Additive Manufactured Polymers in Dentistry, Current State-of-the-Art and Future Perspectives-A Review
Abstract
:1. Introduction
2. 3D Printing Technologies in Dentistry
3. 3D Bioprinting Technologies for Dental and Maxillo-Facial Applications
4. 3D Printed Polymers for Dental Applications
5. Polymeric Bioinks
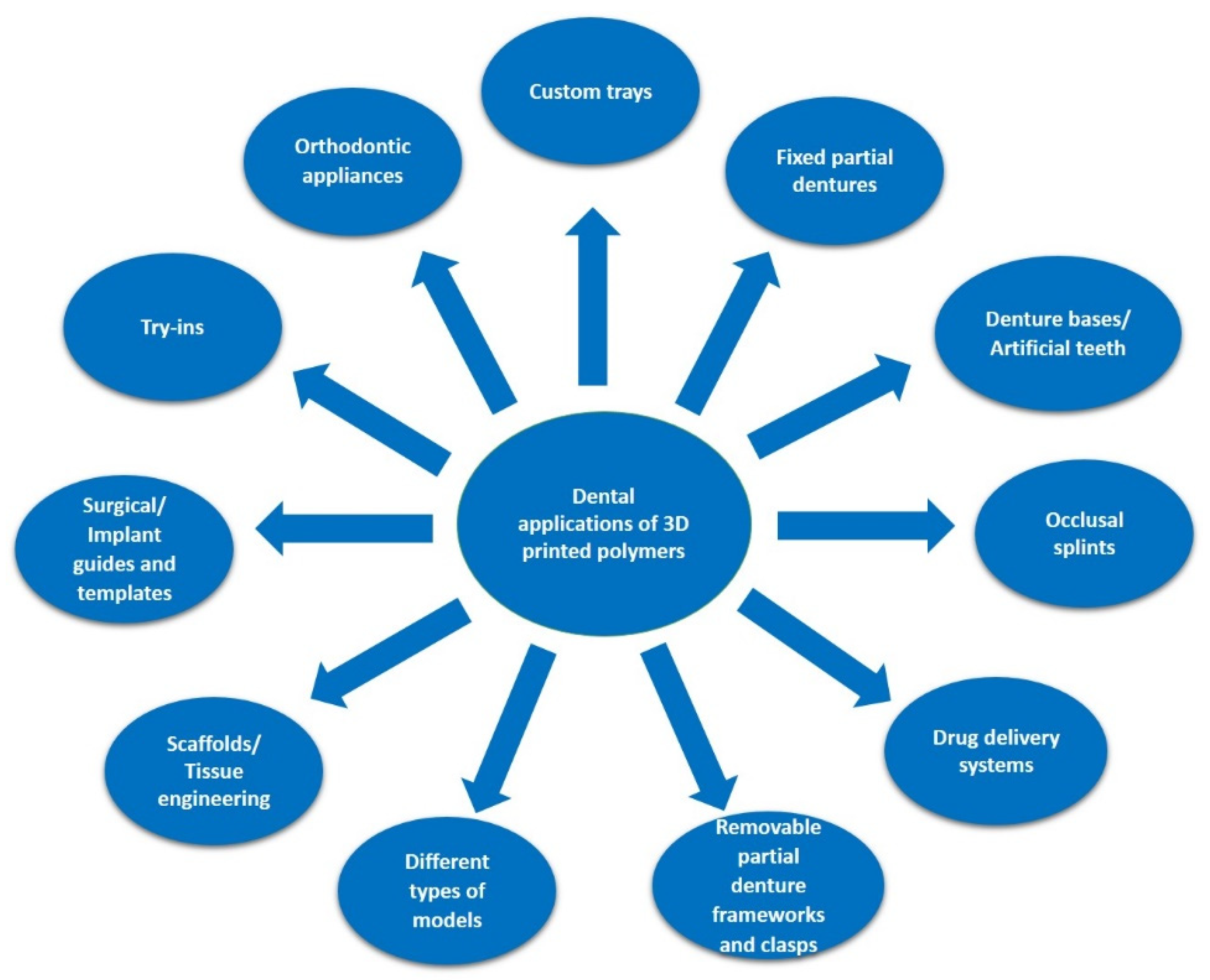
6. 3D-Printing/Bioprinting Applications in Dentistry and Maxillofacial Surgery
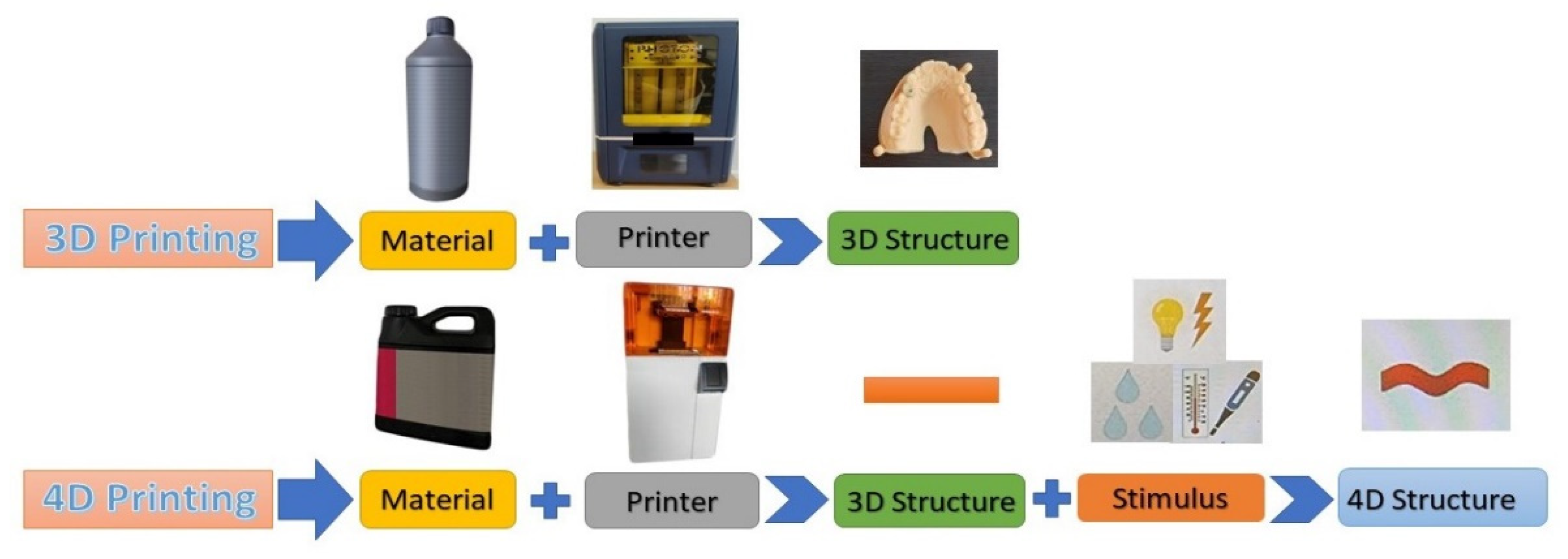
7. 4D Printing and Bioprinting
8. Concluding Remarks: Current Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomé, T.; Erhardt, M.C.G.; Leme, A.A.; Al Bakri, I.; Bedran-Russo, A.K.; Bertassoni, L.E. Emerging Polymers in Dentistry. In Advanced Polymers in Medicine; Puoci, F., Ed.; Springer: Cham, Switzerland, 2015; pp. 265–296. [Google Scholar] [CrossRef]
- Rokaya, D.; Srimaneepong, V.; Sapkota, J.; Qin, J.; Siraleartmukul, K.; Siriwongrungson, V. Polymeric materials and films in dentistry: An overview. J. Adv. Res. 2018, 14, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.R.; Lamprou, D.A. Polymer coatings for biomedical applications: A review. Trans. Ins. Met. 2014, 92, 9–19. [Google Scholar] [CrossRef]
- Nathanael, A.J.; Oh, T.H. Biopolymer Coatings for Biomedical Applications. Polymers 2020, 12, 3061. [Google Scholar] [CrossRef]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef]
- Al-Rabab’ah, M.; Hamadneh, W.; Alsalem, I.; Khraisat, A.; Karaky, A.A. Use of high performance polymers as dental implant abutments and frameworks: A case series report. J. Prosthodont. 2019, 28, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Abduo, J.; Lyons, K.; Bennamoun, M. Trends in Computer-Aided Manufacturing in Prosthodontics: A Review of the Available Streams. Int. J. Dent. 2014, 2014, 783948. [Google Scholar] [CrossRef] [PubMed]
- Clark Ligon, S.; Liska, R.; Stampfl, J.; Gurr, M.; Mulhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Okolie, O.; Stachurek, I.; Kandasubramanian, B.; Njuguna, J. 3D Printing for Hip Implant Applications: A Review. Polymers 2020, 12, 2682. [Google Scholar] [CrossRef]
- Pillai, S.; Upadhyay, A.; Khayambashi, P.; Farooq, I.; Sabri, H.; Tarar, M.; Lee, K.T.; Harb, I.; Zhou, S.; Wang, Y.; et al. Dental 3D-Printing: Transferring Art from the Laboratories to the Clinics. Polymers 2021, 13, 157. [Google Scholar] [CrossRef]
- Ujfalusi, Z.; Pentek, A.; Told, R.; Schiffer, A.; Nyitrai, M.; Maroti, P. Detailed Thermal Characterization of Acrylonitrile Butadiene Styrene and Polylactic Acid Based Carbon Composites Used in Additive Manufacturing. Polymers 2020, 12, 2960. [Google Scholar] [CrossRef]
- Heo, H.; Jin, Y.; Yang, D.; Wier, C.; Minard, A.; Dahotre, N.B.; Neogi, A. Manufacturing and Characterization of Hybrid Bulk Voxelated Biomaterials Printed by Digital Anatomy 3D Printing. Polymers 2021, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Hickel, R.; Reymus, M. 3D printing in dentistry—State of the art. Oper. Dent. 2020, 45, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Torabi, K.; Farjood, E.; Hamedani, E. Rapid prototyping technologies and their applications in prosthodontics, a review of literature. J. Dent. 2015, 16, 1–9. [Google Scholar]
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef]
- Theus, A.S.; Ning, L.; Hwang, B.; Gil, C.; Chen, S.; Wombwell, A.; Mehta, R.; Serpooshan, V. Bioprintability: Physiomechanical and Biological Requirements of Materials for 3D Bioprinting Processes. Polymers 2020, 12, 2262. [Google Scholar] [CrossRef]
- Jockusch, J.; Özcan, M. Additive manufacturing of dental polymers: An overview on processes, materials and applications. Dent. Mater. J. 2020, 39, 345–354. [Google Scholar] [CrossRef]
- Nold, J.; Wesemann, C.; Rieg, L.; Binder, L.; Witkowski, S.; Spies, B.C.; Kohal, R.J. Does Printing Orientation Matter? In-Vitro Fracture Strength of Temporary Fixed Dental Prostheses after a 1-Year Simulation in the Artificial Mouth. Materials 2021, 14, 259. [Google Scholar] [CrossRef]
- Groth, C.; Kravitz, N.D.; Jones, P.E.; Graham, J.W.; Redmond, W.R. Three-dimensional printing technology. J. Clin. Orthod. 2014, 48, 475–485. [Google Scholar]
- Etemad-Shahidi, Y.; Qallandar, O.B.; Evenden, J.; Alifui-Segbaya, F.; Ahmed, K.E. Accuracy of 3-Dimensionally Printed Full-Arch Dental Models: A Systematic Review. J. Clin. Med. 2020, 9, 3357. [Google Scholar] [CrossRef]
- Schweiger, J.; Edelhoff, D.; Güth, J.-F. 3D Printing in Digital Prosthetic Dentistry: An Overview of Recent Developments in Additive Manufacturing. J. Clin. Med. 2021, 10, 2010. [Google Scholar] [CrossRef]
- Prasad, A.; Kandasubramanian, B. Fused Deposition Processing Polycaprolactone of Composites for Biomedical Applications. Polym. Plast. Technol. Mater. 2019, 58, 1365–1398. [Google Scholar] [CrossRef]
- Rocha, C.R.; Perez, A.R.T.; Roberson, D.A.; Shemelya, C.M.; MacDonald, E.; Wicker, R.B. Novel ABS-based binary and ternary polymer blends for material extrusion 3D printing. J. Mater. Res. 2014, 29, 1859–1866. [Google Scholar] [CrossRef]
- Rebong, R.E.; Stewart, K.T.; Utreja, A.; Ghoneima, A.A. Accuracy of three-dimensional dental resin models created by fused deposition modeling, stereolithography, and Polyjet prototype technologies: A comparative study. Angle Orthod. 2018, 88, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhou, C.; Chen, Y. A Fast Mask Projection Stereolithography Process for Fabricating Digital Models in Minutes. J. Manuf. Sci. Eng. 2012, 134, 051011. [Google Scholar] [CrossRef]
- Hazeveld, A.; Huddleston Slater, J.J.R.; Ren, Y. Accuracy and reproducibility of dental replica models reconstructed by different rapid prototyping techniques. Am. J. Orthod. Dentofac. Orthop. 2014, 145, 108–115. [Google Scholar] [CrossRef]
- Greco, G.B.; Popi, D.; Di Stefano, D.A. Accuracy of 3-dimensional printing of dental casts: A proposal for quality standardization. J. Prosthet. Dent. 2022, 127, 899–910. [Google Scholar] [CrossRef]
- Alharbi, N.; Osman, R.; Wismeijer, D. Effects of build direction on the mechanical properties of 3D-printed complete coverage interim dental restorations. J. Prosthet. Dent. 2016, 115, 760–767. [Google Scholar] [CrossRef]
- Li, X.; Xie, B.; Jin, J.; Chai, Y.; Chen, Y. 3D Printing Temporary Crown and Bridge by Temperature Controlled Mask Image Projection Stereolithography. Procedia Manuf. 2018, 26, 1023–1033. [Google Scholar] [CrossRef]
- Dikova, T. Production of high-quality temporary crowns and bridges by stereolithography. Scr. Sci. Med. Dent. 2019, 5, 33–38. [Google Scholar] [CrossRef]
- Maeda, Y.; Minoura, M.; Tsutsumi, S.; Okada, M.; Nokubi, T. A CAD/CAM system for removable denture. Part I: Fabrication of complete dentures. Int. J. Prosthodont. 1994, 7, 17–21. [Google Scholar]
- Hada, T.; Kanazawa, M.; Iwaki, M.; Arakida, T.; Soeda, Y.; Katheng, A.; Otake, R.; Minakuchi, S. Effect of printing direction on the accuracy of 3D-printed dentures using stereolithography technology. Materials 2020, 13, 3405. [Google Scholar] [CrossRef] [PubMed]
- Piedra Cascon, W.; Revilla-León, M. Digital workflow for the design and additively manufacture of a splinted framework and custom tray for the impression of multiple implants: A dental technique. J. Prosthet. Dent. 2018, 120, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Esposito Corcione, C.; Paladini, F.; Gallo, A.L.; Montagna, F.; Jamaledin, R.; Pollini, M.; Maffezzoli, A. Antimicrobial modified hydroxyapatite composite dental bite by stereolithography. Polym. Adv. Technol. 2018, 29, 364–371. [Google Scholar] [CrossRef]
- Yu, J.H.; Wang, Y.T.; Lin, C.L. Customized surgical template fabrication under biomechanical consideration by integrating CBCT image, CAD system and finite element analysis. Dent. Mater. J. 2018, 37, 6–14. [Google Scholar] [CrossRef]
- Sun, Y.; Luebbers, H.; Agbaje, J.O.; Schepers, S.; Politis, C.; Van Slycke, S.; Vrielinck, L. Accuracy of dental implant placement using CBCT-derived mucosa-supported stereolithographic template. Clin. Implant Dent. Relat. Res. 2015, 17, 862–870. [Google Scholar] [CrossRef]
- Ersoy, A.E.; Turkyilmaz, I.; Ozan, O.; McGlumphy, E.A. Reliability of implant placement with stereolithographic surgical guides generated from computed tomography: Clinical data from 94 implants. J. Periodontol. 2008, 79, 1339–1345. [Google Scholar] [CrossRef]
- Cassetta, M.; Di Carlo, S.; Pranno, N.; Sorrentino, V.; Di Giorgio, G.; Pompa, G. The use of stereolithographic surgical templates in oral implantology. Ann. Ital. Chir. 2013, 84, 589–593. [Google Scholar]
- Ozan, O.; Turkyilmaz, I.; Ersoy, A.E.; McGlumphy, E.A.; Rosenstiel, S.F. Clinical accuracy of 3 different types of computed tomography-derived stereolithographic surgical guides in implant placement. J. Oral Maxillofac. Surg. 2009, 67, 394–401. [Google Scholar] [CrossRef]
- D’haese, J.; Van De Velde, T.; Komiyama, A.; Hultin, M.; De Bruyn, H. Accuracy and complications using computer-designed stereolithographic surgical guides for oral rehabilitation by means of dental implants: A review of the literature. Clin. Implant Dent. Relat. Res. 2012, 14, 321–335. [Google Scholar] [CrossRef]
- Herschdorfer, L.; Negreiros, W.; Gallucci, G.; Hamilton, A. Comparison of the accuracy of implants placed with CAD-CAM surgical templates manufactured with various 3D printers: An in vitro study. J. Prosthet. Dent. 2020, 125, 905–910. [Google Scholar] [CrossRef]
- Piedra Cascon, W.; Parra Nunez, A.; Charlen Diez, I.; Revilla León, M. Laboratory workflow to obtain long-term injected resin composite interim restorations from an additive manufactured esthetic diagnostic template. J. Esthet. Restor. Dent. 2019, 31, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Al Mortadi, N.; Jones, Q.; Eggbeer, D.; Lewis, J.; Williams, R.J. Fabrication of a resin appliance with alloy components using digital technology without an analog impression. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 862–867. [Google Scholar] [CrossRef]
- Elomaa, L.; Teixeira, S.; Hakala, R.; Korhonen, H.; Grijpma, D.W.; Seppala, J.V. Preparation of poly(epsilon-caprolactone)-based tissue engineering scaffolds by stereolithography. Acta Biomater. 2011, 7, 3850–3856. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Yeatts, A.; Dean, D.; Fisher, J.P. Stereolithographic bone scaffold design parameters: Osteogenic differentiation and signal expression. Tissue Eng. Part B Rev. 2010, 16, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Skoog, S.A.; Goering, P.L.; Narayan, R.J. Stereolithography in tissue engineering. J. Mater. Sci. Mater. Med. 2014, 25, 845–856. [Google Scholar] [CrossRef]
- Arifin, N.; Sudin, I.; Ngadiman, N.H.A.; Ishak, M.S.A. A Comprehensive Review of Biopolymer Fabrication in Additive Manufacturing Processing for 3D-Tissue-Engineering Scaffolds. Polymers 2022, 14, 2119. [Google Scholar] [CrossRef]
- Osman, R.B.; Alharbi, N.; Wismeijer, D. Build angle: Does it influence the accuracy of 3D-printed dental restorations using digital light-processing technology? Int. J. Prosthodont. 2017, 30, 182–188. [Google Scholar] [CrossRef]
- Lee, H.; Son, K.B.D.; Lee, D.-H.; Kim, S.-Y.; Lee, K.-B. Comparison of wear of interim crowns in accordance with the build angle of digital light processing 3-dimensional printing: A pilot in vivo study. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Williams, R.J.; Bibb, R.; Rafik, T. A technique for fabricating patterns for removable partial denture frameworks using digitized casts and electronic surveying. J. Prosthet. Dent. 2004, 91, 85–88. [Google Scholar] [CrossRef]
- Lin, W.-S.; Harris, B.T.; Pellerito, J.; Morton, D. Fabrication of an interim complete removable dental prosthesis with an in-office digital light processing three-dimensional printer: A proof-of-concept technique. J. Prosthet. Dent. 2018, 120, 331–334. [Google Scholar] [CrossRef]
- Bilgin, M.S.; Erdem, A.; Aglarci, O.S.; Dilber, E. Fabricating complete dentures with CAD/CAM and RP technologies. J. Prosthodont. 2015, 24, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Revilla-León, M.; Besné-Torre, A.; Sánchez-Rubio, J.L.; Fábrega, J.J.; Özcan, M. Digital tools and 3D printing technologies integrated into the workflow of restorative treatment: A clinical report. J. Prosthet. Dent. 2019, 121, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Revilla-León, M.; Sánchez-Rubio, J.L.; Oteo-Calatayud, J.; Özcan, M. Impression technique for a complete-arch prosthesis with multiple implants using additive manufacturing technologies. J. Prosthet. Dent. 2017, 117, 714–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagheri Saed, A.; Behravesh, A.H.; Hasannia, S.; Alavinasab Ardebili, S.A.; Akhoundi, B.; Pourghayoumi, M. Functionalized poly l-lactic acid synthesis and optimization of process parameters for 3D printing of porous scaffolds via digital light processing (DLP) method. J. Manuf. Process. 2020, 56, 550–561. [Google Scholar] [CrossRef]
- Werz, S.M.; Zeichner, S.J.; Berg, B.I.; Zeilhofer, H.F.; Thieringer, F. 3D printed surgical simulation models as educational tool by maxillofacial surgeons. Eur. J. Dent. Educ. 2018, 22, e500–e505. [Google Scholar] [CrossRef]
- Park, M.E.; Shin, S.Y. Three-dimensional comparative study on the accuracy and reproducibility of dental casts fabricated by 3D printers. J. Prosthet. Dent. 2018, 119, 861.e1–861.e7. [Google Scholar] [CrossRef]
- Mai, H.-N.; Lee, K.-B.; Lee, D.-H. Fit of interim crowns fabricated using photopolymer-jetting 3D printing. J. Prosthet. Dent. 2017, 118, 208–215. [Google Scholar] [CrossRef]
- Bibb, R.; Eggbeer, D.; Williams, R. Rapid manufacture of removable partial denture frameworks. Rapid Prototyp. J. 2006, 12, 95–99. [Google Scholar] [CrossRef]
- Bibb, R.J.; Eggbeer, D.; Williams, R.; Woodward, A. Trial fitting of a removable partial denture framework made using computer-aided design and rapid prototyping techniques. Proc. Inst. Mech. Eng. H. 2006, 220, 793–797. [Google Scholar] [CrossRef]
- Inokoshi, M.; Kanazawa, M.; Minakuchi, S. Evaluation of a complete denture trial method applying rapid prototyping. Dent. Mater. J. 2012, 31, 40–46. [Google Scholar] [CrossRef]
- Kanazawa, M.; Iwaki, M.; Arakida, T.; Minakuchi, S. Digital impression and jaw relation record for the fabrication of CAD/CAM custom tray. J. Prosthodont. Res. 2018, 62, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Juneja, M.; Thakur, N.; Kumar, D.; Gupta, A.; Bajwa, B.; Jindal, P. Accuracy in dental surgical guide fabrication using different 3-D printing techniques. Addit. Manuf. 2018, 22, 243–255. [Google Scholar] [CrossRef]
- Panayotov, I.V.; Orti, V.; Cuisinier, F.; Yachouh, J. Polyetheretherketone (PEEK) for medical applications. J. Mater. Sci. Mater. Med. 2016, 27, 118. [Google Scholar] [CrossRef] [PubMed]
- Qu, H. Additive manufacturing for bone tissue engineering scaffolds. Mater. Today Commun. 2020, 24, 101024. [Google Scholar] [CrossRef]
- Silva, D.S.; Wallace, D.B.; Cooley, P.W.; Radulescu, D.; Hayes, D.J. An inkjet printing station for neuroregenerative tissue engineering. In Proceedings of the 2007 IEEE Dallas Engineering in Medicine and Biology Workshop, Dallas, TX, USA, 11–12 November 2007. [Google Scholar]
- Marga, F.; Jakab, K.; Khatiwala, C.; Shepherd, B.; Dorfman, S.; Hubbard, B.; Colbert, S.; Forgacs, G. Toward engineering functional organ modules by additive manufacturing. Biofabrication 2012, 4, 022001. [Google Scholar] [CrossRef]
- Athirasala, A.; Tahayeri, A.; Thrivikraman, G.; Franca, C.M.; Monteiro, N.; Tran, V.; Ferracane, J.; Bertassoni, L.E. A dentin-derived hydrogel bioink for 3D bioprinting of cell laden scaffolds for regenerative dentistry. Biofabrication 2018, 10, 024101. [Google Scholar] [CrossRef]
- Wendel, B.; Rietzel, D.; Kühnlein, F.; Feulner, R.; Hülder, G.; Schmachtenberg, E. Additive processing of polymers. Macromol. Mater. Eng. 2008, 293, 799–809. [Google Scholar] [CrossRef]
- Muta, S.; Ikeda, M.; Nikaido, T.; Sayed, M.; Sadr, A.; Suzuki, T.; Tagami, J. Chairside fabrication of provisional crowns on FDM 3D-printed PVA model. J. Prosthodont. Res. 2020, 64, 401–407. [Google Scholar] [CrossRef]
- Singh, R.; Dureja, J.S. Dental Crowns by FDM Assisted Vapour Smoothing and Silicon Moulding. In 3D Printing in Biomedical Engineering. Materials Horizons: From Nature to Nanomaterials; Singh, S., Prakash, C., Singh, R., Eds.; Springer: Singapore, 2020; pp. 231–250. [Google Scholar] [CrossRef]
- Lo Russo, L.; Lo Muzio, E.; Troiano, G.; Salamini, A.; Zhurakivska, K.; Guida, L. Accuracy of trial complete dentures fabricated by using fused deposition modeling 3-dimensional printing: An in vitro study. J. Prosthet. Dent. 2021, S0022–3913, 416–419. [Google Scholar] [CrossRef]
- Solaberrieta, E.; Minguez, R.; Barrenetxea, L.; Sierra, E.; Etxaniz, O. Computer-aided dental prostheses construction using reverse engineering. Comput. Methods Biomech. Biomed. Eng. 2014, 17, 1335–1346. [Google Scholar] [CrossRef]
- Chen, H.; Yang, X.; Chen, L.; Wang, Y.; Sun, Y. Application of FDM three-dimensional printing technology in the digital manufacture of custom edentulous mandible trays. Sci. Rep. 2016, 6, 19207. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, D.; Young Lee, S.; Yang, H.; Park, S.W.; Lim, H.P.; Yun, K.D.; Park, C. Denture flask fabrication using fused deposition modeling three-dimensional printing. J. Prosthodont. Res. 2020, 64, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Gronet, P.M.; Waskewicz, G.A.; Richardson, C. Preformed acrylic cranial implants using fused deposition modeling: A clinical report. J. Prosthet. Dent. 2002, 90, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Zein, I.; Hutmacher, D.W.; Tan, K.C.; Teoh, S.H. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 2002, 23, 1169–1185. [Google Scholar] [CrossRef]
- Serra, T.; Mateos-Timoneda, M.A.; Planell, J.A.; Navarro, M. 3D printed PLA-based scaffolds: A versatile tool in regenerative medicine. Organogenesis 2013, 9, 239–244. [Google Scholar] [CrossRef]
- Liang, K.; Carmone, S.; Brambilla, D.; Leroux, J.C. 3D printing of a wearable personalized oral delivery device: A first-in-human study. Sci. Adv. 2018, 4, eaat2544. [Google Scholar] [CrossRef] [PubMed]
- Fuenmayor, E.; Forde, M.; Healy, A.V.; Devine, D.M.; Lyons, J.G.; McConville, C.; Major, I. Material considerations for fused-filament fabrication of solid dosage forms. Pharmaceutics 2018, 10, 44. [Google Scholar] [CrossRef]
- Zhang, Q.; Bei, H.-P.; Zhao, M.; Dong, Z.; Zhao, X. Shedding light on 3D printing: Printing photo-crosslinkable constructs for tissue engineering. Biomaterials 2022, 286, 121566. [Google Scholar] [CrossRef]
- Roi, A.; Ardelean, L.C.; Roi, C.I.; Boia, E.-R.; Boia, S.; Rusu, L.-C. Oral Bone Tissue Engineering: Advanced Biomaterials for Cell Adhesion, Proliferation and Differentiation. Materials 2019, 12, 2296. [Google Scholar] [CrossRef]
- Papaioannou, T.G.; Manolesou, D.; Dimakakos, E.; Tsoucalas, G.; Vavuranakis, M.; Tousoulis, D. 3D Bioprinting Methods and Techniques: Applications on Artificial Blood Vessel Fabrication. Acta Cardiol. Sin. 2019, 35, 284–289. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [PubMed]
- Kyle, S.; Jessop, Z.M.; Al-Sabah, A.; Whitaker, I.S. ‘Printability’ of Candidate Biomaterials for Extrusion Based 3D Printing: State-of-the-Art. Adv. Healthc. Mater. 2017, 6, 1700264. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, F.; Zhao, H.; Gao, Q.; Xia, B.; Fu, J. Research on the printability of hydrogels in 3D bioprinting. Sci. Rep. 2016, 6, 29977. [Google Scholar] [CrossRef] [PubMed]
- Hölzl, K.; Lin, S.; Tytgat, L.; van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Martin, J.A.; Ozbolat, I.T. Evaluation of cell viability and functionality in vessel-like bioprintable cell-laden tubular channels. J. Biomech. Eng. 2013, 135, 91011. [Google Scholar] [CrossRef]
- Ning, L.; Chen, X. A brief review of extrusion-based tissue scaffold bio-printing. Biotechnol. J. 2017, 12, 1600671. [Google Scholar] [CrossRef]
- Costa, P.F.; Vaquette, C.; Zhang, Q.; Reis, R.L.; Ivanovski, S.; Hutmacher, D.W. Advanced tissue engineering scaffold design for regeneration of the complex hierarchical periodontal structure. J. Clin. Periodontol. 2014, 41, 283–294. [Google Scholar] [CrossRef]
- Sun, W.; Starly, B.; Daly, A.C.; Burdick, J.A.; Groll, J.; Skeldon, G.; Shu, W.; Sakai, Y.; Shinohara, M.; Nishikawa, M.; et al. The bioprinting roadmap. Biofabrication 2020, 12, 022002. [Google Scholar] [CrossRef]
- Raman, R.; Bashir, R. Stereolithographic 3D Bioprinting for Biomedical Applications. In Essentials of 3D Biofabrication and Translation; Atala, A., Yoo, J.J., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 89–121. [Google Scholar]
- Kim, S.H.; Yeon, Y.K.; Lee, J.M.; Chao, J.R.; Lee, Y.J.; Seo, Y.B.; Sultan, M.T.; Lee, O.J.; Lee, J.S.; Yoon, S.I.; et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat. Commun. 2018, 9, 1620. [Google Scholar] [CrossRef]
- Rider, P.; Kacarevic, Z.P.; Alkildani, S.; Retnasingh, S.; Barbeck, M. Bioprinting of tissue engineering scaffolds. J. Tissue Eng. 2018, 9, 2041731418802090. [Google Scholar] [CrossRef]
- Xu, T.; Jin, J.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing of viable mammalian cells. Biomaterials 2005, 26, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Saunders, R.E.; Derby, B. Inkjet printing biomaterials for tissue engineering: Bioprinting. Int. Mater. Rev. 2014, 59, 430–448. [Google Scholar] [CrossRef]
- Guillotin, B.; Souquet, A.; Catros, S.; Duocastella, M.; Pippenger, B.; Bellance, S.; Bareille, R.; Remy, M.; Bordenave, L.; Amedee, J.; et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 2010, 31, 7250–7256. [Google Scholar] [CrossRef] [PubMed]
- Guillotin, B.; Ali, M.; Ducom, A.; Catros, S.; Keriquel, V.; Souquet, A.; Remy, M.; Fricain, J.-C.; Guillemot, F. Laser-Assisted Bioprinting for Tissue Engineering. In Biofabrication Micro- and Nano-Fabrication, Printing, Patterning, and Assemblies; Forgacs, G., Sun, W., Eds.; William Andrew Publishing: Boston, MA, USA, 2013; pp. 95–118. [Google Scholar]
- An, J.; Ee Mei Teoh, J.; Suntornnond, R.; Kai Chua, C. Design and 3D Printing of Scaffolds and Tissues. Engineering 2015, 1, 261–268. [Google Scholar] [CrossRef]
- Tzeng, J.-J.; Yang, T.-S.; Lee, W.-F.; Chen, H.; Chang, H.-M. Mechanical Properties and Biocompatibility of Urethane Acrylate-Based 3D-Printed Denture Base Resin. Polymers 2021, 13, 822. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.; He, Z.; Hong, J.; Bao, J. Urethane acrylate-based photosensitive resin for three-dimensional printing of stereolithographic elastomer. J. Appl. Polym. Sci. 2020, 137, 49294. [Google Scholar] [CrossRef]
- Chen, S.-G.; Yang, J.; Jia, Y.-G.; Lu, B.; Ren, L. TiO2 and PEEK reinforced 3D printing PMMA composite resin for dental denture base applications. Nanomaterials 2019, 9, 1049. [Google Scholar] [CrossRef]
- Khorsandi, D.; Fahimipour, A.; Abasian, P.; Saber, S.S.; Seyedi, M.; Ghanavati, S.; Ahmad, A.; De Stephanis, A.A.; Taghavinezhaddilami, F.; Leonova, A.; et al. 3D and 4D printing in dentistry and maxillofacial surgery: Printing techniques, materials, and applications. Acta Biomater. 2021, 122, 26–49. [Google Scholar] [CrossRef]
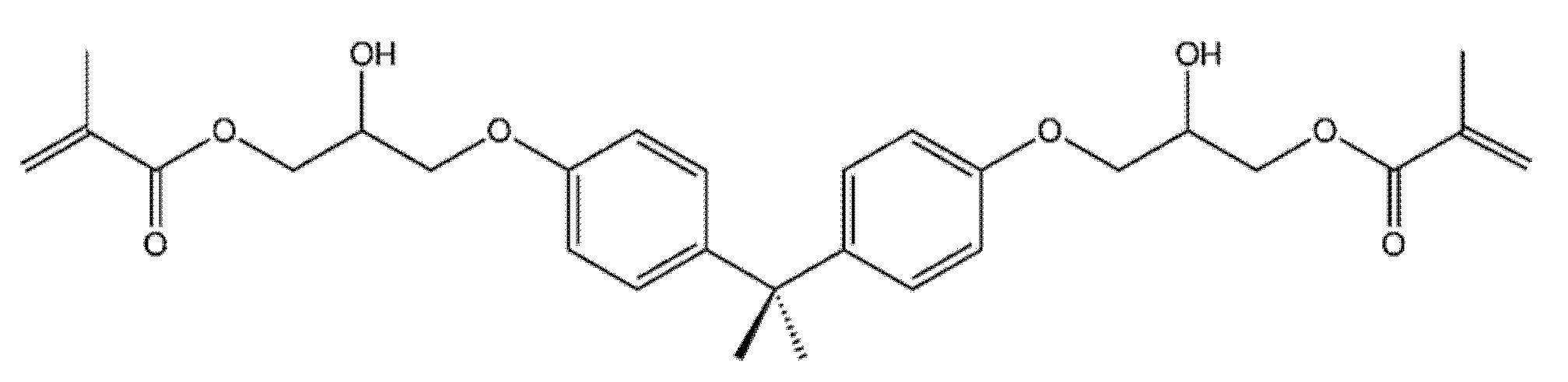
- Lin, C.-H.; Lin, Y.-M.; Lai, Y.-L.; Lee, S.-Y. Mechanical properties, accuracy, and cytotoxicity of UV-polymerized 3D printing resins composed of Bis-EMA, UDMA, and TEGDMA. J. Prosthet. Dent. 2020, 123, 349–354. [Google Scholar] [CrossRef]
- Rusu, L.-C.; Ardelean, L.C.; Jitariu, A.-A.; Miu, C.A.; Streian, C.G. An Insight into the Structural Diversity and Clinical Applicability of Polyurethanes in Biomedicine. Polymers 2020, 12, 1197. [Google Scholar] [CrossRef]
- Chen, H.; Lee, S.-Y.; Lin, Y.-M. Synthesis and formulation of PCL-based urethane acrylates for DLP 3D printers. Polymers 2020, 12, 1500. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.; Kashani, A.; Imbalzano, G.; Nguyen, K.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Molinero-Mourelle, P.; Canals, S.; Gómez-Polo, M.; Fernanda Solá-Ruiz, M.; del Río Highsmith, J.; Celemín Viñuela, A.; Solá-Ruiz, M.F.; Viñuela, A.C. Polylactic acid as a material for three-dimensional printing of provisional restorations. Int. J. Prosthodont. 2018, 31, 349–350. [Google Scholar] [CrossRef]
- Benli, M.; Eker-Gümüş, B.; Kahraman, Y.; Huck, O.; Özcan, M. Can polylactic acid be a CAD/CAM material for provisional crown restorations in terms of fit and fracture strength? Dent. Mater. J. 2021, 40, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Ziąbka, M.; Dziadek, M.; Pielichowska, K. Surface and Structural Properties of Medical Acrylonitrile Butadiene Styrene Modified with Silver Nanoparticles. Polymers 2020, 12, 197. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Zuhri, M.Y.M.; Aisyah, H.A.; Asyraf, M.R.M.; Hassan, S.A.; Zainudin, E.S.; Sapuan, S.M.; Sharma, S.; Bangar, S.P.; Jumaidin, R.; et al. Natural Fiber-Reinforced Polylactic Acid, Polylactic Acid Blends and Their Composites for Advanced Applications. Polymers 2022, 14, 202. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Naakh, M.; Marco, C.; Ellis, G.; Gómez-Fatou, M.A. High-performance nanocomposites based on polyetherketones. Prog. Mater. Sci. 2012, 57, 1106–1190. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef]
- Ardelean, L.C.; Rusu, L.-C.; Tigmeanu, C.V. Alternative Denture Base Materials for Allergic Patients. In Oral Health Care—An Important Issue of the Modern Society; Ardelean, L.C., Rusu, L.-C., Eds.; IntechOpen: London, UK, 2022. [Google Scholar]
- Skirbutis, G.; Dzingute, A.; Masiliunaite, V.; Sulcaite, G.; Zilinskas, G. A review of PEEK polymer’s properties and its use in prosthodontics. Stomatologija 2017, 19, 19–23. [Google Scholar]
- Vaezi, M.; Yang, S. Extrusion-based additive manufacturing of PEEK for biomedical applications. Virtual Phys. Prototyp. 2015, 10, 123–135. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Polyetheretherketone and Its Composites for Bone Replacement and Regeneration. Polymers 2020, 12, 2858. [Google Scholar] [CrossRef] [PubMed]
- Rahmitasari, F.; Ishida, Y.; Kurahashi, K.; Matsuda, T.; Watanabe, M.; Ichikawa, T. PEEK with reinforced materials and modifications for dental implant applications. Dent. J. 2017, 5, 35. [Google Scholar] [CrossRef]
- Farzin, A.; Bahrami, N.; Mohamadnia, N.; Mousavi, S.; Gholami, M.; Ai, J.; Moayeri, R.S. Scaffolds in Dental Tissue Engineering: A Review. Arch. Neurosci. 2020, 7, e97014. [Google Scholar] [CrossRef] [Green Version]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jin, Y.; Yin, J.; Xu, C.; Xiong, R.; Christensen, K.; Ringeisen, B.R.; Chrisey, D.B.; Huang, Y. Evaluation of bioink printability for bioprinting applications. Appl. Phys. Rev. 2018, 5, 041304. [Google Scholar] [CrossRef]
- Visser, J.; Peters, B.; Burger, T.J.; Boomstra, J.; Dhert, W.J.; Melchels, F.P.; Malda, J. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication 2013, 5, 035007. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Jang, J.; Chae, S.; Gao, G.; Kong, J.-S.; Ahn, M.; Cho, D.-W. Three-dimensional bioprinting of cell-laden constructs with polycaprolactone protective layers for using various thermoplastic polymers. Biofabrication 2016, 8, 035013. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, J.Y.; Cho, D.W. Solid Free-form Fabrication Technology and Its Application to Bone Tissue Engineering. Int. J. Stem Cells 2010, 3, 85–95. [Google Scholar] [CrossRef]
- Du, B.L.; Zeng, C.G.; Zhang, W.; Quan, D.P.; Ling, E.A.; Zeng, Y.S. A comparative study of gelatin sponge scaffolds and PLGA scaffolds transplanted to completely transected spinal cord of rat. J. Biomed. Mater. Res. A 2014, 102, 1715–1725. [Google Scholar] [CrossRef]
- Putnam, D. Drug delivery: The heart of the matter. Nat. Mater. 2008, 7, 836–837. [Google Scholar] [CrossRef]
- Yu, C.; Schimelman, J.; Wang, P.; Miller, K.L.; Ma, X.; You, S.; Guan, J.; Sun, B.; Zhu, W.; Chen, S. Photopolymerizable biomaterials and light-based 3D printing strategies for biomedical applications. Chem. Rev. 2020, 120, 10695–10743. [Google Scholar] [CrossRef] [PubMed]
- Soliman, B.G.; Major, G.S.; Atienza-Roca, P.; Murphy, C.A.; Longoni, A.; Alcala- Orozco, C.R.; Rnjak-Kovacina, J.; Gawlitta, D.; Woodfield, T.B.; Lim, K.S. Development and characterization of gelatin-norbornene bioink to understand the interplay between physical architecture and micro-capillary formation in biofabricated vascularized constructs. Adv. Healthc. Mater. 2022, 11, 2101873. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kang, B.; Cui, X.; Lee, S.H.; Lee, K.; Cho, D.W.; Hwang, W.; Woodfield, T.B.; Lim, K.S.; Jang, J. Light-activated decellularized extracellular matrix-based bioinks for volumetric tissue analogs at the centimeter scale. Adv. Funct. Mater. 2021, 31, 2011252. [Google Scholar] [CrossRef]
- Kuhnt, T.; García, R.M.; Camarero-Espinosa, S.; Dias, A.; Ten Cate, A.T.; van Blitterswijk, C.A.; Moroni, L.; Baker, M.B. Poly (caprolactone-co-trimethylene carbonate) urethane acrylate resins for digital light processing of bioresorbable tissue engineering implants. Biomater. Sci. 2019, 7, 4984–4989. [Google Scholar] [CrossRef] [PubMed]
- Do, A.V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Donate, R.; Monzón, M.; Alemán-Domínguez, M.E. Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties. e-Polymers 2020, 20, 571–599. [Google Scholar] [CrossRef]
- Woodard, L.N.; Grunlan, M.A. Hydrolytic degradation and erosion of polyester biomaterials. ACS Macro Lett. 2018, 7, 976–982. [Google Scholar] [CrossRef]
- Abdullah, M.R.; Goharian, A.; Abdul Kadir, M.R.; Wahit, M.U. (2015). Biomechanical and bioactivity concepts of polyetheretherketone composites for use in orthopedic implants-a review. J. Biomed. Mater. Res. Part A 2015, 103, 3689–3702. [Google Scholar] [CrossRef]
- Feng, P.; Jia, J.; Peng, S.; Yang, W.; Bin, S.; Shuai, C. Graphene oxide-driven interfacial coupling in laser 3D printed PEEK/PVA scaffolds for bone regeneration. Virtual Phys. Prototyp. 2020, 15, 211–226. [Google Scholar] [CrossRef]
- Rosenzweig, D.H.; Carelli, E.; Steffen, T.; Jarzem, P.; Haglund, L. 3D-Printed ABS and PLA Scaffolds for Cartilage and Nucleus Pulposus Tissue Regeneration. Int. J. Mol. Sci. 2015, 16, 15118–15135. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, F.; Wang, X.; Zhang, J.; Wang, D.; Huang, X. A photocurable hybrid chitosan/acrylamide bioink for DLP based 3D bioprinting. Mater. Des. 2021, 202, 109588. [Google Scholar] [CrossRef]
- Li, Y.; Liao, C.; Tjong, S.C. Synthetic biodegradable aliphatic polyester nanocomposites reinforced with nanohydroxyapatite and/or graphene oxide for bone tissue engineering applications. Nanomaterials 2019, 9, 590. [Google Scholar] [CrossRef] [PubMed]
- Cortes, A.; Cosola, A.; Sangermano, M.; Campo, M.; Gonzalez Prolongo, S.; Pirri, C.F.; Jimenez-Suarez, A.; Chiappone, A. DLP 4D-printing of remotely, modularly, and selectively controllable shape memory polymer nanocomposites embedding carbon nanotubes. Adv. Funct. Mater. 2021, 31, 2106774. [Google Scholar] [CrossRef]
- Siebert, L.; Luna-Ceron, E.; García-Rivera, L.E.; Oh, J.; Jang, J.; Rosas-Gomez, D.A.; Perez-Gomez, M.D.; Maschkowitz, G.; Fickenscher, H.; Oceguera-Cuevas, D.; et al. Light-controlled growth factors release on tetrapodal ZnO-incorporated 3D-printed hydrogels for developing smart wound scaffold. Adv. Funct. Mater. 2021, 31, 2007555. [Google Scholar] [CrossRef]
- Miao, J.-T.; Ge, M.; Wu, Y.; Peng, S.; Zheng, L.; Chou, T.Y.; Wu, L. 3D printing of sacrificial thermosetting mold for building near-infrared irradiation induced self-healable 3D smart structures. Chem. Eng. J. 2022, 427, 131580. [Google Scholar] [CrossRef]
- Nesic, D.; Schaefer, B.M.; Sun, Y.; Saulacic, N.; Sailer, I. 3D Printing Approach in Dentistry: The Future for Personalized Oral Soft Tissue Regeneration. J. Clin. Med. 2020, 9, 2238. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Mohammadi Amirabad, L.; Zarrintaj, P.; Lindemuth, A.; Tayebi, L. Whole Tooth Engineering. In Applications of Biomedical Engineering in Dentistry; Tayebi, L., Ed.; Springer: Cham. Switzerland, 2010; pp. 443–462. [Google Scholar] [CrossRef]
- Shaikh, S.; Nahar, P.; Shaikh Yakub, S.; Sayed, A.J.; Habibullah, M.A. Current perspectives of 3d printing in dental applications. Braz. Dent. Sci. 2021, 24, 2481. [Google Scholar] [CrossRef]
- Marty, M.; Broutin, A.; Vergnes, J.-N.; Vaysse, F. Comparison of student’s perceptions between 3D printed models versus series models in paediatric dentistry hands-on session. Eur. J. Dent. Educ. 2019, 23, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Höhne, C.; Schmitter, M. 3D Printed Teeth for the Preclinical Education of Dental Students. J. Dent. Educ. 2019, 83, 1100–1106. [Google Scholar] [CrossRef]
- Reymus, M.; Fotiadou, C.; Kessler, A.; Heck, K.; Hickel, R.; Diegritz, C. 3D printed replicas for endodontic education. Int. Endod. J. 2019, 52, 123–130. [Google Scholar] [CrossRef]
- Oberoi, G.; Nitsch, S.; Edelmayer, M.; Janjić, K.; Muller, A.S.; Agis, H. 3D Printing—encompassing the facets of dentistry. Front. Bioeng. Biotechnol. 2018, 6, 172. [Google Scholar] [CrossRef] [PubMed]
- Assche, N.; Vercruyssen, M.; Coucke, W.; Teughels, W.; Jacobs, R.; Quirynen, M. Accuracy of computer-aided implant placement. Clin. Oral Implant. Res. 2012, 23, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Van Kurbad, A. Tooth-supported surgical guides for guided placement of single tooth implants. Int. J. Comput. Dent. 2017, 20, 93–105. [Google Scholar]
- Rinaldi, M.; Ganz, S.D. Computer-guided approach for placement of zygomatic implants: Novel protocol and surgical guide. Compend. Contin. Educ. Dent. 2019, 40, e1–e4. [Google Scholar]
- Chandran, S.; Sakkir, N. Implant–supported full mouth rehabilitation: A guided surgical and prosthetic protocol. J. Clin. Diagn. Res. 2016, 10, ZJ05–ZJ06. [Google Scholar] [CrossRef]
- Louvrier, A.; Marty, P.; Barrabe, A.; Euvrard, E.; Chatelain, B.; Weber, E.; Mayer, C. How useful is 3D printing in maxillofacial surgery? J. Stomatol. Oral Maxillofac. Surg. 2017, 118, 206–212. [Google Scholar] [CrossRef]
- Ghai, S.; Sharma, Y.; Jain, N.; Satpathy, M.; Pillai, A.K. Use of 3-D printing technologies in craniomaxillofacial surgery: A review. Oral Maxillofac. Surg. 2018, 22, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.F.; Alfi, D.; Alfi, J.; Huang, A.T. The use of patient-specific implants in oral and maxillofacial surgery. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 593–600. [Google Scholar] [CrossRef]
- Han, X.; Yang, D.; Yang, C.; Spintzyk, S.; Scheideler, L.; Li, P.; Li, D.; Geis-Gerstorfer, J.; Rupp, F. Carbon fiber reinforced PEEK composites based on 3D-printing technology for orthopedic and dental applications. J. Clin. Med. 2019, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Tahayeri, A.; Morgan, M.; Fugolin, A.P.; Bompolaki, D.; Athirasala, A.; Pfeifer, C.S.; Ferracane, J.L.; Bertassoni, L.E. 3D printed versus conventionally cured provisional crown and bridge dental materials. Dent. Mater. 2018, 34, 192–200. [Google Scholar] [CrossRef]
- Alharbi, N.; Alharbi, S.; Cuijpers, V.M.; Osman, R.B.; Wismeijer, D. Three-dimensional evaluation of marginal and internal fit of 3D-printed interim restorations fabricated on different finish line designs. J. Prosthodont. Res. 2018, 62, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Jackson, T. 3D technologies for precision in orthodontics. Semin. Orthod. 2018, 24, 386–392. [Google Scholar] [CrossRef]
- Charalampakis, O.; Iliadi, A.; Ueno, H.; Oliver, D.R.; Kim, K.B. Accuracy of clear aligners: A retrospective study of patients who needed refinement. Am. J. Orthod. Dentofac. Orthop. 2018, 154, 47–54. [Google Scholar] [CrossRef]
- Salmi, M.; Paloheimo, K.-S.; Tuomi, J.; Ingman, T.; Makitie, A. A digital process for additive manufacturing of occlusal splints: A clinical pilot study. J. R. Soc. Interface 2013, 10, 20130203. [Google Scholar] [CrossRef]
- Jheon, A.H.; Oberoi, S.; Solem, R.C.; Kapila, S. Moving towards precision orthodontics: An evolving paradigm shift in the planning and delivery of customized orthodontic therapy. Orthod. Craniofacial Res. 2017, 20, 106–113. [Google Scholar] [CrossRef]
- Shah, P.; Chong, B.S. 3D imaging, 3D printing and 3D virtual planning in endodontics. Clin. Oral Investig. 2018, 22, 641–654. [Google Scholar] [CrossRef]
- Bae, E.-J.; Jeong, I.-D.; Kim, W.-C.; Kim, J.-H. A comparative study of additive and subtractive manufacturing for dental restorations. J. Prosthet. Dent. 2017, 118, 187–193. [Google Scholar] [CrossRef]
- Eftekhar Ashtiani, R.; Nasiri Khanlar, L.; Mahshid, M.; Moshaverinia, A. Comparison of dimensional accuracy of conventionally and digitally manufactured intracoronal restorations. J. Prosthet. Dent. 2018, 119, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ji, Y.; Huang, G.; Ling, K.; Zhang, X.; Xu, F. Bioprinting 3D cell-laden hydrogel microarray for screening human periodontal ligament stem cell response to extracellular matrix. Biofabrication 2015, 7, 044105. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Chiu, Y.C.; Lee, A.K.; Lin, Y.A.; Lin, P.Y.; Shie, M.Y. Biofabrication of Gingival Fibroblast Cell-Laden Collagen/Strontium-Doped Calcium Silicate 3D-Printed Bi-Layered Scaffold for Osteoporotic Periodontal Regeneration. Biomedicines 2021, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, N.T.; Vaquette, C.; Meinert, C.; Ipe, D.S.; Ivanovski, S. Optimization of 3D bioprinting of periodontal ligament cells. Dent. Mater. 2019, 35, 1683–1694. [Google Scholar] [CrossRef]
- Lee, U.L.; Yun, S.; Cao, H.L.; Ahn, G.; Shim, J.H.; Woo, S.H.; Choung, P.H. Bioprinting on 3D Printed Titanium Scaffolds for Periodontal Ligament Regeneration. Cells 2021, 10, 1337. [Google Scholar] [CrossRef]
- Ono, T.; Tomokiyo, A.; Ipposhi, K.; Yamashita, K.; Alhasan, M.A.; Miyazaki, Y.; Kunitomi, Y.; Tsuchiya, A.; Ishikawa, K.; Maeda, H. Generation of biohybrid implants using a multipotent human periodontal ligament cell line and bioactive core materials. J. Cell. Physiol. 2021, 236, 6742–6753. [Google Scholar] [CrossRef]
- Park, J.H.; Gillispie, G.J.; Copus, J.S.; Zhang, W.B.; Atala, A.; Yoo, J.J.; Yelick, P.C.; Lee, S.J. The effect of BMP-mimetic peptide tethering bioinks on the differentiation of dental pulp stem cells (DPSCs) in 3D bioprinted dental constructs. Biofabrication 2020, 12, 035029. [Google Scholar] [CrossRef]
- Han, J.; Jeong, W.; Kim, M.K.; Nam, S.H.; Park, E.K.; Kang, H.W. Demineralized Dentin Matrix Particle-Based Bio-Ink for Patient-Specific Shaped 3D Dental Tissue Regeneration. Polymers 2021, 13, 1294. [Google Scholar] [CrossRef]
- Dutta, S.D.; Bin, J.; Ganguly, K.; Patel, D.K.; Lim, K.T. Electromagnetic field-assisted cell-laden 3D printed poloxamer-407 hydrogel for enhanced osteogenesis. RSC Adv. 2021, 11, 20342–20354. [Google Scholar] [CrossRef]
- Kim, D.; Lee, H.; Lee, G.-H.; Hoang, T.-H.; Kim, H.-R.; Kim, G.H. Fabrication of bone-derived decellularized extracellular matrix/ceramic-based biocomposites and their osteo/odontogenic differentiation ability for dentin regeneration. Bioeng. Transl. Med. 2022, e10317. [Google Scholar] [CrossRef]
- Han, J.; Kim, D.S.; Jang, H.; Kim, H.-R.; Kang, H.-W. Bioprinting of three-dimensional dentin-pulp complex with local differentiation of human dental pulp stem cells. J. Tissue Eng. 2019, 10, 2041731419845849. [Google Scholar] [CrossRef] [PubMed]
- Duarte Campos, D.F.; Zhang, S.; Kreimendahl, F.; Köpf, M.; Fischer, H.; Vogt, M.; Blaeser, A.; Apel, C.; Esteves-Oliveira, M. Hand-held bioprinting for de novo vascular formation applicable to dental pulp regeneration. Connect. Tissue Res. 2020, 61, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Hsu, T.T.; Liu, Y.W.; Kao, C.T.; Huang, T.H. Bidirectional Differentiation of Human-Derived Stem Cells Induced by Biomimetic Calcium Silicate-Reinforced Gelatin Methacrylate Bioink for Odontogenic Regeneration. Biomedicines 2021, 9, 929. [Google Scholar] [CrossRef]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Kuss, M.A.; Harms, R.; Wu, S.; Wang, Y.; Untrauer, J.B.; Carlson, M.A.; Duan, B. Short-term hypoxic preconditioning promotes prevascularization in 3D bioprinted bone constructs with stromal vascular fraction derived cells. RSC Adv. 2017, 7, 29312–29320. [Google Scholar] [CrossRef]
- Aguilar, I.N.; Smith, L.J.; Olivos, D.J., 3rd; Chu, T.G.; Kacena, M.A.; Wagner, D.R. Scaffold-free Bioprinting of Mesenchymal Stem Cells with the Regenova Printer: Optimization of Printing Parameters. Bioprinting 2019, 15, e00048. [Google Scholar] [CrossRef]
- Aguilar, I.N.; Olivos, D.J., III; Brinker, A.; Alvarez, M.B.; Smith, L.J.; Chu, T.G.; Kacena, M.A.; Wagner, D.R. Scaffold-free bioprinting of mesenchymal stem cells using the Regenova printer: Spheroid characterization and osteogenic differentiation. Bioprinting 2019, 15, e00050. [Google Scholar] [CrossRef]
- Dubey, N.; Ferreira, J.A.; Malda, J.; Bhaduri, S.B.; Bottino, M.C. Extracellular Matrix/Amorphous Magnesium Phosphate Bioink for 3D Bioprinting of Craniomaxillofacial Bone Tissue. ACS Appl. Mater. Interfaces 2020, 12, 23752–23763. [Google Scholar] [CrossRef]
- Moncal, K.K.; Gudapati, H.; Godzik, K.P.; Heo, D.N.; Kang, Y.; Rizk, E.; Ravnic, D.J.; Wee, H.; Pepley, D.F.; Ozbolat, V.; et al. Intra-Operative Bioprinting of Hard, Soft, and Hard/Soft Composite Tissues for Craniomaxillofacial Reconstruction. Adv. Funct. Mater. 2021, 31, 2010858. [Google Scholar] [CrossRef]
- Moncal, K.K.; Aydin, R.S.T.; Godzik, K.P.; Acri, T.M.; Heo, D.N.; Rizk, E.; Wee, H.; Lewis, G.S.; Salem, A.K.; Ozbolat, I.T. Controlled Co-delivery of pPDGF-B and pBMP-2 from intraoperatively bioprinted bone constructs improves the repair of calvarial defects in rats. Biomaterials 2022, 281, 121333. [Google Scholar] [CrossRef] [PubMed]
- Keriquel, V.; Oliveira, H.; Rémy, M.; Ziane, S.; Delmond, S.; Rousseau, B.; Rey, S.; Catros, S.; Amédée, J.; Guillemot, F.; et al. In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications. Sci. Rep. 2017, 7, 1778. [Google Scholar] [CrossRef] [PubMed]
- Kérourédan, O.; Ribot, E.J.; Fricain, J.-C.; Devillard, R.; Miraux, S. Magnetic Resonance Imaging for tracking cellular patterns obtained by Laser-Assisted Bioprinting. Sci. Rep. 2018, 8, 15777. [Google Scholar] [CrossRef]
- Kérourédan, O.; Hakobyan, D.; Rémy, M.; Ziane, S.; Dusserre, N.; Fricain, J.C.; Delmond, S.; Thébaud, N.B.; Devillard, R. In situ prevascularization designed by laser-assisted bioprinting: Effect on bone regeneration. Biofabrication 2019, 11, 045002. [Google Scholar] [CrossRef] [PubMed]
- Touya, N.; Devun, M.; Handschin, C.; Casenave, S.; Ahmed Omar, N.; Gaubert, A.; Dusserre, N.; De Oliveira, H.; Kérourédan, O.; Devillard, R. In vitro and in vivo characterization of a novel tricalcium silicate-based ink for bone regeneration using laser-assisted bioprinting. Biofabrication 2022, 14, 024104. [Google Scholar] [CrossRef]
- Amler, A.K.; Dinkelborg, P.H.; Schlauch, D.; Spinnen, J.; Stich, S.; Lauster, R.; Sittinger, M.; Nahles, S.; Heiland, M.; Kloke, L.; et al. Comparison of the Translational Potential of Human Mesenchymal Progenitor Cells from Different Bone Entities for Autologous 3D Bioprinted Bone Grafts. Int. J. Mol. Sci. 2021, 22, 796. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, M.; Liu, Y.; Shi, C.; Wang, Y.; Liu, T.; Huang, Y.; Zhong, P.; Dai, J.; Liu, X. The performance of 3D bioscaffolding based on a human periodontal ligament stem cell printing technique. J. Biomed. Mater. Res. A 2021, 109, 1209–1219. [Google Scholar] [CrossRef]
- Walladbegi, J.; Schaefer, C.; Pernevik, E.; Sämfors, S.; Kjeller, G.; Gatenholm, P.; Sándor, G.K.; Rasmusson, L. Three-Dimensional Bioprinting Using a Coaxial Needle with Viscous Inks in Bone Tissue Engineering—An In vitro Study. Ann. Maxillofac. Surg. 2020, 10, 370–376. [Google Scholar] [CrossRef]
- Amler, A.K.; Thomas, A.; Tüzüner, S.; Lam, T.; Geiger, M.A.; Kreuder, A.E.; Palmer, C.; Nahles, S.; Lauster, R.; Kloke, L. 3D bioprinting of tissue-specific osteoblasts and endothelial cells to model the human jawbone. Sci. Rep. 2021, 11, 4876. [Google Scholar] [CrossRef]
- Ma, Y.; Ji, Y.; Zhong, T.; Wan, W.; Yang, Q.; Li, A.; Zhang, X.; Lin, M. Bioprinting-Based PDLSC-ECM Screening for in Vivo Repair of Alveolar Bone Defect Using Cell-Laden, Injectable and Photocrosslinkable Hydrogels. ACS Biomater. Sci. Eng. 2017, 3, 3534–3545. [Google Scholar] [CrossRef]
- Kort-Mascort, J.; Bao, G.; Elkashty, O.; Flores-Torres, S.; Munguia-Lopez, J.G.; Jiang, T.; Ehrlicher, A.J.; Mongeau, L.; Tran, S.D.; Kinsella, J.M. Decellularized Extracellular Matrix Composite Hydrogel Bioinks for the Development of 3D Bioprinted Head and Neck in Vitro Tumor Models. ACS Biomater. Sci. Eng. 2021, 7, 5288–5300. [Google Scholar] [CrossRef] [PubMed]
- Salar Amoli, M.; EzEldeen, M.; Jacobs, R.; Bloemen, V. Materials for Dentoalveolar Bioprinting: Current State of the Art. Biomedicines 2022, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Rider, P.; Kačarević, Ž.P.; Alkildani, S.; Retnasingh, S.; Schnettler, R.; Barbeck, M. Additive manufacturing for guided bone regeneration: A perspective for alveolar ridge augmentation. Int. J. Mol. Sci. 2018, 19, 3308. [Google Scholar] [CrossRef]
- Li, Y.C.; Zhang, Y.S.; Akpek, A.; Shin, S.R.; Khademhosseini, A. 4D bioprinting: The next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication 2016, 9, 012001. [Google Scholar] [CrossRef] [Green Version]
- Tibbits, S. 4D Printing: Multi-Material shape change. Archit. Des. 2014, 84, 116–121. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, P.; Liu, Y.; Lv, L.; Zhou, Y. Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomater. 2020, 101, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Apsite, I.; Uribe, J.M.; Posada, A.F.; Rosenfeldt, S.; Salehi, S.; Ionov, L. 4D biofabrication of skeletal muscle microtissues. Biofabrication 2019, 12, 015016. [Google Scholar] [CrossRef]
- Esworthy, T.J.; Miao, S.D.; Lee, S.J.; Zhou, X.; Cui, H.T.; Zuo, Y.Y.; Zhang, L.J.G. Advanced 4D-bioprinting technologies for brain tissue modeling and study. Int. J. Smart Nano Mater. 2019, 10, 177–204. [Google Scholar] [CrossRef]
- Grinberg, D.; Siddique, S.; Le, M.Q.; Liang, R.; Capsal, J.F.; Cottinet, P.J. 4D Printing based piezoelectric composite for medical applications. J. Polym. Sci. B–Polym. Phys. 2019, 57, 109–115. [Google Scholar] [CrossRef]
- Zhang, Z.; Demir, K.G.; Gu, G.X. Developments in 4D-printing: A review on current smart materials, technologies, and applications. Int. J. Soc. Netw. Min. 2019, 10, 205–224. [Google Scholar] [CrossRef]
- Choong, Y.Y.; Maleksaeedi, S.; Eng, H.; Wei, J.; Su, P.C. 4D printing of high performance shape memory polymer using stereolithography. Mater. Des. 2017, 126, 219–225. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Woo, B.H.; Kim, N.; Jun, Y.C. Multicolor 4D printing of shape-memory polymers for light-induced selective heating and remote actuation. Sci. Rep. 2020, 10, 6258. [Google Scholar] [CrossRef] [PubMed]
- Pei, E.; Loh, G.H. Technological considerations for 4D printing: An overview. Prog. Addit. Manuf. 2018, 3, 95–107. [Google Scholar] [CrossRef]
- Su, J.W.; Tao, X.; Deng, H.; Zhang, C.; Jiang, S.; Lin, Y.; Lin, J. 4D printing of a self-morphing polymer driven by a swellable guest medium. Soft Matter 2018, 14, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Lowenberg, C.; Balk, M.; Wischke, C.; Behl, M.; Lendlein, A. Shape-Memory hydrogels: Evolution of structural principles to enable shape switching of hydrophilic polymer networks. Acc. Chem. Res. 2017, 50, 723–732. [Google Scholar] [CrossRef]
- Dental 4D Printing: An Innovative Approach. Available online: https://innovation.ctor.press/articles/dental-4d-printing-an-innovative-approach/ (accessed on 7 July 2022). [CrossRef]
- Liu, G.; He, Y.; Liu, P.; Chen, Z.; Chen, X.; Wan, L.; Li, Y.; Lu, J. Development of bio implants with 2D, 3D, and 4D additive manufacturing materials. Engineering 2020, 6, 1232–1243. [Google Scholar] [CrossRef]
- Manikandan, N.; Rajesh, P.K.; Harish, V. An analysis of the methods and materials for 4-dimensional printing. Mater. Today Proc. 2021, 38, 2167–2173. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, F.; Liu, Y.; Leng, J. 4D printed shape memory polymers and their structures for biomedical applications. Sci. China Technol. Sci. 2020, 63, 545–560. [Google Scholar] [CrossRef]
- Sharma, D.; Mathur, V.P.; Satapathy, B.K. Biodegradable and biocompatible 3D constructs for dental applications: Manufacturing options and perspectives. Ann. Biomed. Eng. 2021, 49, 2030–2056. [Google Scholar] [CrossRef]
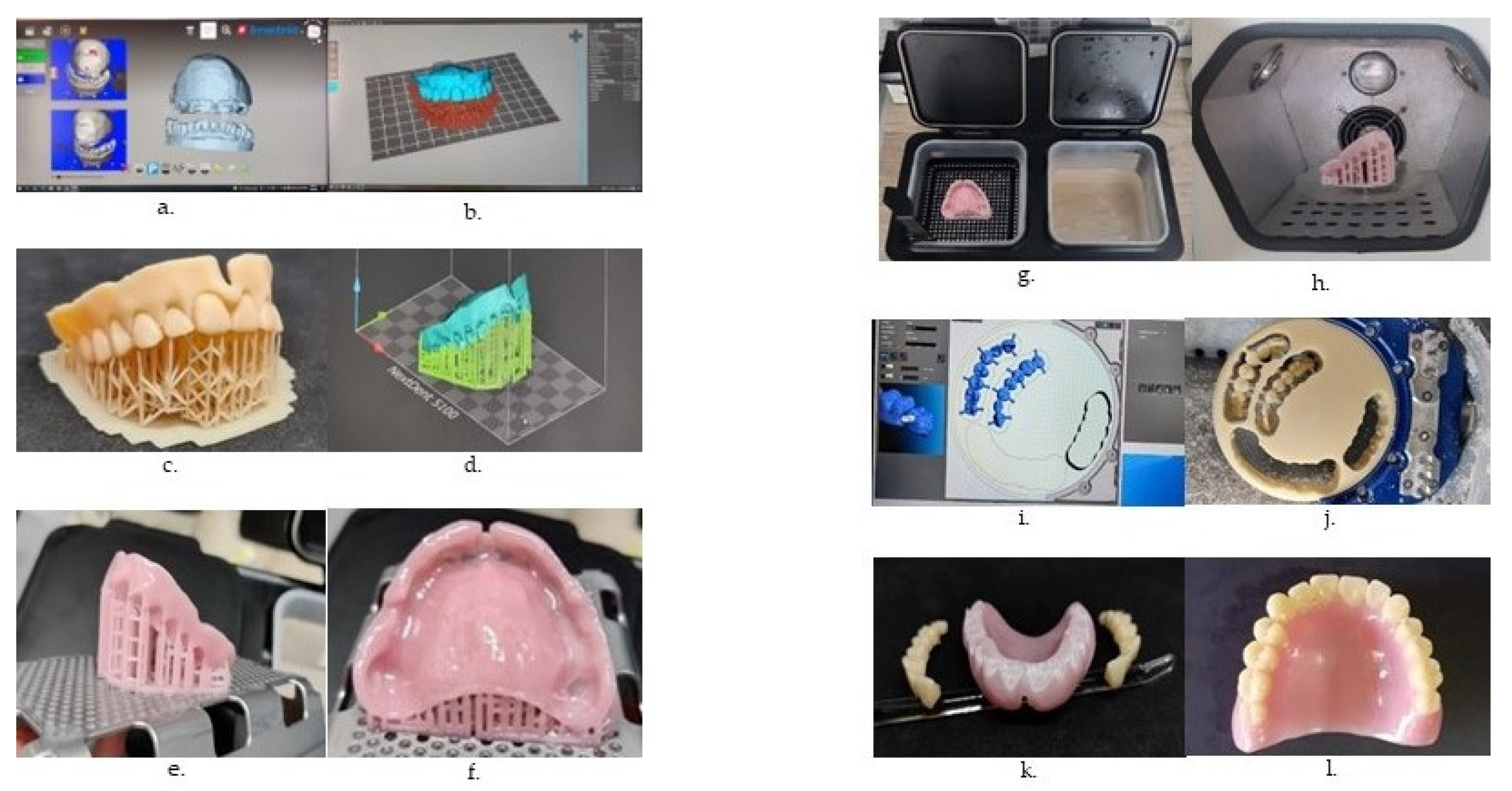
- Piedra-Cascon, W.; Krishnamurthy, V.R.; Att, W.; Revilla-Leon, M. 3D printing parameters, supporting structures, slicing, and post-processing procedures of vat-polymerisation additive manufacturing technologies: A narrative review. J. Dent. 2021, 109, 103630. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Pratap Singh, R.; Rab, S.; Suman, R.; Kumar, L. Significance of 4D printing for dentistry: Materials, process, and potentials. J. Oral Biol. Craniofacial Res. 2022, 12, 388–395. [Google Scholar] [CrossRef]
- Miao, S.; Castro, N.; Nowicki, M.; Xia, L.; Cui, H.; Zhou, X.; Zhu, W.; Jun Lee, S.; Sarkar, K.; Vozzi, G.; et al. 4D printing of polymeric materials for tissue and organ regeneration. Mater. Today 2017, 20, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.A.; Siddiqui, Z.; Carney, G.J.; Naaldijk, Y.; Guiro, K.; Ferrer, A.I.; Sherman, L.S.; Guvendiren, M.; Kumar, V.A.; Rameshwar, P. A 3D Bioprinted Material That Recapitulates the Perivascular Bone Marrow Structure for Sustained Hematopoietic and Cancer Models. Polymers 2021, 13, 480. [Google Scholar] [CrossRef] [PubMed]
- Serpooshan, V.; Guvendiren, M. Editorial for the Special Issue on 3D Printing for Tissue Engineering and Regenerative Medicine. Micromachines 2020, 11, 366. [Google Scholar] [CrossRef]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef]
- Revilla-León, M.; Özcan, M. Additive manufacturing technologies used for processing polymers: Current status and potential application in prosthetic dentistry. J. Prosthodont. 2019, 28, 146–158. [Google Scholar] [CrossRef]
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and Properties of Gelatin Methacryloyl (GelMA) Hydrogels and Their Recent Applications in Load-Bearing Tissue. Polymers 2018, 10, 1290. [Google Scholar] [CrossRef]
- Guan, J.; Yuan, F.-Z.; Mao, Z.-M.; Zhu, H.-L.; Lin, L.; Chen, H.H.; Yu, J.-K. Fabrication of 3D-Printed Interpenetrating Hydrogel Scaffolds for Promoting Chondrogenic Differentiation. Polymers 2021, 13, 2146. [Google Scholar] [CrossRef]
- Chimene, D.; Miller, L.; Cross, L.M.; Jaiswal, M.K.; Singh, I.; Gaharwar, A.K. Nanoengineered Osteoinductive Bioink for 3D Bioprinting Bone Tissue. ACS Appl. Mater. Interfaces 2020, 12, 15976–15988. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Theus, A.S.; Kamalakar, A.; Ning, L.; Cao, C.; Tomov, M.L.; Kaiser, J.M.; Goudy, S.; Willett, N.J.; Jang, H.W.; et al. 3D Bioprinted Bacteriostatic Hyperelastic Bone Scaffold for Damage-Specific Bone Regeneration. Polymers 2021, 13, 1099. [Google Scholar] [CrossRef]
- Huang, Y.H.; Jakus, A.E.; Jordan, S.W.; Dumanian, Z.; Parker, K.; Zhao, L.; Patel, P.K.; Shah, R.N. Three-Dimensionally Printed Hyperelastic Bone Scaffolds Accelerate Bone Regeneration in Critical-Size Calvarial Bone Defects. Plast. Reconstr. Surg. 2019, 143, 1397–1407. [Google Scholar] [CrossRef] [PubMed]



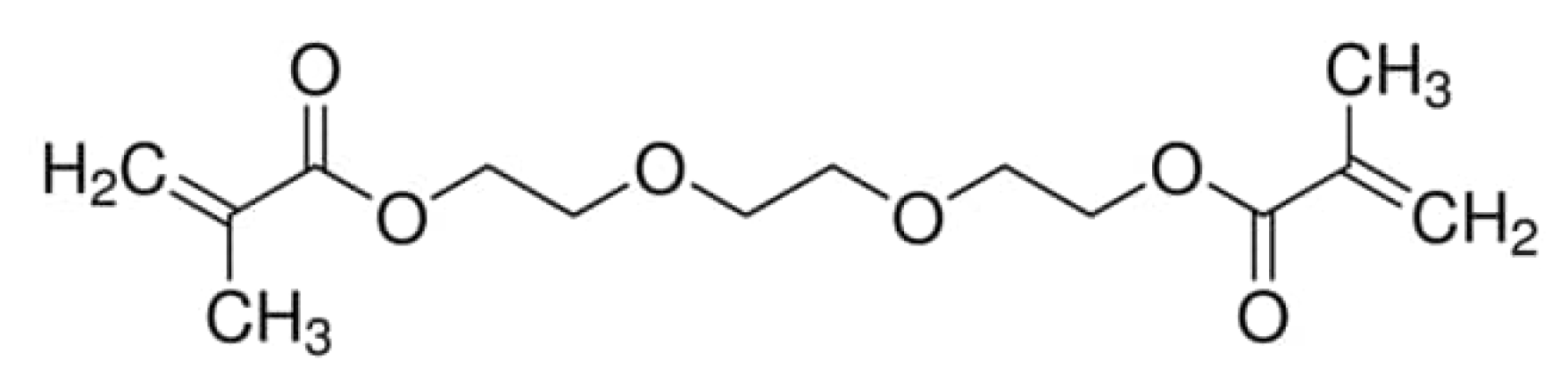
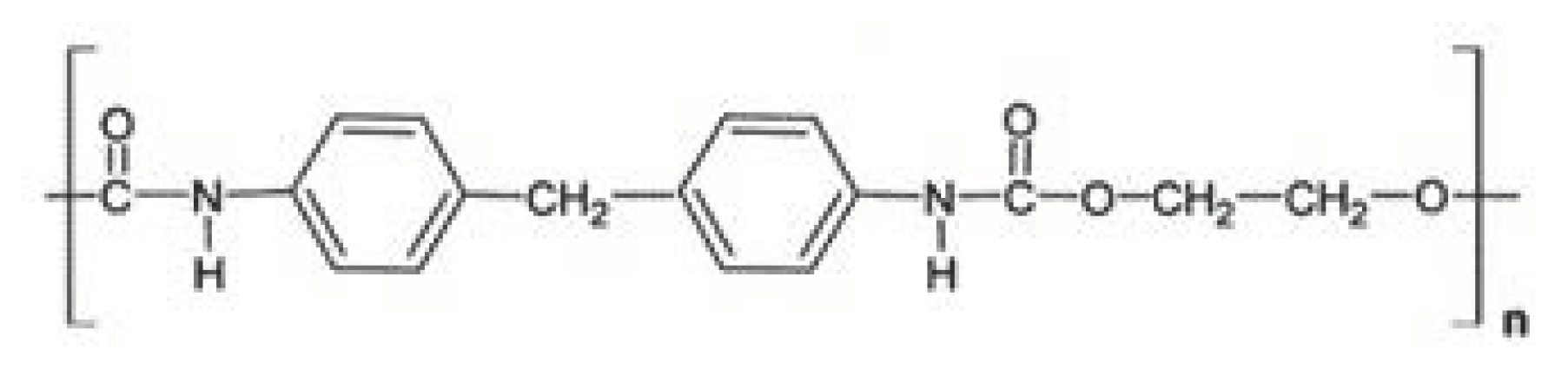
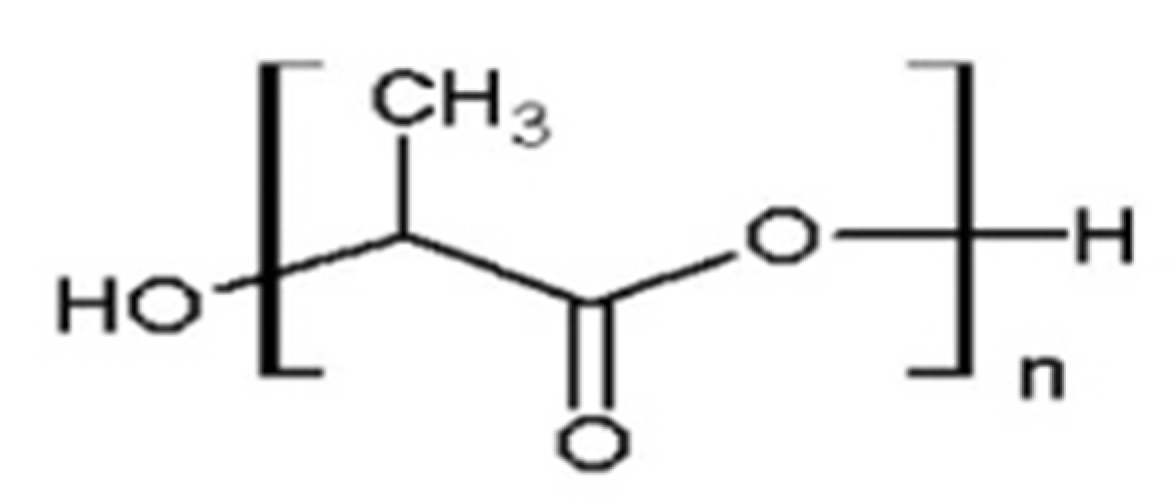
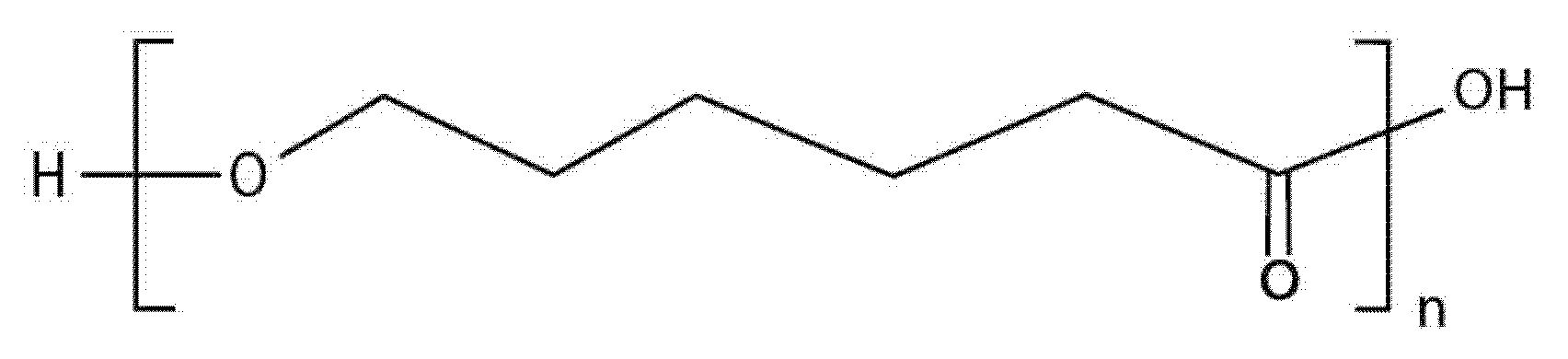


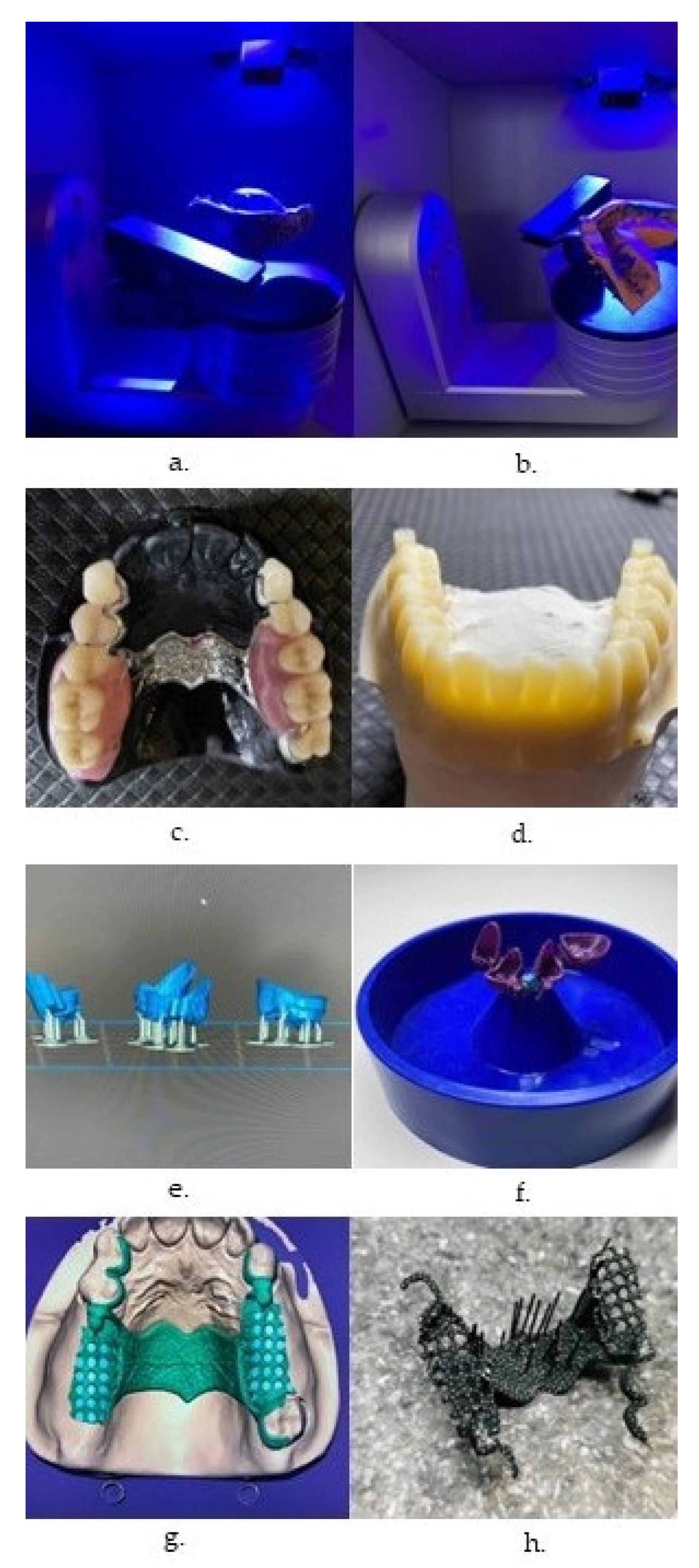
| Characteristic | SLA | DLP | MJT | FDM |
|---|---|---|---|---|
| Type | vat photo-polymerization | vat photo-polymerization | material jetting | material extrusion |
| Resolution | high | high | high | low |
| Accuracy | medium | high | high | medium |
| Speed | medium | high | high | medium |
| Object size | scalable | scalable | scalable | scalable |
| Cost | medium | medium | high | low |
| Reference | Targeted Tissue | 3D Bioprinting Technology | Bioink |
|---|---|---|---|
| [170] | Periodontal regeneration | Inkjet | GelMA + PEGDA |
| [171] | Periodontal regeneration | Extrusion | Collagen |
| [172] | Periodontal regeneration | Extrusion | GelMA |
| [173] | PDL regeneration | Extrusion | Collagen |
| [174] | PDL regeneration | Scaffold-free | - |
| [175] | Dental tissue regeneration | Extrusion | Gelatin + GelMA + HAc + glycerol |
| [176] | Dental tissue regeneration | Extrusion | Demineralized dentin matrix particles + fibrinogen + gelatin |
| [177] | Dental tissue regeneration | Extrusion | Poloxamer-407 |
| [178] | Dental tissue regeneration | Extrusion | Collagen type 1 or dECM + β-TCP |
| [68] | Dentin/dental pulp regeneration | Extrusion | Alginate + dentin matrix |
| [179] | Dentin/dental pulp regeneration | Extrusion | Fibrinogen + gelatin + HAc + glycerol |
| [180] | Dental pulp regeneration | Inkjet | Collagen type 1 + agarose |
| [181] | Dentin regeneration | Extrusion | Calcium silicate + GelMA |
| [182] | Alveolar bone/bone regeneration | Extrusion | Gelatin + fibrinogen + HA + glycerol |
| [183] | Alveolar bone/bone regeneration | Extrusion | MeHAc + GelMA + HAc |
| [184] | Bone regeneration | Scaffold-free | - |
| [185] | Bone regeneration | Scaffold-free | - |
| [186] | Bone regeneration | Extrusion | ECM + AMP |
| [187] | Bone regeneration | Extrusion | Collagen + chitosan + β-glycer-ophosphate + nHA |
| [188] | Bone regeneration | Extrusion | Collagen + chitosan + β-glycerophosphate + nHA |
| [189] | Bone regeneration | Laser-based | Collagen type 1 + nHA |
| [190] | Bone regeneration | Laser-based | Collagen type 1 |
| [191] | Bone regeneration | Laser-based | Collagen type 1 |
| [192] | Bone regeneration | Laser-based | Collagen type 1 + TCP |
| [193] | Bone regeneration | SLA | GelMA |
| [194] | Bone regeneration | Extrusion | Sodium alginate + gelatin + nHA |
| [195] | Bone regeneration | Extrusion | Nanofibrillated cellulose + alginate |
| [196] | Alveolar bone in vitro model | SLA | GelMA + PEGDA |
| [197] | Alveolar bone regeneration | Inkjet | GelMA + PEGDA |
| [198] | Head and neck cancer in vitro model | Extrusion | Alginate + gelatin + dECM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tigmeanu, C.V.; Ardelean, L.C.; Rusu, L.-C.; Negrutiu, M.-L. Additive Manufactured Polymers in Dentistry, Current State-of-the-Art and Future Perspectives-A Review. Polymers 2022, 14, 3658. https://doi.org/10.3390/polym14173658
Tigmeanu CV, Ardelean LC, Rusu L-C, Negrutiu M-L. Additive Manufactured Polymers in Dentistry, Current State-of-the-Art and Future Perspectives-A Review. Polymers. 2022; 14(17):3658. https://doi.org/10.3390/polym14173658
Chicago/Turabian StyleTigmeanu, Codruta Victoria, Lavinia Cosmina Ardelean, Laura-Cristina Rusu, and Meda-Lavinia Negrutiu. 2022. "Additive Manufactured Polymers in Dentistry, Current State-of-the-Art and Future Perspectives-A Review" Polymers 14, no. 17: 3658. https://doi.org/10.3390/polym14173658
APA StyleTigmeanu, C. V., Ardelean, L. C., Rusu, L. -C., & Negrutiu, M. -L. (2022). Additive Manufactured Polymers in Dentistry, Current State-of-the-Art and Future Perspectives-A Review. Polymers, 14(17), 3658. https://doi.org/10.3390/polym14173658









