Robust Impact Effect and Super-Lyophobic Reduced Galinstan on Polymers Applied for Energy Harvester
Abstract
:1. Introduction
2. Results and Discussion
2.1. Wetting Behaviors of Galinstan
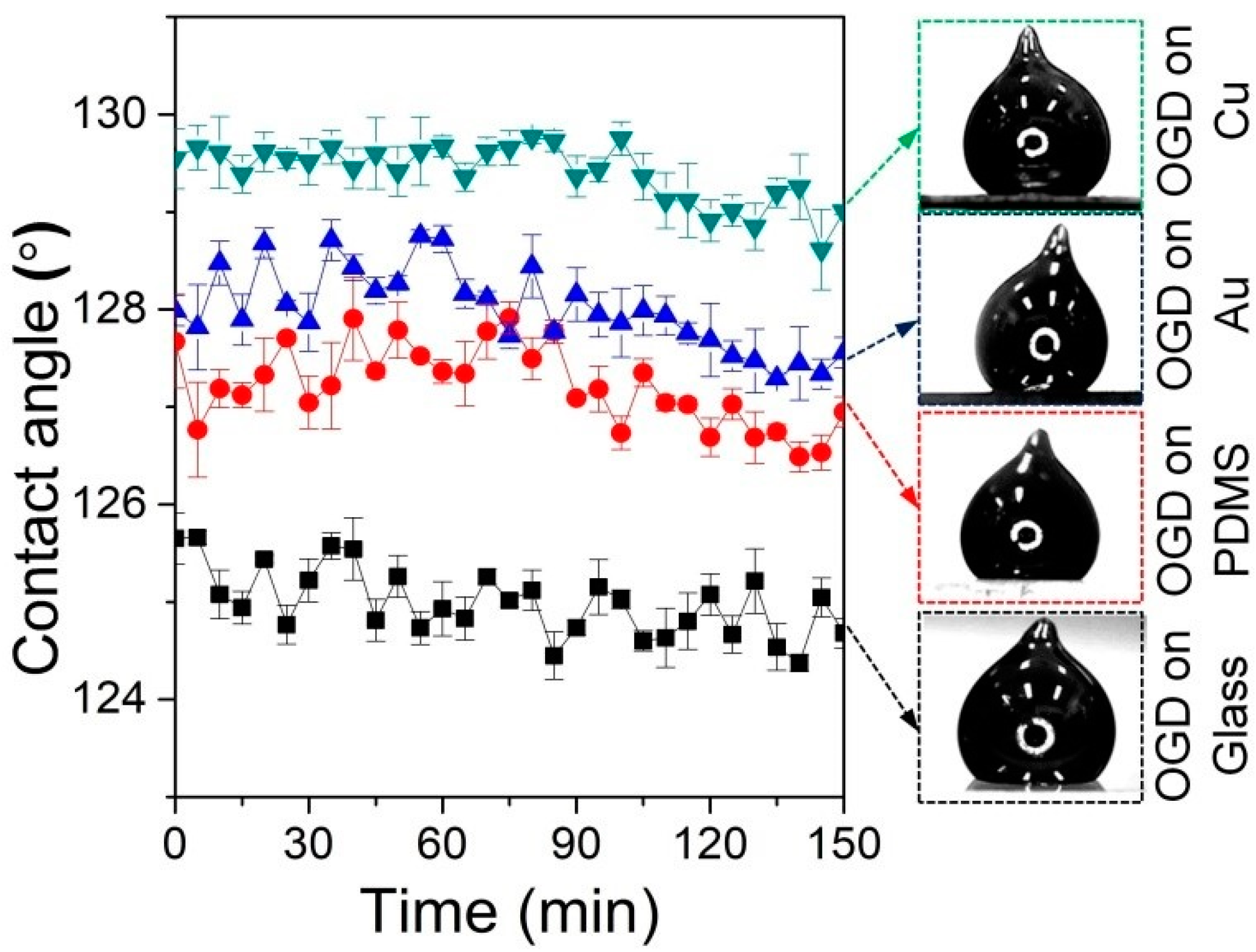
2.1.1. Wetting Behaviors of Oxidized Galinstan
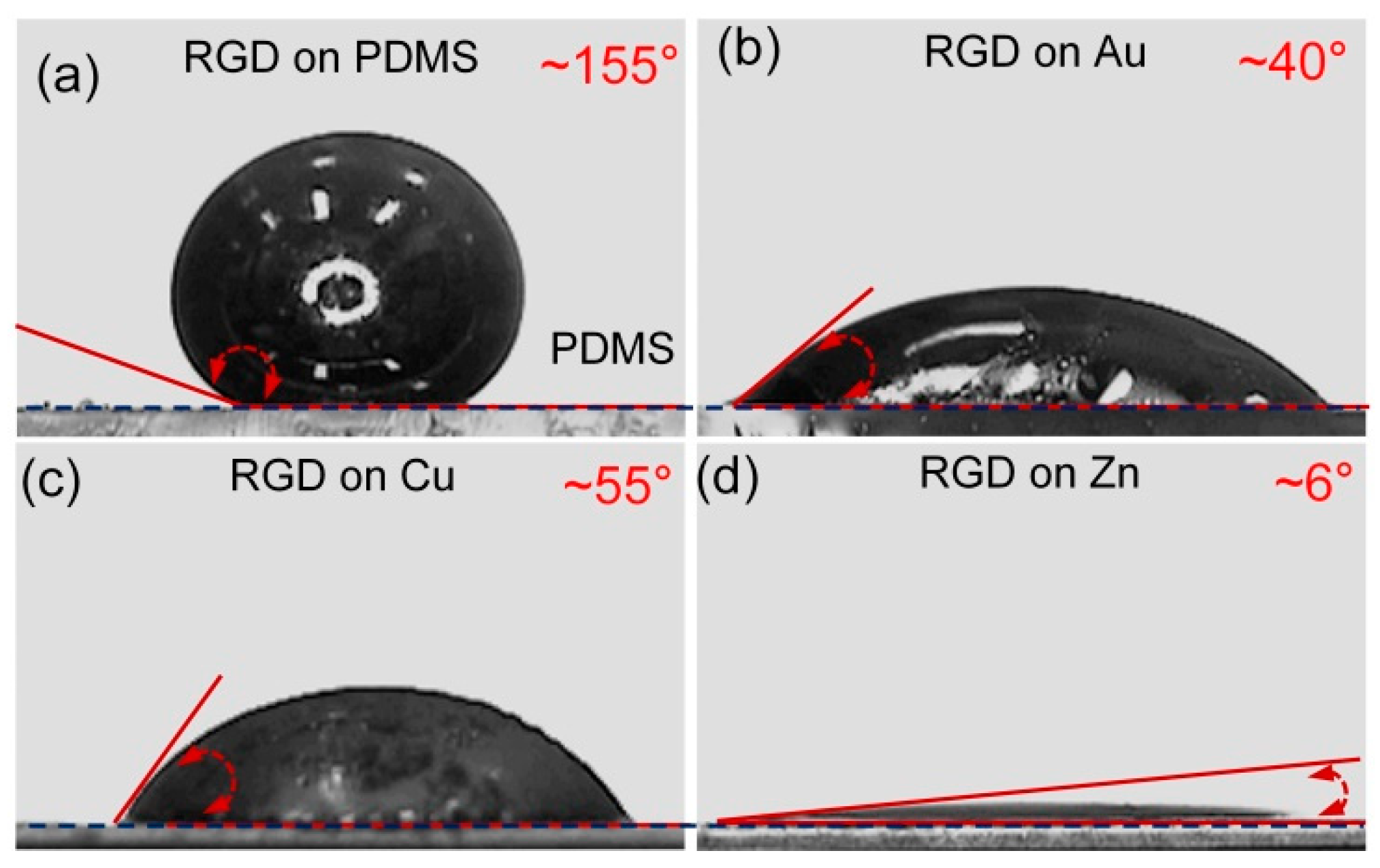
2.1.2. Wetting Behaviors of Reduced Galinstan
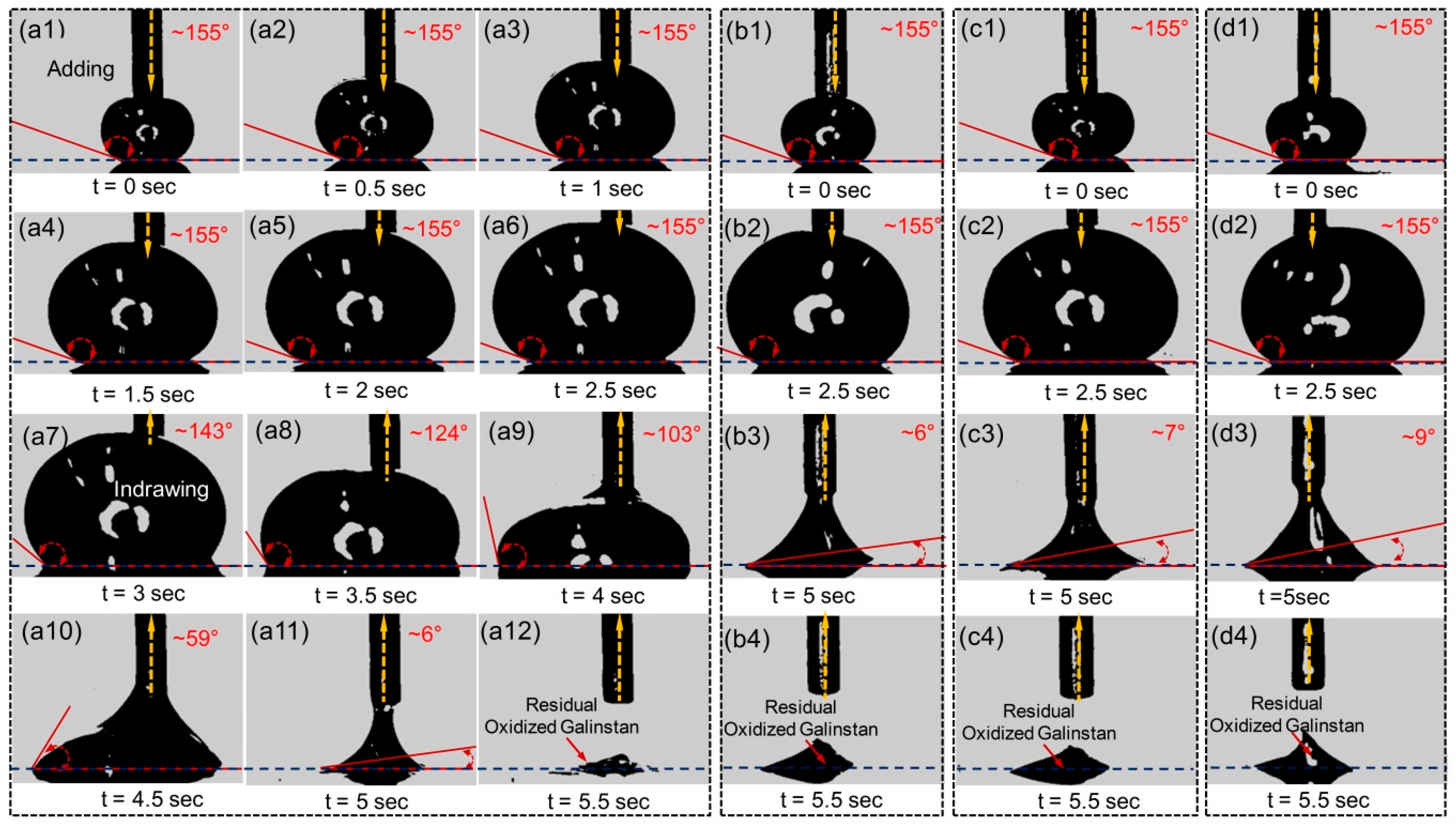
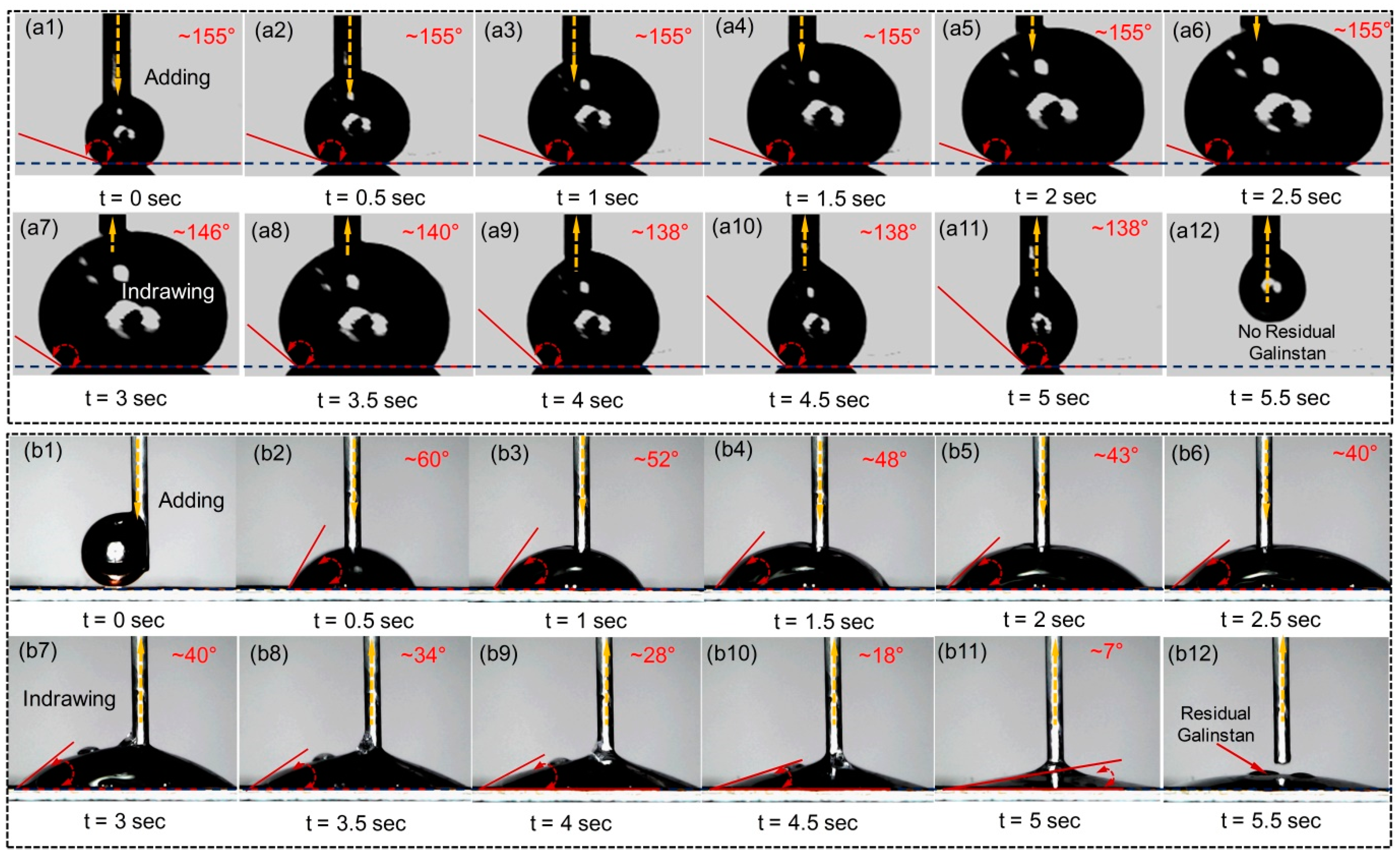
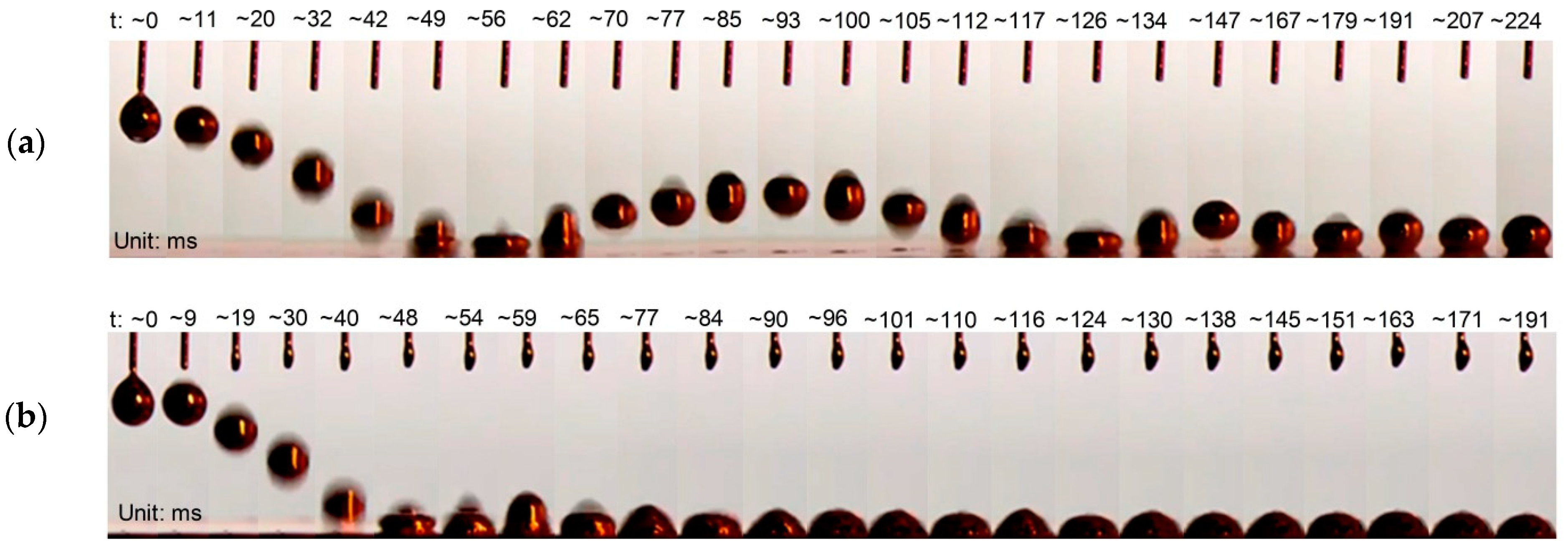
2.1.3. Impact Effect of Reduced Galinstan
2.2. Microfluidic Energy Harvester Application
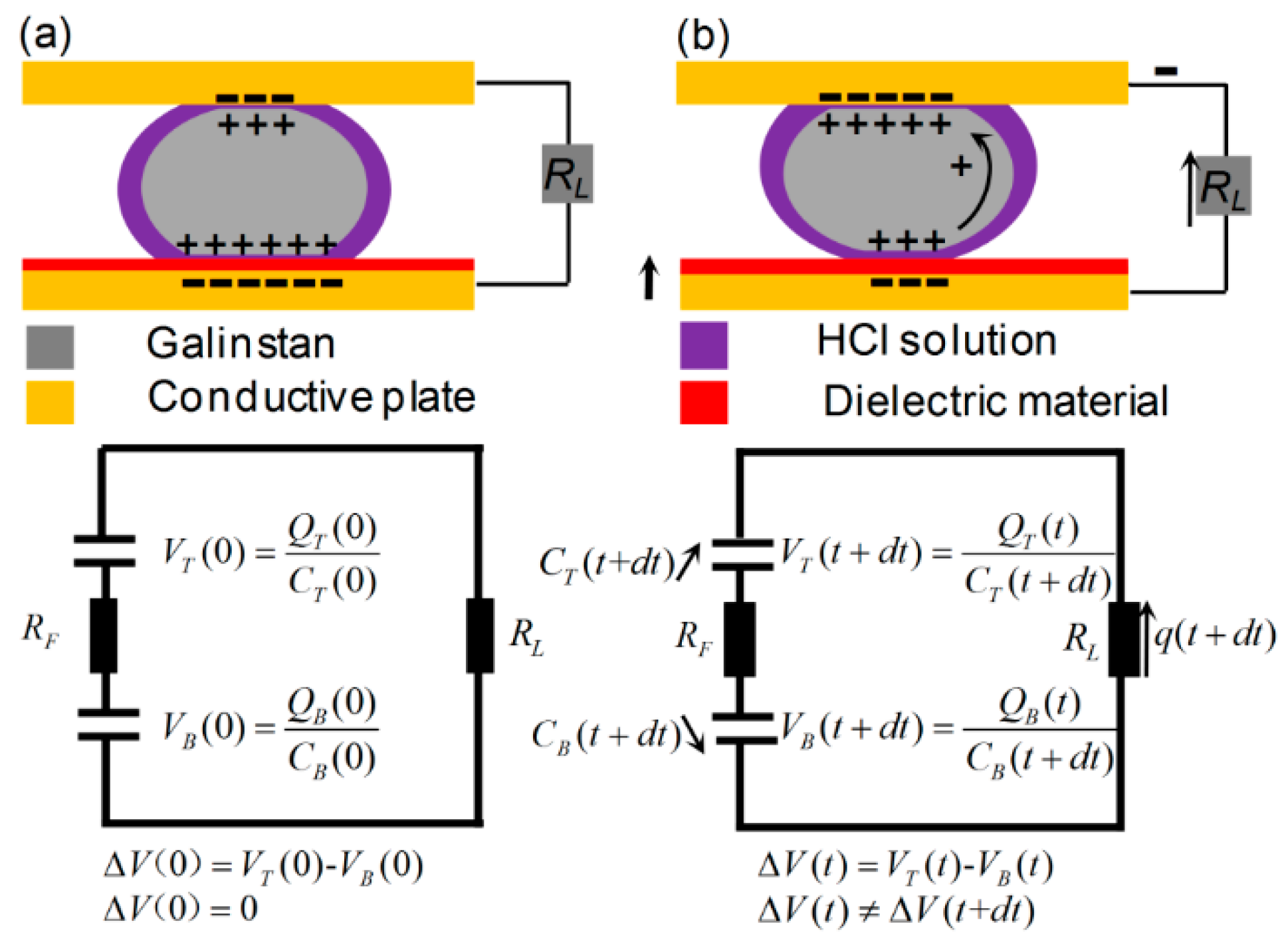
2.2.1. Electric Circuit Modeling of the Reduced Galinstan-Based Energy Harvester
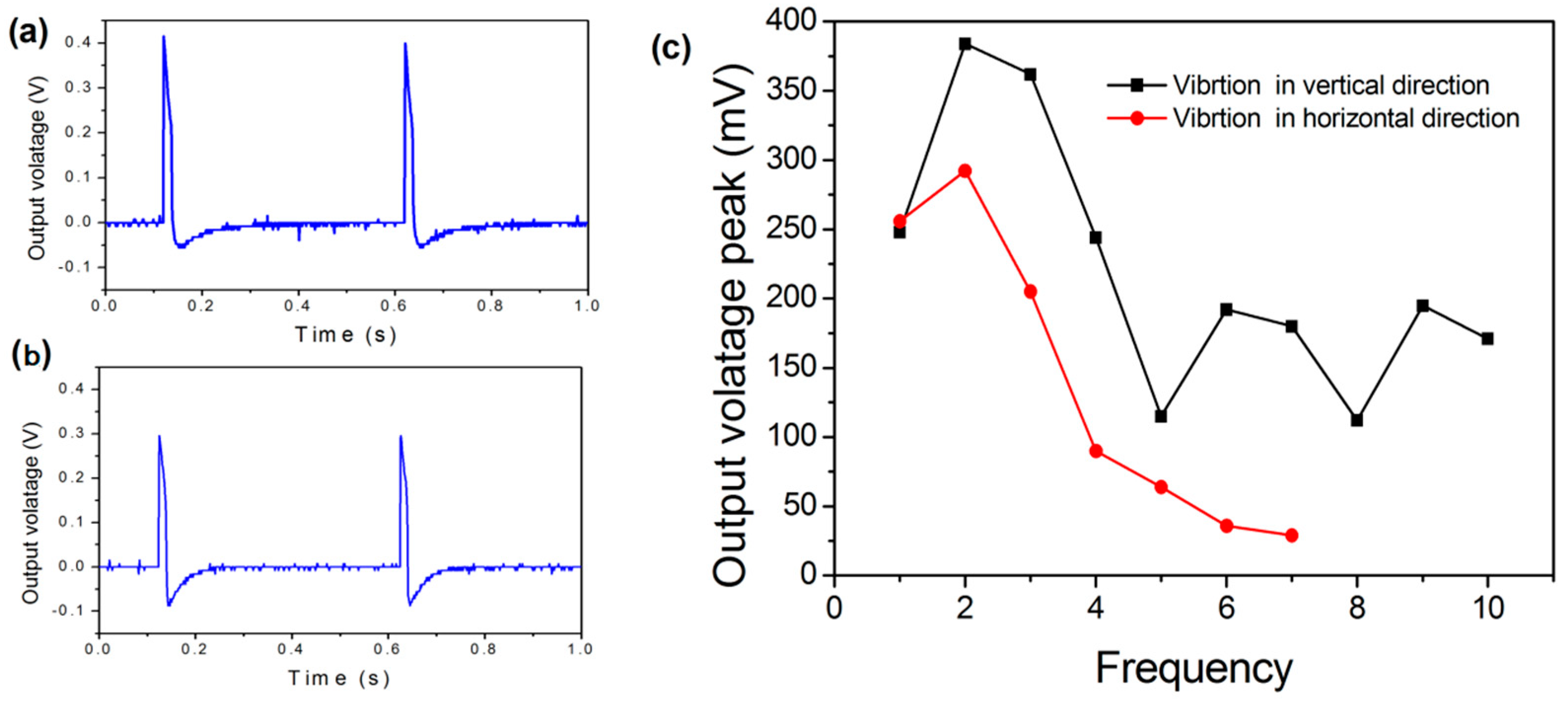
2.2.2. Reduced Galinstan-Based Energy Harvester
3. Conclusions
4. Experimental Section
4.1. Preparation of Reduced Galinstan
4.2. Fabrication of Reduced Galinstan-Based Energy Harvester
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, J.; Ma, T. Triboelectricity-based self-charging droplet capacitor for harvesting low-level ambient energy. Nano Energy 2020, 74, 104795. [Google Scholar] [CrossRef]
- Wu, X.; Li, G.; Lee, D.-W. A novel energy conversion method based on hydrogel material for self-powered sensor system applications. Appl. Energy 2016, 173, 103–110. [Google Scholar] [CrossRef]
- Wu, H.; Mendel, N.; van der Ham, S.; Shui, L.; Zhou, G.; Mugele, F. Charge Trapping-Based Electricity Generator (CTEG): An Ultrarobust and High Efficiency Nanogenerator for Energy Harvesting from Water Droplets. Adv. Mater. 2020, 32, 2001699. [Google Scholar] [CrossRef] [PubMed]
- Wijewardhana, K.R.; Shen, T.-Z.; Song, J.-K. Energy harvesting using air bubbles on hydrophobic surfaces containing embedded charges. Appl. Energy 2017, 206, 432–438. [Google Scholar] [CrossRef]
- Liang, J.; Liao, W.-H. Impedance Modeling and Analysis for Piezoelectric Energy Harvesting Systems. IEEE/ASME Trans. Mechatron. 2011, 17, 1145–1157. [Google Scholar] [CrossRef]
- Hua, R.; Liu, H.; Yang, H.; Wang, Y.; Ferrante, J. A nonlinear interface integrated lever mechanism for piezoelectric footstep energy harvesting. Appl. Phys. Lett. 2018, 113, 053902. [Google Scholar] [CrossRef]
- Helseth, L.E.; Guo, X.D. Contact Electrification and Energy Harvesting Using Periodically Contacted and Squeezed Water Droplets. Langmuir 2015, 31, 3269–3276. [Google Scholar] [CrossRef]
- Al Ahmad, M.; Jabbour, G. Electronically droplet energy harvesting using piezoelectric cantilevers. Electron. Lett. 2012, 48, 647–649. [Google Scholar] [CrossRef]
- Krupenkin, T.N.; Taylor, J.A. Reverse electrowetting as a new approach to high-power energy harvesting. Nat. Commun. 2011, 2, 448. [Google Scholar] [CrossRef]
- Moon, J.K.; Jeong, J.; Lee, D.; Pak, H.K. Electrical power generation by mechanically modulating electrical double layers. Nat. Commun. 2013, 4, 1487. [Google Scholar] [CrossRef] [Green Version]
- Kong, W.; Cao, P.; He, X.; Yu, L.; Ma, X.; He, Y.; Lu, L.; Zhang, X.; Deng, Y. Ionic liquid based vibrational energy harvester by periodically squeezing the liquid bridge. RSC Adv. 2014, 4, 19356–19361. [Google Scholar] [CrossRef]
- Hsu, T.-H.; Taylor, J.A.; Krupenkin, T.N. Energy harvesting from aperiodic low-frequency motion using reverse electrowetting. Faraday Discuss. 2017, 199, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Conner, C.; de Visser, T.; Loessberg, J.; Sherman, S.; Smith, A.; Ma, S.; Napoli, M.T.; Pennathur, S.; Weld, D. Energy Harvesting with a Liquid-Metal Microfluidic Influence Machine. Phys. Rev. Appl. 2018, 9, 044008. [Google Scholar] [CrossRef]
- Adhikari, P.R.; Tasneem, N.T.; Reid, R.C.; Mahbub, I. Electrode and electrolyte configurations for low frequency motion energy harvesting based on reverse electrowetting. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Chen, G.; Liu, X.; Li, S.; Dong, M.; Jiang, D. A droplet energy harvesting and actuation system for self-powered digital microfluidics. Lab Chip 2018, 18, 1026–1034. [Google Scholar] [CrossRef]
- Lin, Z.-H.; Cheng, G.; Lee, S.; Pradel, K.C.; Wang, Z.L. Harvesting Water Drop Energy by a Sequential Contact-Electrification and Electrostatic-Induction Process. Adv. Mater. 2014, 26, 4690–4696. [Google Scholar] [CrossRef]
- Zhu, G.; Su, Y.; Bai, P.; Chen, J.; Jing, Q.; Yang, W.; Wang, Z.L. Harvesting Water Wave Energy by Asymmetric Screening of Electrostatic Charges on a Nanostructured Hydrophobic Thin-Film Surface. ACS Nano 2014, 8, 6031–6037. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Park, J.; Kim, W.K.; Yang, Y.; Lee, E.; Han, C.J.; Park, S.Y.; Lee, J.; Kim, Y.S. An effective energy harvesting method from a natural water motion active transducer. Energy Environ. Sci. 2014, 7, 3279–3283. [Google Scholar] [CrossRef]
- Helseth, L.E.; Guo, X.D. Hydrophobic polymer covered by a grating electrode for converting the mechanical energy of water droplets into electrical energy. Smart Mater. Struct. 2016, 25, 045007. [Google Scholar] [CrossRef]
- Su, Y.; Wen, X.; Zhu, G.; Yang, J.; Chen, J.; Bai, P.; Wu, Z.; Jiang, Y.; Wang, Z.L. Hybrid triboelectric nanogenerator for harvesting water wave energy and as a self-powered distress signal emitter. Nano Energy 2014, 9, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Pu, X.; Guo, H.; Chen, J.; Wang, X.; Xi, Y.; Hu, C.; Wang, Z.L. Eye motion triggered self-powered mechnosensational communication system using triboelectric nanogenerator. Sci. Adv. 2017, 3, e1700694. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yin, Y.; Wang, B.; Zhou, T.; Wang, J.; Luo, J.; Tang, W.; Cao, R.; Yuan, Z.; Li, N.-W.; et al. Self-Powered Electrospinning System Driven by a Triboelectric Nanogenerator. ACS Nano 2017, 11, 10439–10445. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, K.; Wang, Z.L.; Yang, Y. Unity Convoluted Design of Solid Li-Ion Battery and Triboelectric Nanogenerator for Self-Powered Wearable Electronics. Adv. Energy Mater. 2017, 7, 1701629. [Google Scholar] [CrossRef]
- Yu, H.; He, X.; Ding, W.; Hu, Y.; Yang, D.; Lu, S.; Wu, C.; Zou, H.; Liu, R.; Lu, C.; et al. A Self-Powered Dynamic Displacement Monitoring System Based on Triboelectric Accelerometer. Adv. Energy Mater. 2017, 7, 1700565. [Google Scholar] [CrossRef]
- He, X.; Zi, Y.; Yu, H.; Zhang, S.L.; Wang, J.; Ding, W.; Zou, H.; Zhang, W.; Lu, C.; Wang, Z.L. An ultrathin paper-based self-powered system for portable electronics and wireless human-machine interaction. Nano Energy 2017, 39, 328–336. [Google Scholar] [CrossRef]
- Yang, Z.; Halvorsen, E.; Dong, T. Electrostatic Energy Harvester Employing Conductive Droplet and Thin-Film Electret. J. Microelectromech. Syst. 2014, 23, 315–323. [Google Scholar] [CrossRef]
- Deng, H.; Zhao, Z.; Jiao, C.; Ye, J.; Zhao, S.; Ma, M.; Zhong, X. A Liquid-Metal-Based Freestanding Triboelectric Generator for Low-Frequency and Multidirectional Vibration. Front. Mater. 2021, 8, 692273. [Google Scholar] [CrossRef]
- Li, G.; Sun, F.; Chen, H.; Jin, Y.; Zhang, A.; Du, J. High-Efficiency Large-Area Printed Multilayer Liquid Metal Wires for Stretchable Biomedical Sensors with Recyclability. ACS Appl. Mater. Interfaces 2021, 13, 56961–56971. [Google Scholar] [CrossRef]
- West, D.; Taylor, J.A.; Krupenkin, T. Alternating Current Liquid Metal Vortex Magnetohydrodynamic Generator. Energ. Convers. Manag. 2020, 223, 113223. [Google Scholar] [CrossRef]
- Vallem, V.; Roosa, E.; Ledinh, T.; Jung, W.; Kim, T.I.; Rashid-Nadimi, S.; Kiani, A.; Dickey, M.D. A Soft Variable-Area Electrical-Double-Layer Energy Harvester. Adv. Mater. 2021, 33, 2103142. [Google Scholar] [CrossRef]
- Jeon, J.; Chung, S.K.; Lee, J.-B.; Doo, S.J.; Kim, D. Acoustic wave-driven oxidized liquid metal-based energy harvester. Eur. Phys. J. Appl. Phys. 2018, 81, 20902. [Google Scholar] [CrossRef]
- Hsu, T.-H.; Manakasettharn, S.; Taylor, J.A.; Krupenkin, T. Bubbler: A Novel Ultra-High Power Density Energy Harvesting Method Based on Reverse Electrowetting. Sci. Rep. 2015, 5, 16537. [Google Scholar] [CrossRef] [PubMed]
- Dickey, M.D. Stretchable and Soft Electronics using Liquid Metals. Adv. Mater. 2017, 29, 1606425. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Du, J.; Zhang, A.; Lee, D.-W. Electrochemically controllable actuation of liquid metal droplets based on Marangoni effect. J. Appl. Phys. 2019, 126, 084505. [Google Scholar] [CrossRef]
- Matsuhisa, N.; Chen, X.; Bao, Z.; Someya, T. Materials and structural designs of stretchable conductors. Chem. Soc. Rev. 2019, 48, 2946–2966. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Parmar, M.; Lee, D.-W. An oxidized liquid metal-based microfluidic platform for tunable electronic device applications. Lab Chip 2015, 15, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Hagman, A.; Hjort, K.; Jobs, M.; Sundqvist, J.; Wu, Z. Liquid alloy printing of microfluidic stretchable electronics. Lab Chip 2012, 12, 4657–4664. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Du, J.; Zhang, A.; Lee, D. Artificial Heart Based on Electrically Controlled Non-Toxic Liquid Metal Pump. Adv. Eng. Mater. 2019, 21, 1900381. [Google Scholar] [CrossRef]
- Jeong, S.H.; Hjort, K.; Wu, Z. Tape Transfer Printing of a Liquid Metal Alloy for Stretchable RF Electronics. Sensors 2014, 14, 16311–16321. [Google Scholar] [CrossRef]
- Jeong, S.; Hjort, K.; Wu, Z. Tape Transfer Atomization Patterning of Liquid Alloys for Microfluidic Stretchable Wireless Power Transfer. Sci. Rep. 2015, 5, srep08419. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, Y.; Liu, J. Atomized spraying of liquid metal droplets on desired substrate surfaces as a generalized way for ubiquitous printed electronics. Appl. Phys. A 2014, 116, 1091–1097. [Google Scholar] [CrossRef]
- Li, G.; Parmar, M.; Kim, D.; Lee, J.-B.; Lee, D.-W. PDMS based coplanar microfluidic channels for the surface reduction of oxidized Galinstan. Lab Chip 2014, 14, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wu, X.; Lee, D.-W. Selectively plated stretchable liquid metal wires for transparent electronics. Sens. Actuators B Chem. 2015, 221, 1114–1119. [Google Scholar] [CrossRef]
- Li, G.; Lee, D.-W. An advanced selective liquid-metal plating technique for stretchable biosensor applications. Lab Chip 2017, 17, 3415–3421. [Google Scholar] [CrossRef]
- Li, G.; Wu, X.; Lee, D.-W. A galinstan-based inkjet printing system for highly stretchable electronics with self-healing capability. Lab Chip 2016, 16, 1366–1373. [Google Scholar] [CrossRef]
- Zuo, L.; Scully, B.; Shestani, J.; Zhou, Y. Design and characterization of an electromagnetic energy harvester for vehicle suspensions. Smart Mater. Struct. 2010, 19, 045003. [Google Scholar] [CrossRef]
- Yang, B.; Lee, C.; Kee, W.L.; Lim, S.-P. Hybrid energy harvester based on piezoelectric and electromagnetic mechanisms. J. Micro/Nanolithogr. MEMS MOEMS 2010, 9, 023002. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Hu, S.; Jin, Y.; Zhang, A.; Hua, L.; Du, J.; Li, G. Robust Impact Effect and Super-Lyophobic Reduced Galinstan on Polymers Applied for Energy Harvester. Polymers 2022, 14, 3633. https://doi.org/10.3390/polym14173633
Chen H, Hu S, Jin Y, Zhang A, Hua L, Du J, Li G. Robust Impact Effect and Super-Lyophobic Reduced Galinstan on Polymers Applied for Energy Harvester. Polymers. 2022; 14(17):3633. https://doi.org/10.3390/polym14173633
Chicago/Turabian StyleChen, Husheng, Shilong Hu, Yuan Jin, Aibing Zhang, Licheng Hua, Jianke Du, and Guangyong Li. 2022. "Robust Impact Effect and Super-Lyophobic Reduced Galinstan on Polymers Applied for Energy Harvester" Polymers 14, no. 17: 3633. https://doi.org/10.3390/polym14173633




