Anti-Colitic Effect of an Exopolysaccharide Fraction from Pediococcus pentosaceus KFT-18 on Dextran Sulfate Sodium-Induced Colitis through Suppression of Inflammatory Mediators
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. PE-EPS Preparation
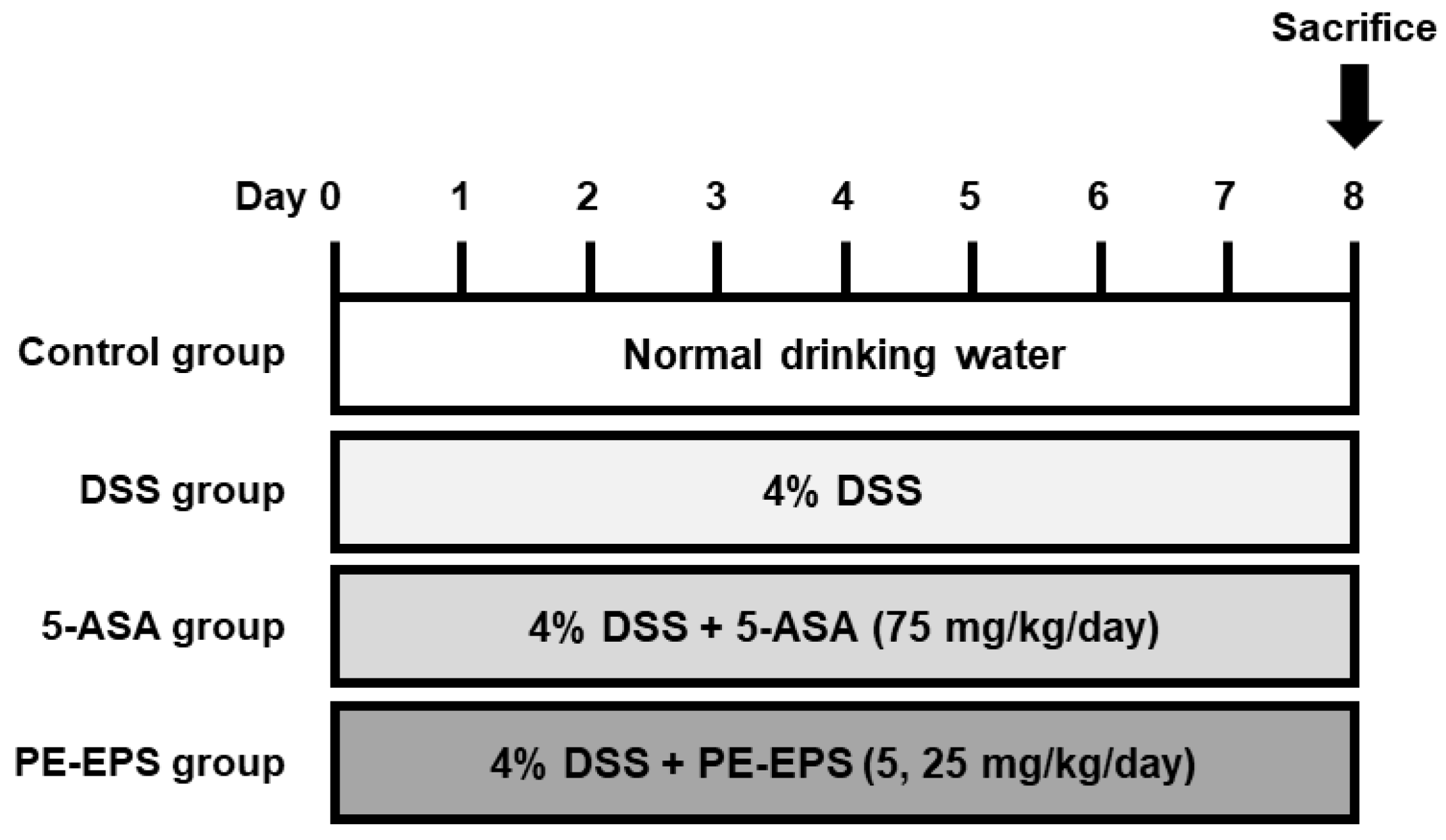
2.3. DSS-Induced Colitis Mouse
2.4. Hematoxylin and Eosin (H&E) Staining
2.5. Protein Extraction and Western Blot Analysis
2.6. Quantitative Real-Time Reverse-Transcriptase Polymerase Chain Reaction (qRT-PCR)
2.7. Statistical Analysis
3. Results
3.1. PE-EPS Restores Clinical Symptoms in DSS-Induced Colitis Mice
3.2. PE-EPS Decreases the mRNA and Protein Levels of iNOS and COX-2 in the DSS-Induced Colitis Mice
3.3. PE-EPS Suppresses Production of Pro-Inflammatory Cytokines of the DSS-Induced Colitis Mice
3.4. PE-EPS Suppresses DSS-Induced Activation of the NF-κB and STAT1 Pathways in Colons of the Colitis Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salas, A.; Hernandez-Rocha, C.; Duijvestein, M.; Faubion, W.; McGovern, D.; Vermeire, S.; Vetrano, S.; Vande Casteele, N. JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Sands, B.E.; Kaplan, G.G. The role of TNFalpha in ulcerative colitis. J. Clin. Pharmacol. 2007, 47, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Atreya, R.; Neurath, M.F. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin. Rev. Allergy Immunol. 2005, 28, 187–196. [Google Scholar] [CrossRef]
- Ogata, H.; Hibi, T. Cytokine and anti-cytokine therapies for inflammatory bowel disease. Curr. Pharm. Des. 2003, 9, 1107–1113. [Google Scholar] [CrossRef]
- van Dullemen, H.M.; van Deventer, S.J.; Hommes, D.W.; Bijl, H.A.; Jansen, J.; Tytgat, G.N.; Woody, J. Treatment of Crohn’s disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2). Gastroenterology 1995, 109, 129–135. [Google Scholar] [CrossRef]
- Al-Bawardy, B.; Shivashankar, R.; Proctor, D.D. Novel and Emerging Therapies for Inflammatory Bowel Disease. Front. Pharmacol. 2021, 12, 651415. [Google Scholar] [CrossRef]
- Vulliemoz, M.; Brand, S.; Juillerat, P.; Mottet, C.; Ben-Horin, S.; Michetti, P.; on behalf of Swiss IBDnet, an official working group of the Swiss Society of Gastroenterology. TNF-Alpha Blockers in Inflammatory Bowel Diseases: Practical Recommendations and a User’s Guide: An Update. Digestion 2020, 101 (Suppl. S1), 16–26. [Google Scholar] [CrossRef]
- Hansel, T.T.; Kropshofer, H.; Singer, T.; Mitchell, J.A.; George, A.J. The safety and side effects of monoclonal antibodies. Nat. Rev. Drug. Discov. 2010, 9, 325–338. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-kappaB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Hsieh, I.N.; Chang, A.S.; Teng, C.M.; Chen, C.C.; Yang, C.R. Aciculatin inhibits lipopolysaccharide-mediated inducible nitric oxide synthase and cyclooxygenase-2 expression via suppressing NF-kappaB and JNK/p38 MAPK activation pathways. J. Biomed. Sci. 2011, 18, 28. [Google Scholar] [CrossRef]
- Choi, J.H.; Chung, K.S.; Jin, B.R.; Cheon, S.Y.; Nugroho, A.; Roh, S.S.; An, H.J. Anti-inflammatory effects of an ethanol extract of Aster glehni via inhibition of NF-kappaB activation in mice with DSS-induced colitis. Food Funct. 2017, 8, 2611–2620. [Google Scholar] [CrossRef]
- Lu, P.D.; Zhao, Y.H. Targeting NF-kappaB pathway for treating ulcerative colitis: Comprehensive regulatory characteristics of Chinese medicines. Chin. Med. 2020, 15, 15. [Google Scholar] [CrossRef]
- Schreiber, S.; Rosenstiel, P.; Hampe, J.; Nikolaus, S.; Groessner, B.; Schottelius, A.; Kuhbacher, T.; Hamling, J.; Folsch, U.R.; Seegert, D. Activation of signal transducer and activator of transcription (STAT) 1 in human chronic inflammatory bowel disease. Gut 2002, 51, 379–385. [Google Scholar] [CrossRef]
- Arkteg, C.B.; Goll, R.; Gundersen, M.D.; Anderssen, E.; Fenton, C.; Florholmen, J. Mucosal gene transcription of ulcerative colitis in endoscopic remission. Scand. J. Gastroenterol. 2020, 55, 139–147. [Google Scholar] [CrossRef]
- Troncone, E.; Marafini, I.; Del Vecchio Blanco, G.; Di Grazia, A.; Monteleone, G. Novel Therapeutic Options for People with Ulcerative Colitis: An Update on Recent Developments with Janus Kinase (JAK) Inhibitors. Clin. Exp. Gastroenterol. 2020, 13, 131–139. [Google Scholar] [CrossRef]
- Andujar, I.; Recio, M.C.; Giner, R.M.; Cienfuegos-Jovellanos, E.; Laghi, S.; Muguerza, B.; Rios, J.L. Inhibition of ulcerative colitis in mice after oral administration of a polyphenol-enriched cocoa extract is mediated by the inhibition of STAT1 and STAT3 phosphorylation in colon cells. J. Agric. Food Chem. 2011, 59, 6474–6483. [Google Scholar] [CrossRef]
- Jiang, S.; Cai, L.; Lv, L.; Li, L. Pediococcus pentosaceus, a future additive or probiotic candidate. Microb. Cell Fact. 2021, 20, 45. [Google Scholar] [CrossRef]
- Lee, M.-J.; Lee, J.-H. Detection of Pediococci in Kimchi Using Pediococci Selective Medium. Microbiol. Biotechnol. Lett. 2009, 37, 238–242. [Google Scholar]
- Shin, J.S.; Jung, J.Y.; Lee, S.G.; Shin, K.S.; Rhee, Y.K.; Lee, M.K.; Hong, H.D.; Lee, K.T. Exopolysaccharide fraction from Pediococcus pentosaceus KFT18 induces immunostimulatory activity in macrophages and immunosuppressed mice. J. Appl. Microbiol. 2016, 120, 1390–1402. [Google Scholar] [CrossRef]
- Chung, K.S.; Shin, J.S.; Lee, J.H.; Park, S.E.; Han, H.S.; Rhee, Y.K.; Cho, C.W.; Hong, H.D.; Lee, K.T. Protective effect of exopolysaccharide fraction from Bacillus subtilis against dextran sulfate sodium-induced colitis through maintenance of intestinal barrier and suppression of inflammatory responses. Int. J. Biol. Macromol. 2021, 178, 363–372. [Google Scholar] [CrossRef]
- Hong, J.Y.; Chung, K.S.; Shin, J.S.; Park, G.; Jang, Y.P.; Lee, K.T. Anti-Colitic Effects of Ethanol Extract of Persea americana Mill. through Suppression of Pro-Inflammatory Mediators via NF-kappaB/STAT3 Inactivation in Dextran Sulfate Sodium-Induced Colitis Mice. Int. J. Mol. Sci. 2019, 20, 177. [Google Scholar] [CrossRef]
- Fabiany, C.G.; Natália, S.; Helena, F.M.; Eduardo, P.P.; Luíse, M.; Elizabeth, C.-L.; Ana, H.R.P. Characterization of acute murine dextran sodium sulfate (DSS) colitis: Severity of inflammation is dependent on the DSS molecular weight and concentration. Acta Sci. Vet. 2013, 41, 1142. [Google Scholar]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15–25. [Google Scholar] [CrossRef]
- Pullan, R.D.; Thomas, G.A.; Rhodes, M.; Newcombe, R.G.; Williams, G.T.; Allen, A.; Rhodes, J. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut 1994, 35, 353–359. [Google Scholar] [CrossRef]
- Gonzalez-Mauraza, H.; Martin-Cordero, C.; Alarcon-de-la-Lastra, C.; Rosillo, M.A.; Leon-Gonzalez, A.J.; Sanchez-Hidalgo, M. Anti-inflammatory effects of Retama monosperma in acute ulcerative colitis in rats. J. Physiol. Biochem. 2014, 70, 163–172. [Google Scholar] [CrossRef]
- Choi, S.; Woo, J.K.; Jang, Y.S.; Kang, J.H.; Jang, J.E.; Yi, T.H.; Park, S.Y.; Kim, S.Y.; Yoon, Y.S.; Oh, S.H. Fermented Pueraria Lobata extract ameliorates dextran sulfate sodium-induced colitis by reducing pro-inflammatory cytokines and recovering intestinal barrier function. Lab. Anim. Res. 2016, 32, 151–159. [Google Scholar] [CrossRef]
- Du, S.Y.; Huang, H.F.; Li, X.Q.; Zhai, L.X.; Zhu, Q.C.; Zheng, K.; Song, X.; Xu, C.S.; Li, C.Y.; Li, Y.; et al. Anti-inflammatory properties of uvaol on DSS-induced colitis and LPS-stimulated macrophages. Chin. Med. 2020, 15, 43. [Google Scholar] [CrossRef]
- Lama, A.; Provensi, G.; Amoriello, R.; Pirozzi, C.; Rani, B.; Mollica, M.P.; Raso, G.M.; Ballerini, C.; Meli, R.; Passani, M.B. The anti-inflammatory and immune-modulatory effects of OEA limit DSS-induced colitis in mice. Biomed. Pharmacother. 2020, 129, 110368. [Google Scholar] [CrossRef]
- Lee, S.; Shin, S.; Kim, H.; Han, S.; Kim, K.; Kwon, J.; Kwak, J.H.; Lee, C.K.; Ha, N.J.; Yim, D.; et al. Anti-inflammatory function of arctiin by inhibiting COX-2 expression via NF-kappaB pathways. J. Inflamm. 2011, 8, 16. [Google Scholar] [CrossRef]
- Liu, P.W.; Chen, M.F.; Tsai, A.P.; Lee, T.J. STAT1 mediates oroxylin a inhibition of iNOS and pro-inflammatory cytokines expression in microglial BV-2 cells. PLoS ONE 2012, 7, e50363. [Google Scholar] [CrossRef]
- Surayot, U.; Wang, J.; Seesuriyachan, P.; Kuntiya, A.; Tabarsa, M.; Lee, Y.; Kim, J.K.; Park, W.; You, S. Exopolysaccharides from lactic acid bacteria: Structural analysis, molecular weight effect on immunomodulation. Int. J. Biol. Macromol. 2014, 68, 233–240. [Google Scholar] [CrossRef]
- Ksonzekova, P.; Bystricky, P.; Vlckova, S.; Patoprsty, V.; Pulzova, L.; Mudronova, D.; Kubaskova, T.; Csank, T.; Tkacikova, L. Exopolysaccharides of Lactobacillus reuteri: Their influence on adherence of E. coli to epithelial cells and inflammatory response. Carbohydr. Polym. 2016, 141, 10–19. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, S.; Cui, J.; Guo, T.; Zhang, J.; Li, B. Alleviative Effects of Exopolysaccharide Produced by Lactobacillus helveticus KLDS1.8701 on Dextran Sulfate Sodium-Induced Colitis in Mice. Microorganisms 2021, 9, 2086. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, M.; Ren, F. A Role of Exopolysaccharide Produced by Streptococcus thermophilus in the Intestinal Inflammation and Mucosal Barrier in Caco-2 Monolayer and Dextran Sulphate Sodium-Induced Experimental Murine Colitis. Molecules 2019, 24, 513. [Google Scholar] [CrossRef]
- Randhawa, P.K.; Singh, K.; Singh, N.; Jaggi, A.S. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J. Physiol. Pharmacol. 2014, 18, 279–288. [Google Scholar] [CrossRef]
- Wirtz, S.; Neurath, M.F. Mouse models of inflammatory bowel disease. Adv. Drug Deliv. Rev. 2007, 59, 1073–1083. [Google Scholar] [CrossRef]
- Wirtz, S.; Neufert, C.; Weigmann, B.; Neurath, M.F. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007, 2, 541–546. [Google Scholar] [CrossRef]
- Umar, S. Intestinal stem cells. Curr. Gastroenterol. Rep. 2010, 12, 340–348. [Google Scholar] [CrossRef]
- Ugalde-Silva, P.; Gonzalez-Lugo, O.; Navarro-Garcia, F. Tight Junction Disruption Induced by Type 3 Secretion System Effectors Injected by Enteropathogenic and Enterohemorrhagic Escherichia coli. Front. Cell Infect. Microbiol. 2016, 6, 87. [Google Scholar] [CrossRef]
- Moita, E.; Gil-Izquierdo, A.; Sousa, C.; Ferreres, F.; Silva, L.R.; Valentao, P.; Dominguez-Perles, R.; Baenas, N.; Andrade, P.B. Integrated analysis of COX-2 and iNOS derived inflammatory mediators in LPS-stimulated RAW macrophages pre-exposed to Echium plantagineum L. bee pollen extract. PLoS ONE 2013, 8, e59131. [Google Scholar] [CrossRef]
- Pervin, M.; Hasnat, M.A.; Lim, J.H.; Lee, Y.M.; Kim, E.O.; Um, B.H.; Lim, B.O. Preventive and therapeutic effects of blueberry (Vaccinium corymbosum) extract against DSS-induced ulcerative colitis by regulation of antioxidant and inflammatory mediators. J. Nutr. Biochem. 2016, 28, 103–113. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, S.H.; Lee, S.Y.; Kim, E.K.; Kwon, J.E.; Seo, H.B.; Lee, H.H.; Lee, B.I.; Park, S.H.; Cho, M.L. Grim19 Attenuates DSS Induced Colitis in an Animal Model. PLoS ONE 2016, 11, e0155853. [Google Scholar] [CrossRef]
- Garcia, F.A.O.; Sales-Campos, H.; Yuen, V.G.; Machado, J.R.; Viana, G.S.B.; Oliveira, C.J.F.; McNeill, J.H. Arthrospira (Spirulina) platensis Attenuates Dextran Sulfate Sodium-induced Colitis in Mice by Suppressing Key Pro-inflammatory Cytokines. Korean J. Gastroenterol. 2020, 76, 150–158. [Google Scholar] [CrossRef]
- de Prati, A.C.; Ciampa, A.R.; Cavalieri, E.; Zaffini, R.; Darra, E.; Menegazzi, M.; Suzuki, H.; Mariotto, S. STAT1 as a new molecular target of anti-inflammatory treatment. Curr. Med. Chem. 2005, 12, 1819–1828. [Google Scholar] [CrossRef]
- Yu, Y.L.; Chen, M.; Zhu, H.; Zhuo, M.X.; Chen, P.; Mao, Y.J.; Li, L.Y.; Zhao, Q.; Wu, M.; Ye, M. STAT1 epigenetically regulates LCP2 and TNFAIP2 by recruiting EP300 to contribute to the pathogenesis of inflammatory bowel disease. Clin. Epigenetics 2021, 13, 127. [Google Scholar] [CrossRef]
- Marrero, J.A.; Matkowskyj, K.A.; Yung, K.; Hecht, G.; Benya, R.V. Dextran sulfate sodium-induced murine colitis activates NF-kappaB and increases galanin-1 receptor expression. Am. J. Physiol. Gastrointest Liver Physiol. 2000, 278, G797-804. [Google Scholar] [CrossRef]
- Liu, D.; Huo, X.; Gao, L.; Zhang, J.; Ni, H.; Cao, L. NF-kappaB and Nrf2 pathways contribute to the protective effect of Licochalcone A on dextran sulphate sodium-induced ulcerative colitis in mice. Biomed. Pharmacother. 2018, 102, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Adelaja, A.; Hoffmann, A. Signaling Crosstalk Mechanisms That May Fine-Tune Pathogen-Responsive NFkappaB. Front. Immunol. 2019, 10, 433. [Google Scholar] [CrossRef]
- Mehvar, R. Recent trends in the use of polysaccharides for improved delivery of therapeutic agents: Pharmacokinetic and pharmacodynamic perspectives. Curr. Pharm. Biotechnol. 2003, 4, 283–302. [Google Scholar] [CrossRef]
- Zhang, W.J.; Wang, S.; Kang, C.Z.; Lv, C.G.; Zhou, L.; Huang, L.Q.; Guo, L.P. Pharmacodynamic material basis of traditional Chinese medicine based on biomacromolecules: A review. Plant Methods 2020, 16, 26. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Chung, K.-S.; Shin, J.-S.; Jung, S.-H.; Lee, S.; Lee, M.-K.; Hong, H.-D.; Rhee, Y.K.; Lee, K.-T. Anti-Colitic Effect of an Exopolysaccharide Fraction from Pediococcus pentosaceus KFT-18 on Dextran Sulfate Sodium-Induced Colitis through Suppression of Inflammatory Mediators. Polymers 2022, 14, 3594. https://doi.org/10.3390/polym14173594
Lee J-H, Chung K-S, Shin J-S, Jung S-H, Lee S, Lee M-K, Hong H-D, Rhee YK, Lee K-T. Anti-Colitic Effect of an Exopolysaccharide Fraction from Pediococcus pentosaceus KFT-18 on Dextran Sulfate Sodium-Induced Colitis through Suppression of Inflammatory Mediators. Polymers. 2022; 14(17):3594. https://doi.org/10.3390/polym14173594
Chicago/Turabian StyleLee, Jeong-Hun, Kyung-Sook Chung, Ji-Sun Shin, Seang-Hwan Jung, Sangmin Lee, Myung-Ki Lee, Hee-Do Hong, Young Kyoung Rhee, and Kyung-Tae Lee. 2022. "Anti-Colitic Effect of an Exopolysaccharide Fraction from Pediococcus pentosaceus KFT-18 on Dextran Sulfate Sodium-Induced Colitis through Suppression of Inflammatory Mediators" Polymers 14, no. 17: 3594. https://doi.org/10.3390/polym14173594






