PubMed]
- Dahlke, J.; Kimmig, J.; Abend, M.; Zechel, S.; Vitz, J.; Schubert, U.S.; Hager, M.D. Quantification of the scratch-healing efficiency for novel zwitterionic polymers. NPG Asia Mater. 2020, 12, 1. [Google Scholar] [CrossRef]
- Purohit, R.; Gupta, N.K.; Purohit, M.R.; Patil, A.; Bharilya, R.K.; Singh, S.K. An Investigation on Manufacturing of Self-Healing Materials. In Materials Today: Proceedings; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; pp. 3371–3377. [Google Scholar] [CrossRef]
- Karami, Z.; Zolghadr, M.; Zohuriaan-Mehr, M.J. Self-healing Diels–Alder engineered thermosets. In Self-Healing Polymer-Based Systems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 209–233. [Google Scholar] [CrossRef]
- Cui, J.; Li, X.; Pei, Z.; Pei, Y. A long-term stable and environmental friendly self-healing coating with polyaniline/sodium alginate microcapsule structure for corrosion protection of water-delivery pipelines. Chem. Eng. J. 2019, 358, 379. [Google Scholar] [CrossRef]
- Padhan, A.K.; Mandal, D. Types of chemistries involved in self-healing polymeric systems. Self-Heal. Polym.-Based Syst. 2020, 17–73. [Google Scholar] [CrossRef]
- Hia, I.L.; Vahedi, V.; Pasbakhsh, P. Self-Healing Polymer Composites: Prospects, Challenges, and Applications. Polym. Rev. 2016, 56, 225. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Rong, M.Z.; Zhang, M.Q. Self-healing polymeric materials based on microencapsulated healing agents: From design to preparation. Prog. Polym. Sci. 2015, 49–50, 175. [Google Scholar] [CrossRef]
- Kosarli, M.; Bekas, D.G.; Tsirka, K.; Baltzis, D.; Vaimakis-Tsogkas, D.; Orfanidis, S.; Papavassiliou, G.; Paipetis, A.S. Microcapsule-based self-healing materials: Healing efficiency and toughness reduction vs. capsule size. Compos. Part B Eng. 2019, 171, 78–86. [Google Scholar] [CrossRef]
- McDonald, S.A.; Coban, S.B.; Sottos, N.R.; Withers, P.J. Tracking capsule activation and crack healing in a microcapsule-based self-healing polymer. Sci. Rep. 2019, 9, 17773. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W. Prospects and Future Directions of Self-Healing Fiber-Reinforced Composite Materials. Polymers 2020, 12, 379. [Google Scholar] [CrossRef] [PubMed]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Van Tittelboom, K.; De Belie, N. Self-Healing in Cementitious Materials—A Review. Materials 2013, 6, 2182–2217. [Google Scholar] [CrossRef] [PubMed]
- Qamar, I.P.S.; Sottos, N.R.; Trask, R.S. Grand challenges in the design and manufacture of vascular self-healing. Multifunct. Mater. 2020, 3, 013001. [Google Scholar] [CrossRef]
- Cuvellier, A.; Torre-Muruzabal, A.; van Assche, G.; de Clerck, K.; Rahier, H. Selection of healing agents for a vascular self-healing application. Polym. Test. 2017, 62, 302. [Google Scholar] [CrossRef]
- Jony, B.; Thapa, M.; Mulani, S.B.; Roy, S. Repeatable self-healing of thermosetting fiber reinforced polymer composites with thermoplastic healant. Smart Mater. Struct. 2019, 28, 025037. [Google Scholar] [CrossRef]
- Garcia, S.J.; Fischer, H.R. Self-healing polymer systems: Properties, synthesis and applications. In Smart Polymers and their Applications. In Smart Polymers and Their Applications; Woodhead Publishing: Sawston, UK, 2014. [Google Scholar] [CrossRef]
- McKeen, L. Polyolefins, Polyvinyls, and Acrylics Permeability Properties of Plastics and Elastomers; Elsevier Ltd.: Amsterdam, The Netherlands, 2012; pp. 145–193. [Google Scholar] [CrossRef]
- Eisenberg, A.; Hird, B.; Moore, R.B. A new multiplet-cluster model for the morphology of random ionomers. Macromolecules 1990, 23, 4098. [Google Scholar] [CrossRef]
- Eisenberg, A. Clustering of Ions in Organic Polymers. A Theoretical Approach. Macromolecules 1970, 3, 147–154. [Google Scholar] [CrossRef]
- Capek, I. Dispersions of polymer ionomers: I. Adv. Colloid Interface Sci. 2004, 112, 1. [Google Scholar] [CrossRef]
- Mora-Barrantes, I.; Malmierca, M.A.; Valentin, J.L.; Rodriguez, A.; Ibarra, L. Effect of covalent cross-links on the network structure of thermo-reversible ionic elastomers. Soft Matter 2012, 8, 5201. [Google Scholar] [CrossRef]
- Kalista, S.J., Jr.; Ward, T.C. Thermal characteristics of the self-healing response in poly(ethylene-co-methacrylic acid) copolymers. J. R. Soc. Interface 2007, 4, 405. [Google Scholar] [CrossRef] [PubMed]
- Kalista, S.J.; Ward, T.C.; Oyetunji, Z. Self-healing of poly(ethylene-co-methacrylic acid) copolymers following projectile puncture. Mech. Adv. Mater. Struct. 2007, 14, 391. [Google Scholar] [CrossRef]
- Pestka, K.A.; Buckley, J.D.; Kalista, S.J.; Bowers, N.R. Elastic evolution of a self-healing ionomer observed via acoustic and ultrasonic resonant spectroscopy. Sci. Rep. 2017, 7, 1. [Google Scholar] [CrossRef]
- Varley, R.J.; van der Zwaag, S. Towards an understanding of thermally activated self-healing of an ionomer system during ballistic penetration. Acta Mater. 2008, 56, 5737–5750. [Google Scholar] [CrossRef]
- Grande, A.M.; Castelnovo, L.; di Landro, L.; Giacomuzzo, C.; Francesconi, A.; Rahman, M.A. Rate-dependent self-healing behavior of an ethylene-co-methacrylic acid ionomer under high-energy impact conditions. J. Appl. Polym. Sci. 2013, 130, 1949–1958. [Google Scholar] [CrossRef]
- Spencer, M.W.; Wetzel, M.D.; Troeltzsch, C.; Paul, D.R. Effects of acid neutralization on the properties of Kand Napoly(ethylene-co-methacrylic acid) ionomers. Polymer 2012, 53, 569. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, R.; Ahuja, I.P.S. Use of Thermosetting Polymers for Smart Civil Structures. Ref. Modul. Mater. Sci. Mater. Eng. 2020. [Google Scholar] [CrossRef]
- Gu, B.; Burgess, D.J. Polymeric Materials in Drug Delivery. Nat. Synth. Biomed. Polym. 2014, 333–349. [Google Scholar] [CrossRef]
- Loo, Y.L.; Wakabayashi, K.; Huang, Y.E.; Register, R.A.; Hsiao, B.S. Thin crystal melting produces the low-temperature endotherm in ethylene/methacrylic acid ionomers. Polymer 2005, 46, 5118. [Google Scholar] [CrossRef]
- Kulkarni, H.P.; Mogilevsky, G.; Mullins, W.M.; Wu, Y. Mechanism of aging effects on viscoelasticity in ethylene-methacrylic acid ionomer studied by local thermal-mechanical analysis. J. Mater. Res. 2009, 24, 1087. [Google Scholar] [CrossRef]
- Rui, Y.; Grady, B.P. Long-time crystallization kinetics in zinc-neutralized ethylene–methacrylic acid ionomers. Thermochim. Acta 2013, 565, 183. [Google Scholar] [CrossRef]
- Zin, M.H.; Abdan, K.; Norizan, M.N. The effect of different fiber loading on flexural and thermal properties of banana/pineapple leaf (PALF)/glass hybrid composite. In Structural Health Monitoring of Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Woodhead Publishing: Cambridge, UK, 2018; pp. 1–17. [Google Scholar] [CrossRef]
- Kalista, S.J.; Pflug, J.R.; Varley, R.J. Effect of ionic content on ballistic self-healing in EMAA copolymers and ionomers. Polym. Chem. 2013, 4, 4910. [Google Scholar] [CrossRef]
- Giles, H.F.; Wagner, J.R.; Mount, E.M. Testing Properties. Extrusion 2005, 195–205. [Google Scholar] [CrossRef]
- Tadano, K.; Hirasawa, E.; Yamamoto, H.; Yano, S. Order-disorder transition of ionic clusters in ionomers. Macromolecules 1989, 22, 226–233. [Google Scholar] [CrossRef]
- Kutsumizu, S.; Tadano, K.; Matsuda, Y.; Goto, M.; Tachino, H.; Hara, H.; Hirasawa, E.; Tagawa, H.; Muroga, Y.; Yano, S. Investigation of Microphase Separation and Thermal Properties of Noncrystalline Ethylene Ionomers. 2. IR, DSC, and Dielectric Characterization. Macromolecules 2000, 33, 9044. [Google Scholar] [CrossRef]
- Zuliki, M.; Zhang, S.; Nyamajaro, K.; Tomkovic, T.; Hatzikiriakos, S.G. Rheology of sodium and zinc ionomers: Effects of neutralization and valency. Phys. Fluids 2020, 32, 023104. [Google Scholar] [CrossRef]
- Najm, M.; Yavitt, B.M.; Hatzikiriakos, S.G. Synergistic ionic interactions in EMAA ionomer blends: A rheological and mechanical property investigation. J. Rheol. 2021, 65, 1373. [Google Scholar] [CrossRef]
- Ahmed, T.J.; Stavrov, D.; Bersee, H.E.N.; Beukers, A. Induction welding of thermoplastic composites—An overview. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1638. [Google Scholar] [CrossRef]






| Sample Name | Base Melt Index (g/10 min) | Ion Type | Neutralized % |
|---|---|---|---|
| PEMAA-13%Li-M33 | 33 | Li | 13.1% |
| PEMAA-20%Li-M33 | 33 | Li | 20.0% |
| PEMAA-20%Li-M122 | 122 | Li | 20.5% |
| PEMAA-26%Li-M122 | 122 | Li | 26.2% |
| PEMAA-53%Li-M122 | 122 | Li | 52.9% |
| PEMAA-57%Li-M190 | 190 | Li | 57.5% |
| PEMAA-20%Zn-M33 | 33 | Zn | 20.2% |
| PEMAA-21%Zn-M122 | 122 | Zn | 20.6% |
| PEMAA-71%Zn-M190 | 190 | Zn | 71.0% |
| PEMAA-20%Na-M33 | 33 | Na | 20.1% |
| PEMAA-21%Na-M122 | 122 | Na | 20.6% |
| PEMAA-78%Na-M122 | 122 | Na | 78.4% |
| PEMAA-21%Mg-M33 | 33 | Mg | 20.9% |
| PEMAA-20%Mg-M122 | 122 | Mg | 20.4% |
| PEMAA-73%Mg-M122 | 122 | Mg | 72.9% |
| PEMAA | |
|---|---|
| Melt Index (g/10 min) | 33, 122 (All), 190 (Zn, Li) |
| Mol % of the copolymer | E(96.35%), MAA(3.65%) (Low MI) E(96.4%), MAA(3.60%) (High MI) |
| Sample Name | Maximum Load [N] | Tensile Stress at Maximum Load [MPa] | Modulus [MPa] | Extension (%) |
|---|---|---|---|---|
| PEMAA-13%Li-M33 | 441 ± 18 | 21.2 ± 0.5 | 98 ± 2 | 2.60 ± 0.25 |
| PEMAA-20%Li-M33 | 459 ± 24.9 | 22.0 ± 1.3 | 124 ± 11 | 1.67 ± 0.27 |
| PEMAA-20%Li-M122 | 367 ± 14 | 17.9 ± 1.0 | 117 ± 3 | 1.88 ± 0.15 |
| PEMAA-26%Li-M122 | 413 ± 16 | 19.8 ± 0.6 | 111 ± 4 | 1.45 ± 0.13 |
| PEMAA-53%Li-M122 | 517 ± 27 | 26.3 ± 1.1 | 144 ± 9 | 1.15 ± 0.17 |
| PEMAA-57%Li-M190 | 484 ± 58 | 23.6 ± 2.3 | 143 ± 7 | 1.43 ± 0.08 |
| PEMAA-20%Zn-M33 | 279 ± 9 | 13.2 ± 0.2 | 67.5 ± 5.9 | 1.82 ± 0.07 |
| PEMAA-21%Zn-M122 | 235 ± 16 | 11.9 ± 0.9 | 69.1 ± 9.8 | 2.12 ± 0.37 |
| PEMAA-71%Zn-M190 | 368 ± 23 | 18.0 ± 1.4 | 200 ± 2 | 1.60 ± 0.33 |
| PEMAA-20%Na-M33 | 418 ± 13 | 20.4 ± 0.53 | 85.9 ± 8.4 | 2.02 ± 0.14 |
| PEMAA-21%Na-M122 | 250 ± 18 | 12.8 ± 0.8 | 102 ± 13 | 1.63 ± 0.21 |
| PEMAA-78%Na-M122 | 340 ± 27 | 16.7 ± 0.9 | 72.6 ± 6.8 | 1.51 ± 0.10 |
| PEMAA-21%Mg-M33 | 425 ± 21 | 20.0 ± 1.3 | 93.7 ± 11.4 | 2.45 ± 0.27 |
| PEMAA-20%Mg-M122 | 309 ± 33 | 15.2 ± 2.3 | 89.0 ± 10.8 | 2.10 ± 0.33 |
| PEMAA-73%Mg-M122 | 484 ± 50 | 22.9 ± 2.03 | 135 ± 3 | 1.44 ± 0.21 |
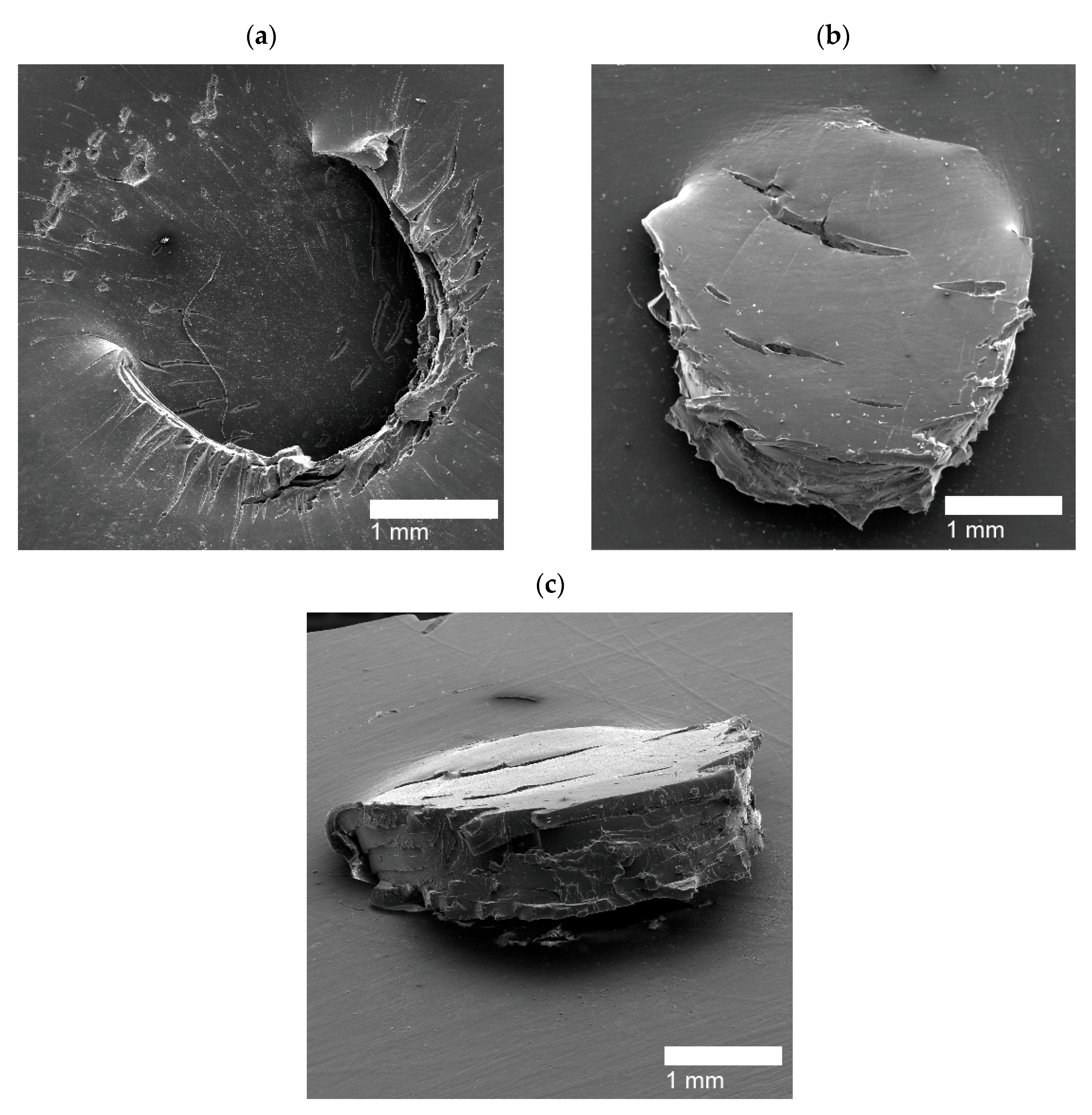
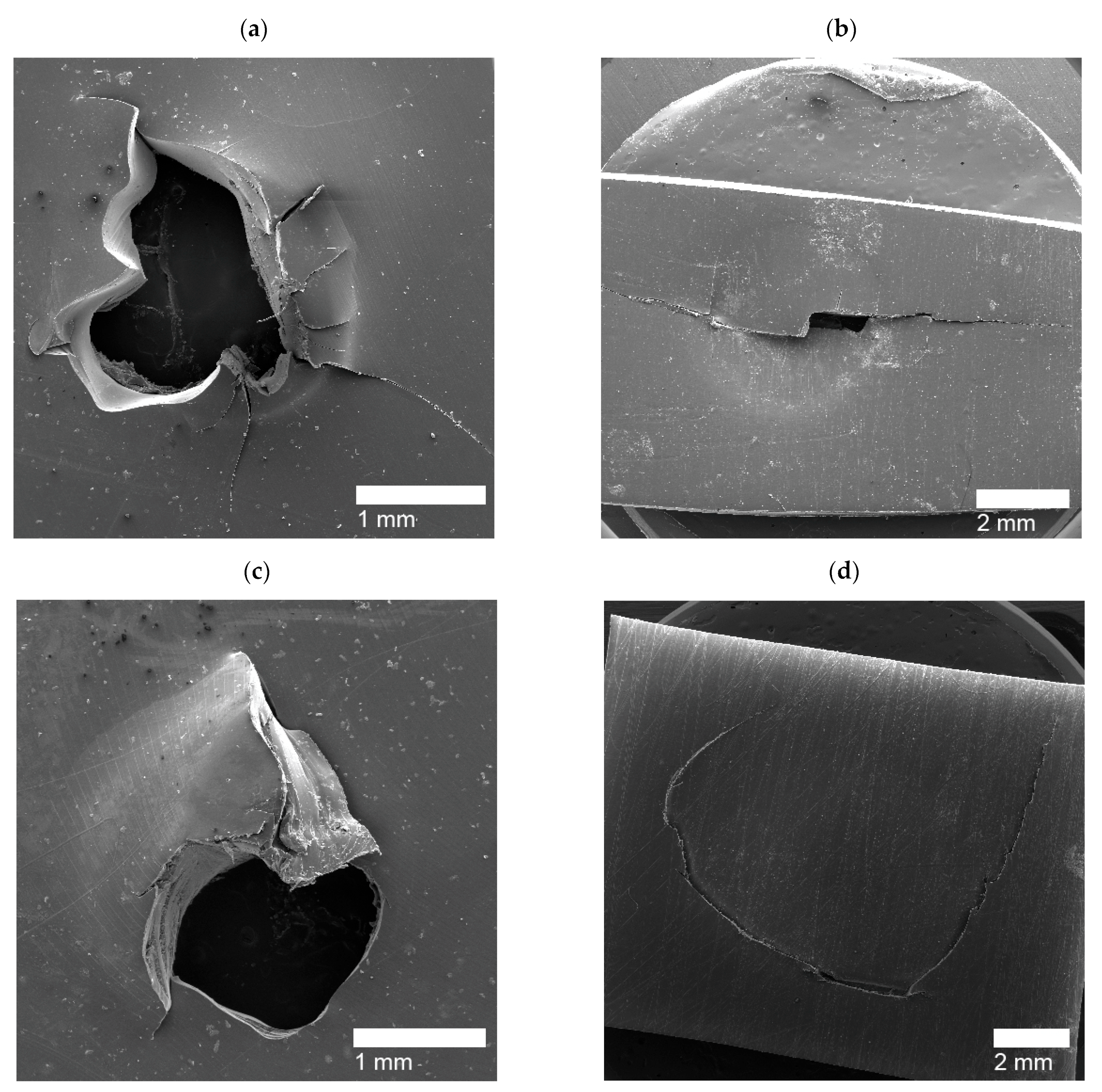
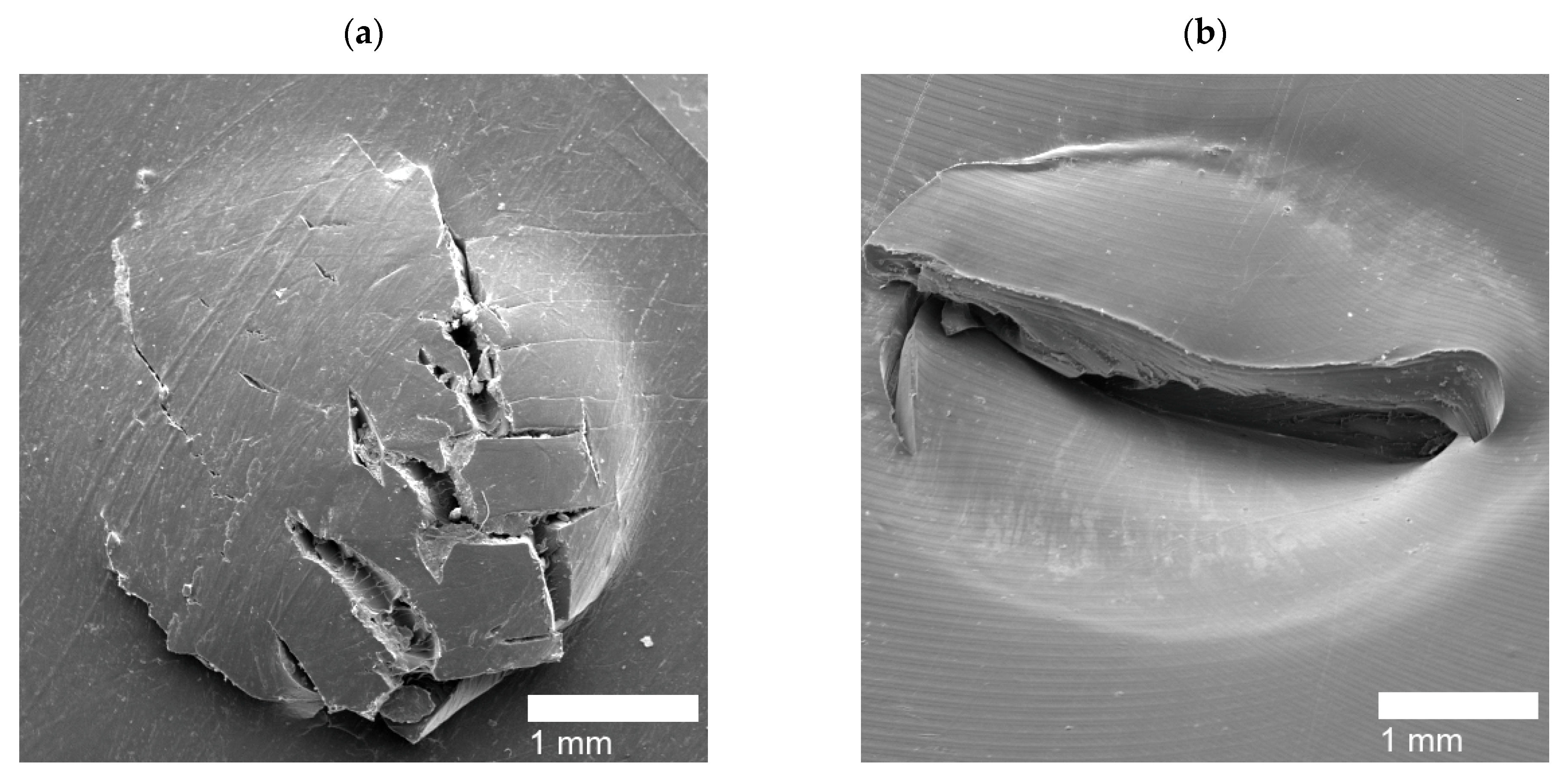
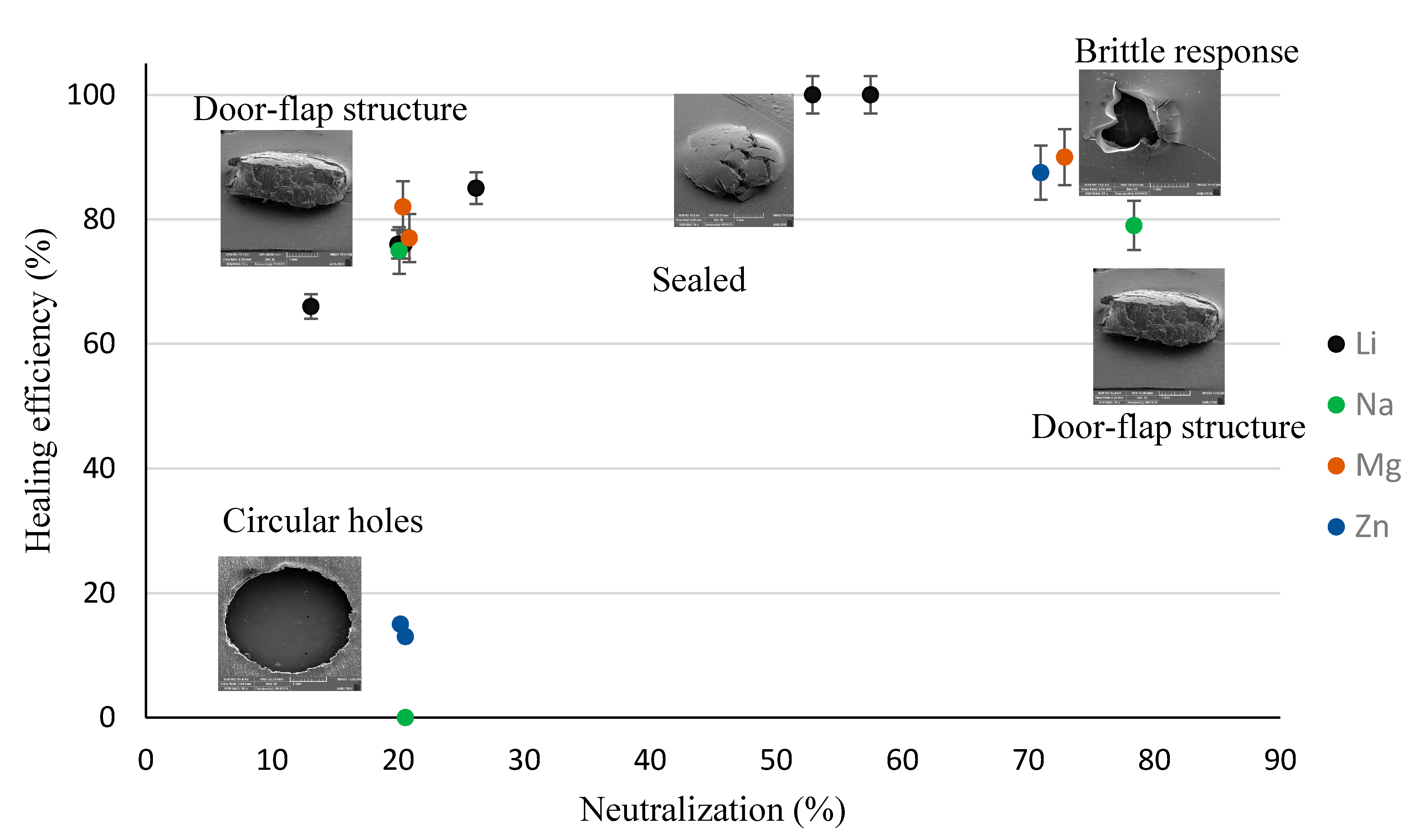
| Puncture Type | Sample Name |
|---|---|
| Circular Holes | PE, PEMAA-21%Na-M122, PEMAA-21%Zn-M122, PEMAA-20%Zn-M33 |
| Door-flap | PEMAA-13%Li-M33, PEMAA-20%Li-M33, PEMAA-20%Li-M122, PEMAA-26%Li-M122, PEMAA-20%Na-M33, PEMAA-78%Na-M122, PEMAA-21%Mg-M33, PEMAA-20%Mg-M122 |
| Brittle Holes | PEMAA-71%Zn-M190, PEMAA-73%Mg-M122 |
| Line Fracture | PEMAA-71%Zn-M190, PEMAA-57%Li-M190, PEMAA-73%Mg-M122 |
| Sealed site | PEMAA-53%Li-M122, PEMAA-57%Li-M190 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Choufi, N.; Mustapha, S.; Tehrani-Bagha, A.R.; Grady, B.P. Self-Healability of Poly(Ethylene-co-Methacrylic Acid): Effect of Ionic Content and Neutralization. Polymers 2022, 14, 3575. https://doi.org/10.3390/polym14173575
El Choufi N, Mustapha S, Tehrani-Bagha AR, Grady BP. Self-Healability of Poly(Ethylene-co-Methacrylic Acid): Effect of Ionic Content and Neutralization. Polymers. 2022; 14(17):3575. https://doi.org/10.3390/polym14173575
Chicago/Turabian StyleEl Choufi, Nadim, Samir Mustapha, Ali R. Tehrani-Bagha, and Brian P. Grady. 2022. "Self-Healability of Poly(Ethylene-co-Methacrylic Acid): Effect of Ionic Content and Neutralization" Polymers 14, no. 17: 3575. https://doi.org/10.3390/polym14173575
APA StyleEl Choufi, N., Mustapha, S., Tehrani-Bagha, A. R., & Grady, B. P. (2022). Self-Healability of Poly(Ethylene-co-Methacrylic Acid): Effect of Ionic Content and Neutralization. Polymers, 14(17), 3575. https://doi.org/10.3390/polym14173575