Inflammation-Responsive Nanovalves of Polymer-Conjugated Dextran on a Hole Array of Silicon Substrate for Controlled Antibiotic Release
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Developing Antibiotic Delivery System with ROS-Triggered Valves
2.3. Loading FITC-Vancomycin into the Chips and Its Release
2.4. Cell In Vitro Studies
3. Results and Discussion
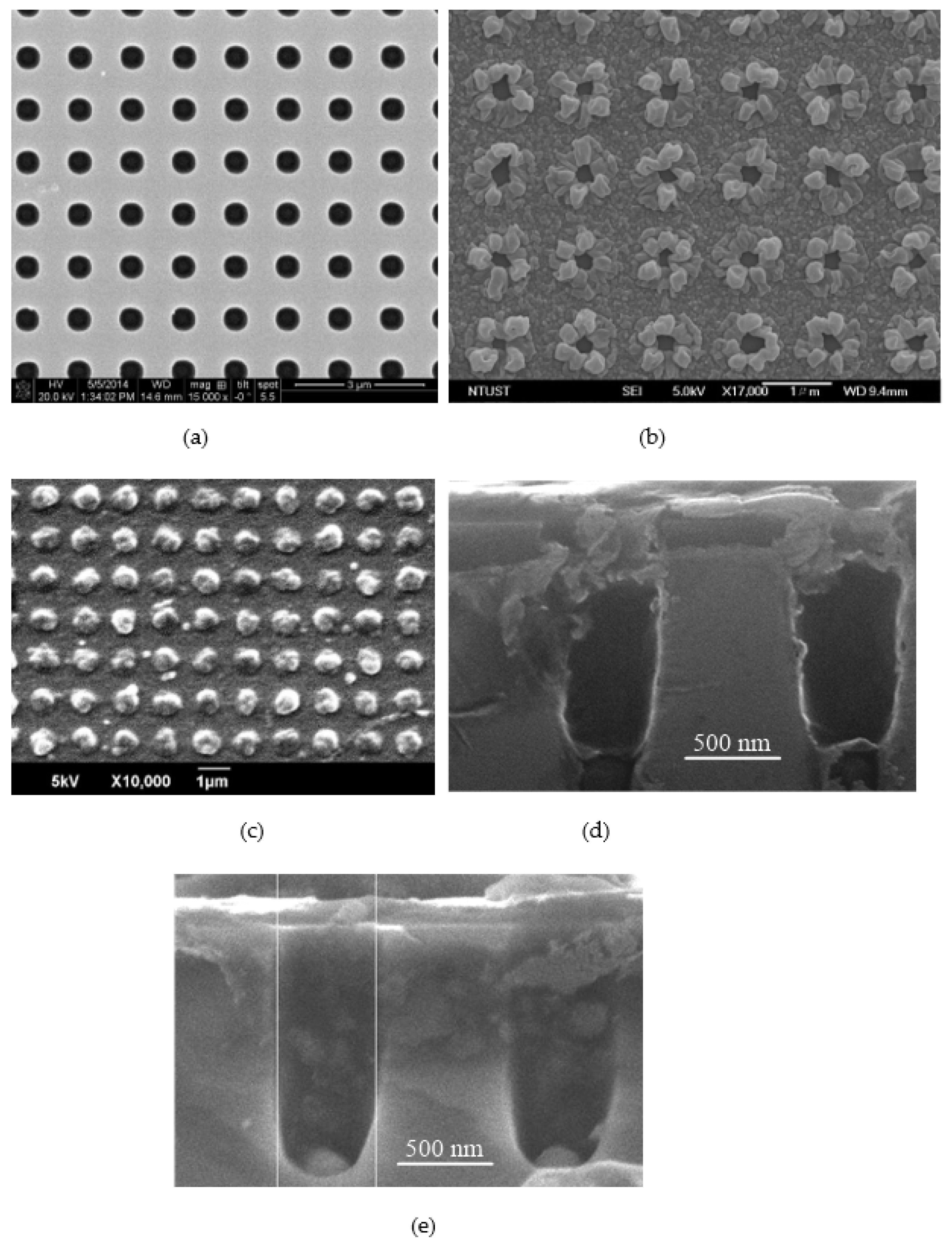
3.1. Morphology of the Drug Delivery Chip
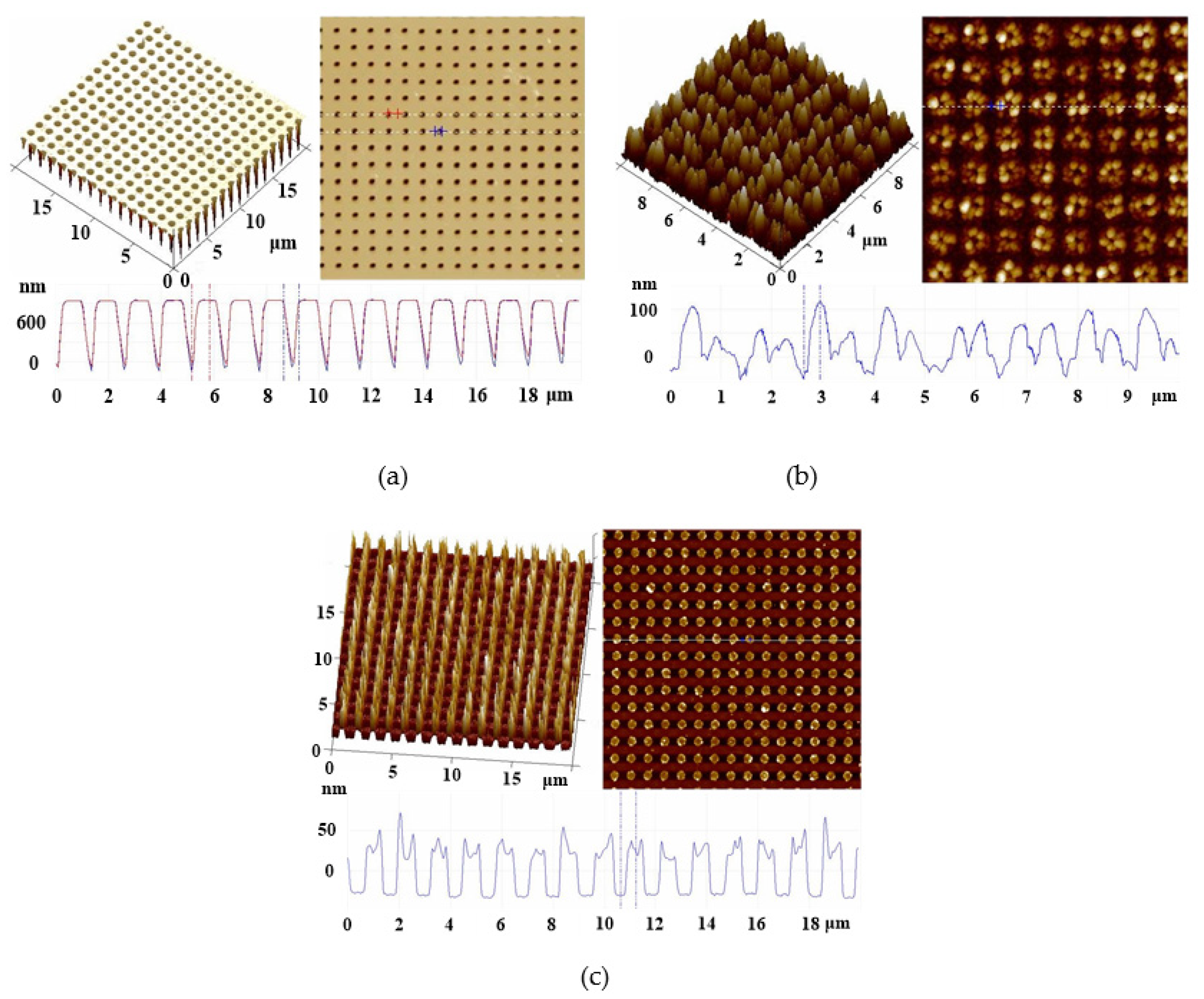
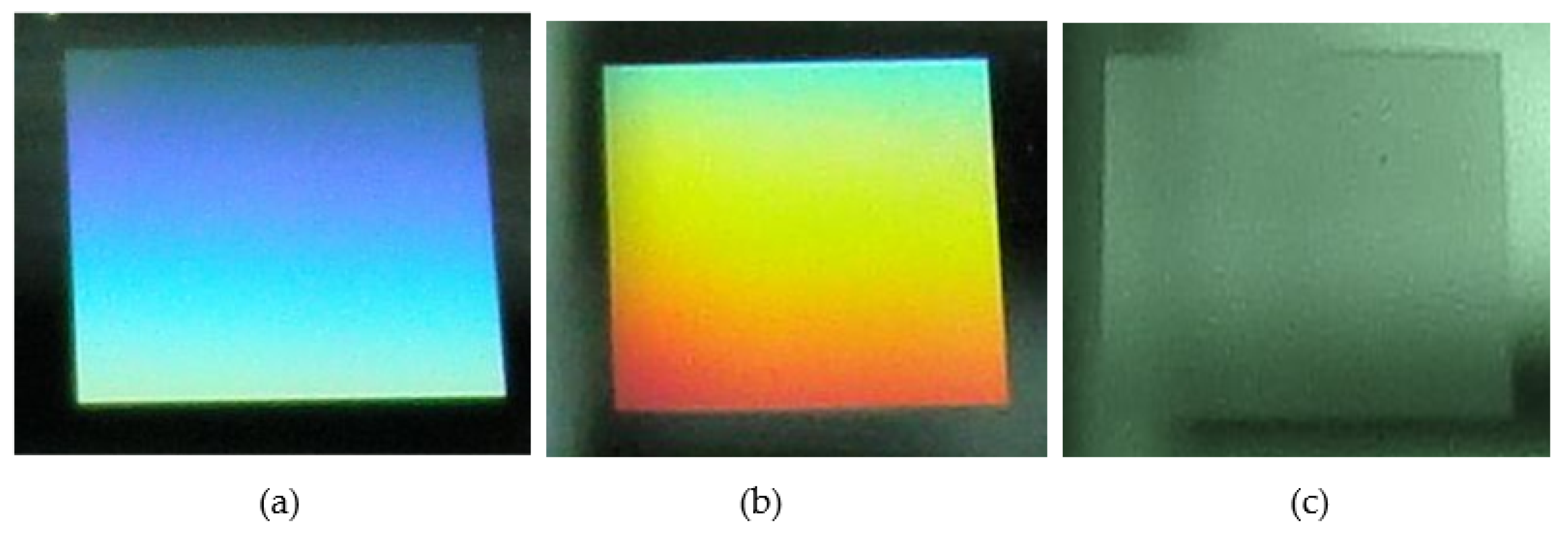
3.2. Surface Characterization
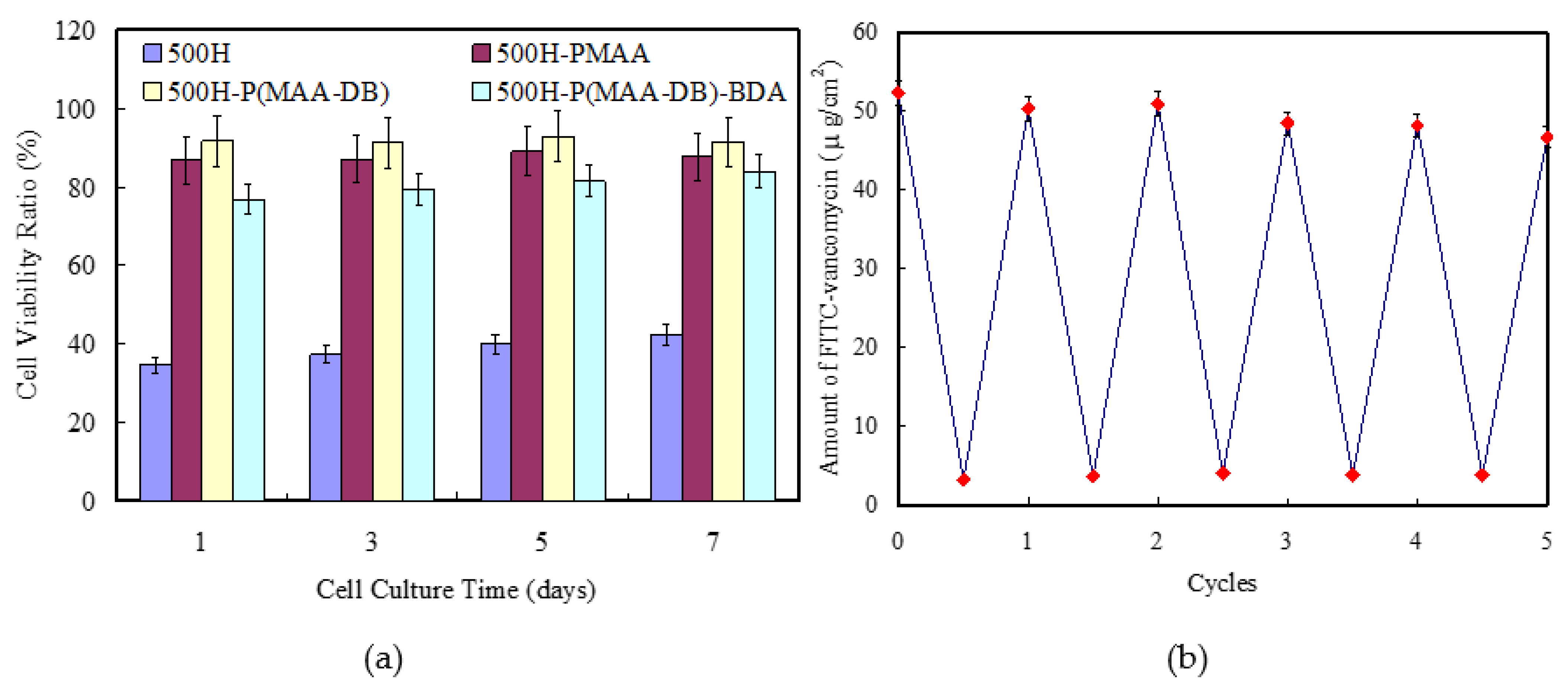
3.3. Loading and Releasing of FITC-Vancomycin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Webster, T.A.; Sismaet, H.J.; Conte, J.L.; Chan, I.J.; Goluch, E.D. Electrochemical detectionof Pseudomonas aeruginosa in human fluid samples viapyocyanin. Biosens. Bioelectron. 2014, 60, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Clemente, A.; Alba-Patiño, A.; Rojo-Molinero, E.; Russell, S.M.; Borges, M.; Oliver, A.; de la Rica, R. Rapid Detection of Pseudomonas aeruginosa Biofilms via Enzymatic Liquefaction of Respiratory Samples. ACS Sens. 2020, 5, 3956–3963. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, I.K.; Bertazzo, S.; O’Callaghan, D.J.; Schlegel, A.A.; Kallepitis, C.; Antcliffe, D.B.; Gordon, A.C.; Stevens, M.M. Differentiating sepsis from non-infectious systemic inflammation based on microvesicle-bacteria aggregation. Nanoscale 2015, 7, 13511–13520. [Google Scholar] [CrossRef] [PubMed]
- Re Silva, R.F.; Paixão, T.R.L.C.; Torres, M.D.T.; de Araujo, W.R. Simple and inexpensive electrochemical paper-based analytical device for sensitive detection of Pseudomonas aeruginosa. Sens. Actuators B Chem. 2020, 308, 127669. [Google Scholar] [CrossRef]
- Niethammer, P.; Grabher, C.; Look, A.T.; Mitchison, T.J. A Tissue-Scale Gradient of Hydrogen Peroxide Mediates Rapid Wound Detection in Zebrafish. Nature 2009, 459, 996–999. [Google Scholar] [CrossRef]
- Jobdeedamrong, A.; Theerasilp, M.; Wongsuwan, N.; Nasongkla, N.; Crespy, D. Inflammation-responsive nanocapsules for the dual-release of antibacterial drugs. Chem. Commun. 2020, 56, 12725–12728. [Google Scholar] [CrossRef]
- Lee, S.; Stubelius, A.; Hamelmann, N.; Tran, V.; Almutairi, A. Inflammation-Responsive Drug-Conjugated Dextran Nanoparticles Enhance Anti-Inflammatory Drug Efficacy. ACS Appl. Mater. Interfaces 2018, 10, 40378–40387. [Google Scholar] [CrossRef]
- Du, B.; Jia, S.; Wang, Q.; Ding, X.; Liu, Y.; Yao, H.; Zhou, J. A Self-Targeting, Dual ROS/pH-Responsive Apoferritin Nanocage for Spatiotemporally Controlled Drug Delivery to Breast Cancer. Biomacromolecules 2018, 19, 1026–1036. [Google Scholar] [CrossRef]
- Tan, L.; Yang, M.Y.; Wu, H.X.; Tang, Z.W.; Xiao, J.Y.; Liu, C.J.; Zhuo, R.X. Glucose- and pH-Responsive Nanogated Ensemble Based on Polymeric Network Capped Mesoporous Silica. ACS Appl. Mater. Interfaces 2015, 7, 6310–6316. [Google Scholar] [CrossRef]
- Yang, H.W.; Chen, J.K.; Kuo, S.W.; Lee, A.W. Degradable coronas comprising polyelectrolyte complexes of PDMAEMA and gelatin for pH-triggered antibiotic release. Polymer 2014, 55, 2678–2687. [Google Scholar] [CrossRef]
- Zhang, Z.; Wells, C.J.; King, A.M.; Bear, J.C.; Davies, G.L.; Williams, G.R. pH-Responsive nanocomposite fibres allowing MRI monitoring of drug release. J. Mater. Chem. B 2020, 8, 7264–7274. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.; Confeld, M.; Borowicz, P.; Wang, T.; Mallik, S.; Quadir, M. PEG-b-poly (carbonate)-derived nanocarrier platform with pH-responsive properties for pancreatic cancer combination therapy. Colloids Surf. B 2018, 174, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.; Zhao, Y.; Ge, N.; Qian, L. A pH/ROS cascade-responsive and self-accelerating drug release nanosystem for the targeted treatment of multi-drug-resistant colon cancer. Drug Deliv. 2020, 27, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, L.; Yu, H.; Ur-Rahman, K. Advances in phenylboronic acid-based closed-loop smart drug delivery system for diabetic therapy. J. Control. Release 2019, 305, 50–64. [Google Scholar] [CrossRef]
- Lee, S.; Stubelius, A.; Olejniczak, J.; Jang, H.; Huu, V.A.N.; Almutairi, A. Chemical Amplification Accelerates Reactive Oxygen Species Triggered Polymeric Degradation. Biomater. Sci. 2018, 6, 107–114. [Google Scholar] [CrossRef]
- Guo, Z.; Shin, I.; Yoon, J. Recognition and Sensing of Various Species Using Boronic Acid Derivatives. Chem. Commun. 2012, 48, 5956–5967. [Google Scholar] [CrossRef]
- Broaders, K.E.; Grandhe, S.; Frechet, J.M.J. A Biocompatible Oxidation-Triggered Carrier Polymer with Potential in Therapeutics. J. Am. Chem. Soc. 2011, 133, 756–758. [Google Scholar] [CrossRef]
- Peng, H.F.; Ning, X.Y.; Wei, G.; Wang, S.P.; Dai, G.L.; Ju, A.Q. The preparations of novel cellulose/phenylboronic acid composite intelligent bio-hydrogel and its glucose, pH-responsive behaviors. Carbohydr. Polym. 2018, 195, 349–355. [Google Scholar] [CrossRef]
- Sun, G.; Mao, J.J. Engineering Dextran-Based Scaffolds for Drug Delivery and Tissue Repair. Nanomedicine 2012, 7, 1771–1784. [Google Scholar] [CrossRef]
- Thongchaivetcharat, K.; Jenjob, R.; Seidi, F.; Crespy, D. Programming pH-responsive release of two payloads from dextran-based nanocapsules. Carbohydr. Polym. 2019, 217, 217–223. [Google Scholar] [CrossRef]
- Feng, W.Q.; Lv, W.P.; Qi, J.J.; Zhang, G.L.; Zhang, F.B.; Fan, X.B. Quadruple-Responsive Nanocomposite Based on Dextran-PMAA-PNIPAM, Iron Oxide Nanoparticles, and Gold Nanorods. Macromol. Rapid Commun. 2012, 33, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Curcio, M.; Cirillo, G.; Paoli, A.; Naimo, G.D.; Mauro, L.; Amantea, D.; Leggio, A.; Nicoletta, F.P.; Lemma, F. Self-assembling Dextran prodrug for redox- and pH-responsive co-delivery of therapeutics in cancer cells. Colloids Surf. B 2020, 185, 110537. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Liu, P.; Pranantyo, D.; Neoh, K.G.; Kang, E.T. Dextran- and Chitosan-Based Antifouling, Antimicrobial Adhesion, and Self-Polishing Multilayer Coatings from pH-Responsive Linkages-Enabled Layer-by-Layer Assembly. ACS Sustain. Chem. Eng. 2018, 6, 3916–3926. [Google Scholar] [CrossRef]
- Rahimi, S.; Stumpf, S.; Grimm, O.; Schacher, F.H.; Schubert, U.S.; Schubert, S. Dual Photo- and pH-Responsive Spirooxazine-Functionalized Dextran Nanoparticles. Biomacromolecules 2020, 21, 3620–3630. [Google Scholar] [CrossRef]
- Chen, J.-K.; Chen, T.-Y. Fabrication of high-aspect-ratio poly (2-hydroxyethyl methacrylate) brushes patterned on silica surfaces by very-large-scale integration process. J. Colloid Interface Sci. 2011, 355, 359–367. [Google Scholar] [CrossRef]
- Chen, J.-K.; Wang, J.-H.; Cheng, C.-C.; Chang, J.-Y. Reversibly thermoswitchable two-dimensional periodic arrays prepared from tethered poly (N-isopropylacrylamide) on silicon surfaces. ACS Appl. Mater. Interface 2013, 5, 2959–2966. [Google Scholar] [CrossRef]
- Chen, J.K.; Zhou, G.Y.; Chang, C.J. Real-time multicolor antigen detection with chemoresponsive diffraction arrays of silicon oxide nanopillar arrays. Sens. Actuators B 2013, 186, 802–810. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, F.-J. Biomolecule-functionalized polymer brushes. Chem. Soc. Rev. 2013, 42, 3394–3426. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, G.; Huang, C.; Chang, J. Two-dimensional periodic relief array as a versatile platform for selective immunosorbent assay and visualizing of antigens. ACS Appl. Mater. Interface 2013, 5, 3348–3355. [Google Scholar] [CrossRef]
- Ishihara, K.; Mitera, K.; Inoue, Y.; Fukazawa, K. Effects of molecular interactions at various polymer brush surfaces on fibronectin adsorption induced cell adhesion. Colloids Surf. B 2020, 194, 111205. [Google Scholar] [CrossRef]
- Chen, J.K.; Wang, J.H.; Cheng, C.C.; Ko, F.H. Fabrication of biomimetic device with PS-b-PNIPAAm copolymer pillars mimicking a gecko foot pad. Sens. Actuator B 2012, 174, 332–341. [Google Scholar] [CrossRef]
- Krishnamoorthy, M.; Hakobyan, S.; Ramstedt, M.; Gautrot, J.E. Surface-initiated polymer brushes in the biomedical field: Applications in membrane science, biosensing, cell culture, regenerative medicine and antibacterial coatings. Chem. Rev. 2014, 114, 10976–11026. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-K.; Wang, J.-H.; Cheng, C.-C.; Chang, J.-Y.; Chang, F.-C. Polarity-indicative two-dimensional periodic relief arrays of tethered poly (methyl methacrylate) on silicon surfaces for visualization in volatile organic compound sensing. Appl. Phys. Lett. 2013, 102, 151906. [Google Scholar] [CrossRef]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef]
- Chen, J.-K.; Bai, B.-J. pH-Switchable Optical Properties of the One-Dimensional Periodic Array of Tethered Poly(2-dimethylaminoethyl methacrylate) Brushes on a Silicon Surface. J. Phys. Chem. C 2011, 115, 21341–21350. [Google Scholar] [CrossRef]
- Lee, A.; Cheng, C.; Chang, C.; Lu, C.; Chen, J. Optical assay of trypsin using a one-dimensional plasmonic array of gelatin-modified poly(methacrylic acid). Microchim. Acta 2020, 187, 280. [Google Scholar] [CrossRef]
- North, S.M.; Armes, S.P. Aqueous solution behavior of stimulus-responsive poly (methacrylic acid)-poly (2-hydroxypropyl methacrylate) diblock copolymer nanoparticles. Polym. Chem. 2020, 11, 2147–2156. [Google Scholar] [CrossRef]
- Yan, Q.; Zheng, H.N.; Jiang, C.; Li, K.; Xiao, S.J. EDC/NHS activation mechanism of polymethacrylic acid: Anhydride versus NHS-ester. RSC Adv. 2015, 5, 69939–69947. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Ataman, N.C.; Mocny, P.; Wang, J.; Moraes, J.; Klok, H.-A. Surface-initiated controlled radical polymerization: State-of-the-art, opportunities, and challenges in surface and interface engineering with polymer brushes. Chem. Rev. 2017, 117, 1105–1318. [Google Scholar] [CrossRef]
- Guo, J.; Wu, Y.; Wei, P.; Huang, Y.; Chen, J. Immobilization of antibody conjugated ZnS quantum dots onto poly(2,6-dimethyl-1,4-phenylene oxide) nanofibers with Poly(Nisopropylacrylamide) grafts as reversibly fluorescence immunoassay. Dye. Pigment. 2018, 159, 198–208. [Google Scholar] [CrossRef]
- Leone, G.; Giovanella, U.; Bertini, F.; Hoseinkhani, S.; Porzio, W.; Ricci, G.; Botta, C.; Galeotti, F. Hierarchically structured, blue-emitting polymer hybrids through surface-initiated nitroxide-mediated polymerization and water templated assembly. J. Mater. Chem. C 2013, 1, 6585–6593. [Google Scholar] [CrossRef]
- Chen, J.; Zhuang, A. Fabrication of a highly dense line patterned polystyrene brush on silicon surfaces using very large scale integration processing. J. Phys. Chem. C 2010, 114, 11801–11809. [Google Scholar] [CrossRef]
- Chen, J.; Hsieh, C.; Huang, C.; Li, P. Characterization of patterned poly (methyl methacrylate) brushes under various structures upon solvent immersion. J. Colloid Interface Sci. 2009, 338, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-K.; Chen, Z.-Y.; Lin, H.-C.; Hong, P.-D.; Chang, F.-C. Patterned poly(2-hydroxyethyl methacrylate) brushes on silicon surfaces behave as “Tentacles” to capture ferritin from aqueous solution. ACS Appl. Mater. Interfaces 2009, 1, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.-P.; Hsu, H.-L.; Chang, C.-J.; Lee, S.-C.; Chen, J.-K. Surface lattice resonance of line array of poly (glycidyl methacrylate) with CdS quantum dots for label-free biosensing. Colloids Surf. B 2019, 179, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.-R.; Cheng, C.-C.; Lee, A.-W.; Wei, P.-L.; Chen, J.-K. Visualization platform of one-dimensional gratings of tethered-polyvinyltetrazole brushes on silicon surfaces for sensing of Cr(III). Microchim. Acta 2017, 184, 2723–2730. [Google Scholar] [CrossRef]
- Hoare, T.; Pelton, R. Engineering Glucose Swelling Responses in Poly(N-isopropylacrylamide)-Based Microgels. Macromolecules 2007, 40, 670–678. [Google Scholar] [CrossRef]
- Griffiths, H.R.; Gao, D.; Pararasa, C. Redox Regulation in Metabolic Programming and Inflammation. Redox Biol. 2017, 12, 50–57. [Google Scholar] [CrossRef]
- Mendes, G.; Faria, M.; Carvalho, A.; Gonçalves, M.C.; de Pinho, M.N. Structure of water in hybrid cellulose acetate-silica ultrafiltration membranes and permeation properties. Carbohydr. Polym. 2018, 189, 342–351. [Google Scholar] [CrossRef]
- Guo, J.-W.; Huang, B.-R.; Lai, J.-Y.; Lu, C.-H.; Chen, J.-K. Reversibly photoswitchable arrays prepared from azobenzene-modified tethered poly (methacrylic acid) brush as colored actuator. Sens. Actuator B 2020, 304, 127275. [Google Scholar] [CrossRef]
- Li, H.; Shan, Y.; Qiao, L.; Dou, A.; Shi, X.; Xu, G. Facile Synthesis of Boronate-Decorated Polyethyleneimine-Grafted Hybrid Magnetic Nanoparticles for the Highly Selective Enrichment of Modified Nucleosides and Ribosylated Metabolites. Anal. Chem. 2013, 85, 11585–11592. [Google Scholar] [CrossRef]
- Wang, B.; Ma, R.; Liu, G.; Li, Y.; Liu, X.; An, Y.; Shi, L. Glucose-Responsive Micelles from Self-Assembly of Poly(ethylene glycol)-b-Poly(acrylic acid-co-acrylamidophenylboronic acid) and the Controlled Release of Insulin. Langmuir 2009, 25, 12522–12528. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huo, W.; He, M.; Lian, J.; Zhang, S.; Gao, Y. On-Chip determination of glycated hemoglobin with a novel boronicacid copolymer. Sens. Actuator B 2017, 253, 542–551. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, A.-W.; Chang, P.-L.; Liaw, S.-K.; Lu, C.-H.; Chen, J.-K. Inflammation-Responsive Nanovalves of Polymer-Conjugated Dextran on a Hole Array of Silicon Substrate for Controlled Antibiotic Release. Polymers 2022, 14, 3611. https://doi.org/10.3390/polym14173611
Lee A-W, Chang P-L, Liaw S-K, Lu C-H, Chen J-K. Inflammation-Responsive Nanovalves of Polymer-Conjugated Dextran on a Hole Array of Silicon Substrate for Controlled Antibiotic Release. Polymers. 2022; 14(17):3611. https://doi.org/10.3390/polym14173611
Chicago/Turabian StyleLee, Ai-Wei, Pao-Lung Chang, Shien-Kuei Liaw, Chien-Hsing Lu, and Jem-Kun Chen. 2022. "Inflammation-Responsive Nanovalves of Polymer-Conjugated Dextran on a Hole Array of Silicon Substrate for Controlled Antibiotic Release" Polymers 14, no. 17: 3611. https://doi.org/10.3390/polym14173611




