Changes in Natural Silk Fibres by Hydration, Tensile Loading and Heating as Studied by 1H NMR: Anisotropy in NMR Relaxation Times
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Samples
2.2. Mechanical Loading Tests
2.2.1. Deformation Measurements on the Normal Silk Fibres
2.2.2. Tensile Tension of Silk Threads with Constant Mechanical Loads
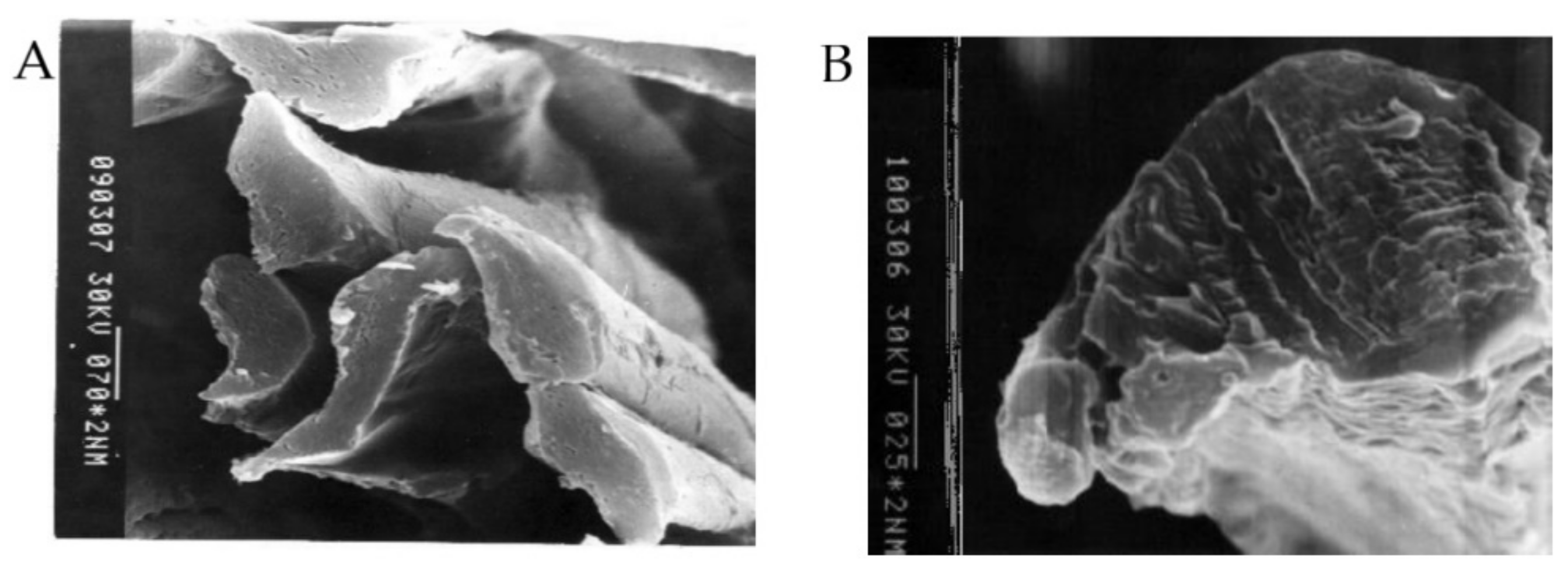
2.3. Electron Microscopy
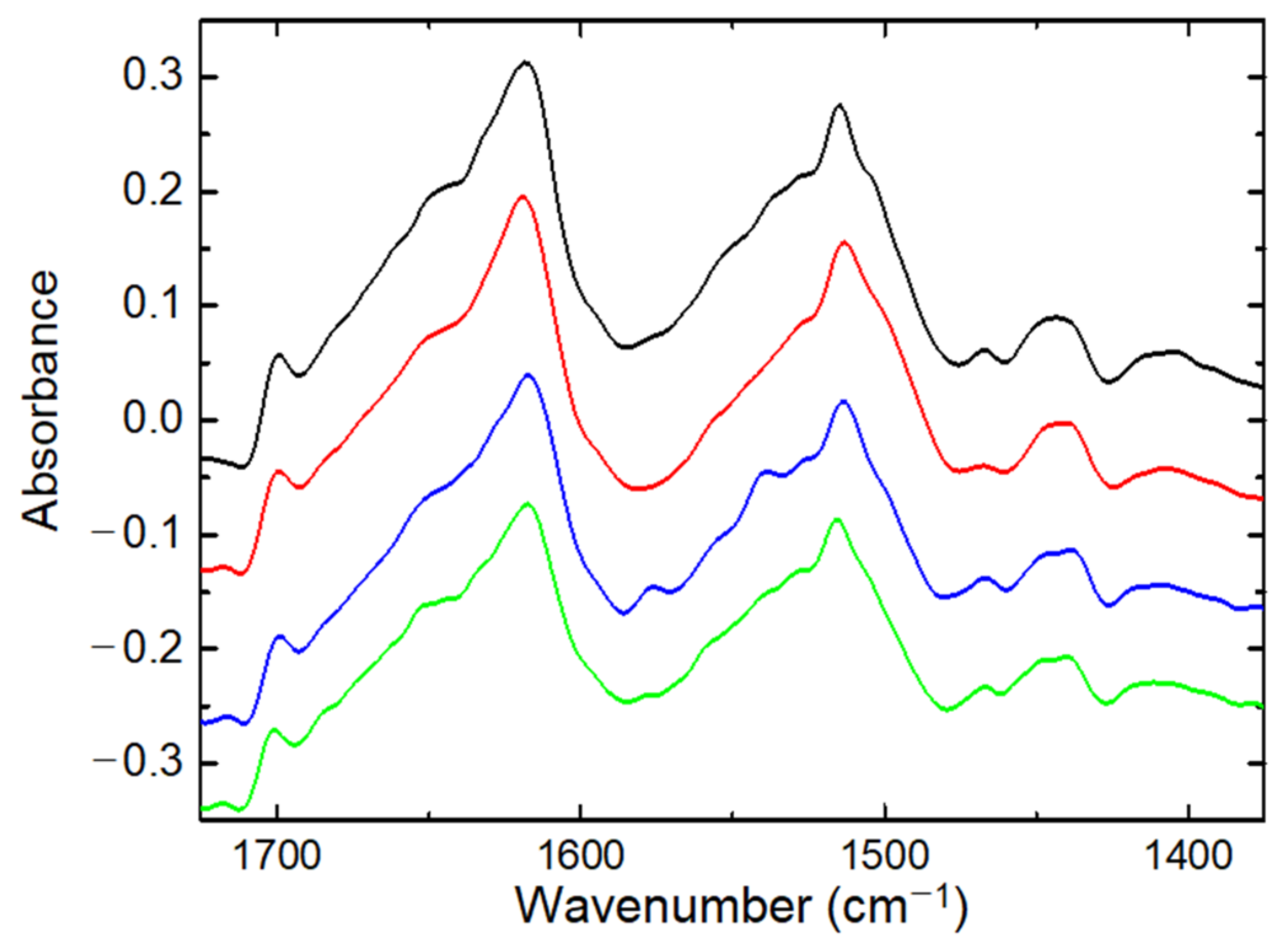
2.4. FTIR Spectroscopy
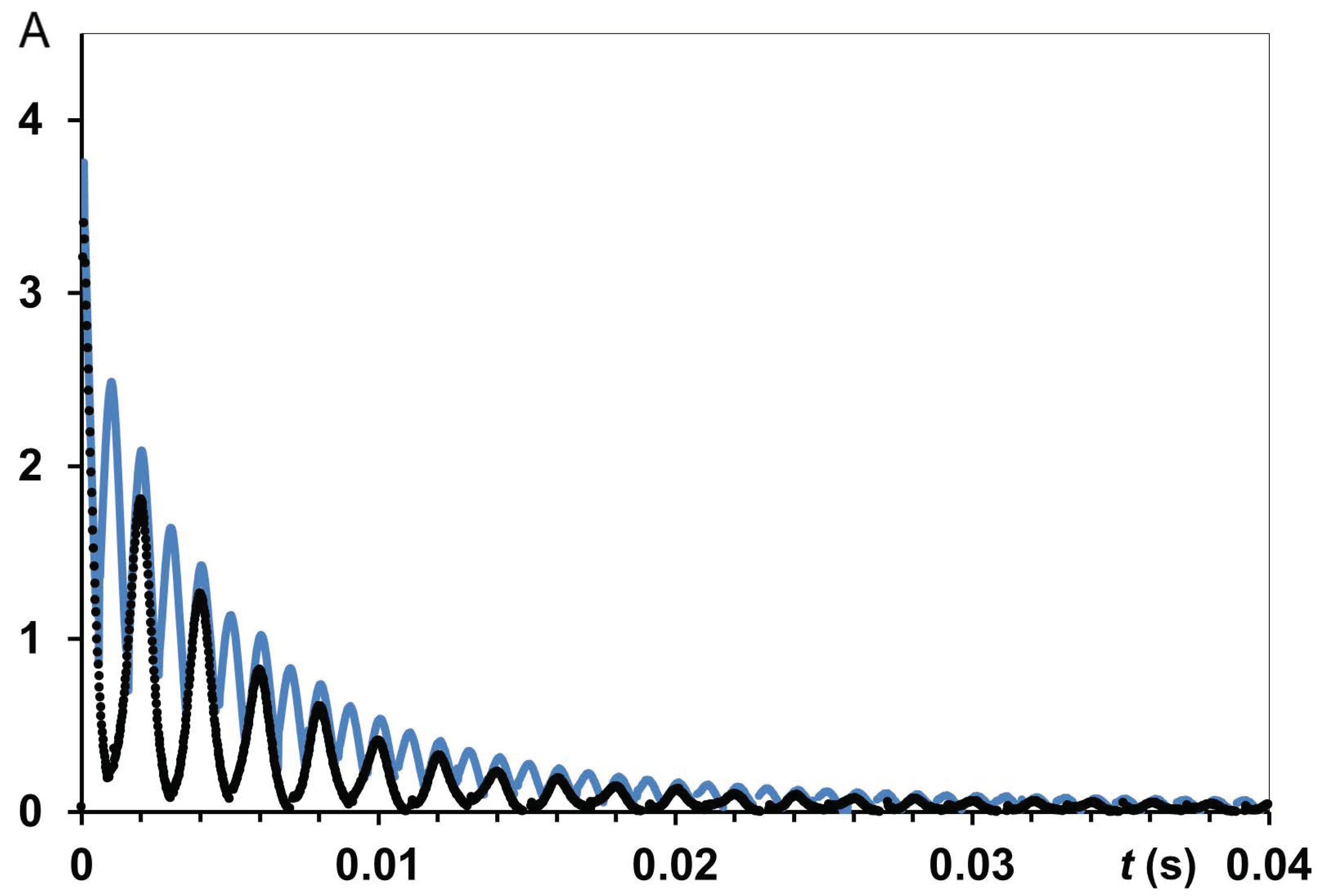
2.5. NMR Methods
3. Results
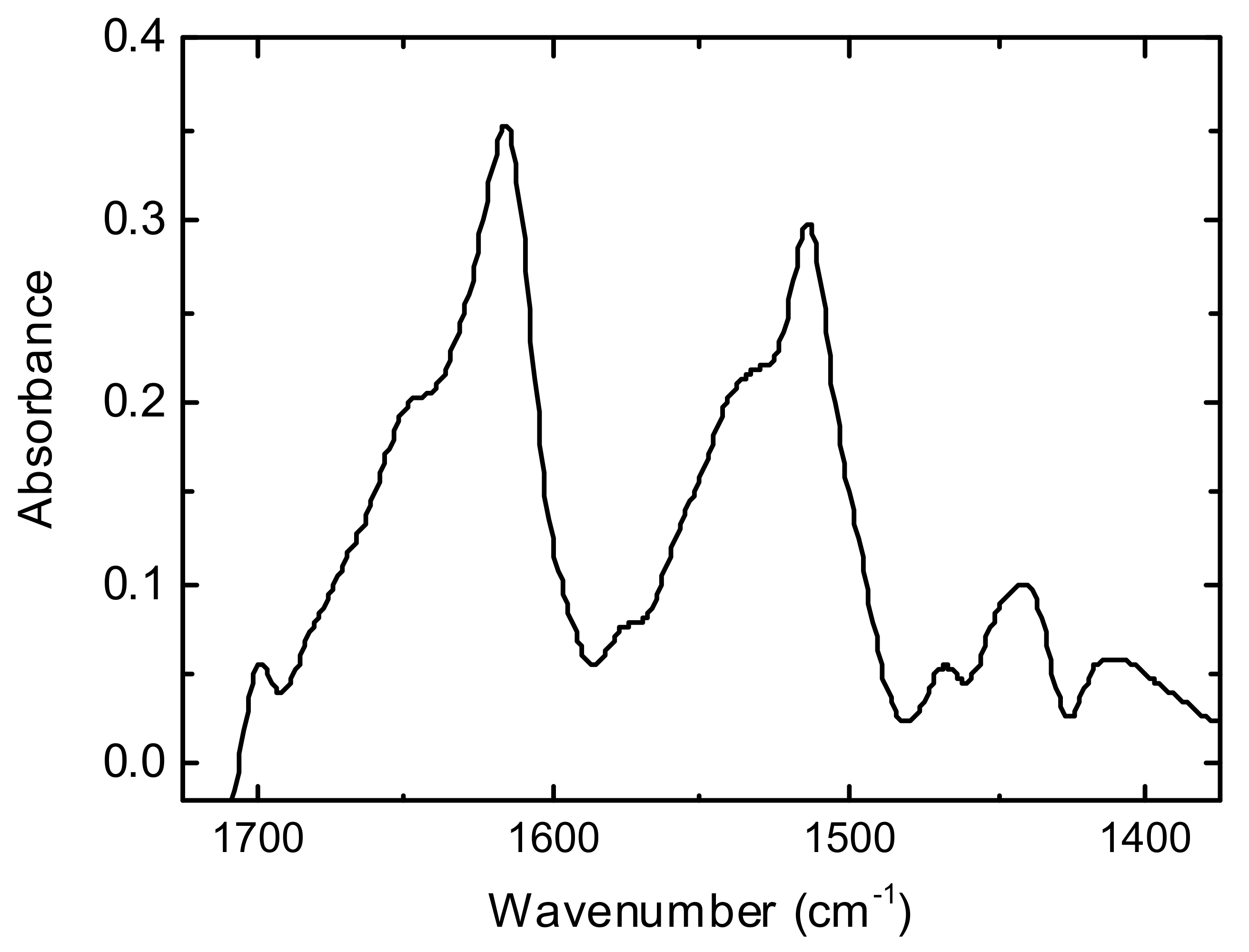
3.1. FTIR Spectroscopy
3.2. NMR Results
3.2.1. NMR Studying Randomly Oriented Silk Fibres, D2O Exchange and Silk B. mori Cocoon
3.2.2. NMR Studying Orientation Anisotropy in Natural Silk B. mori Fibres
3.2.3. NMR Study of Tensile Loading on Orientation Anisotropy in Natural Silk Fibres B. mori
3.2.4. NMR Study of Temperature Effect on Oriented Natural Silk Fibres B. mori
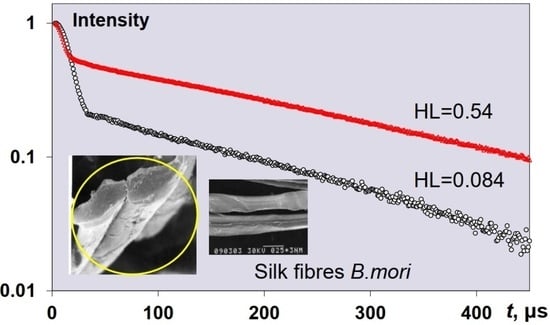
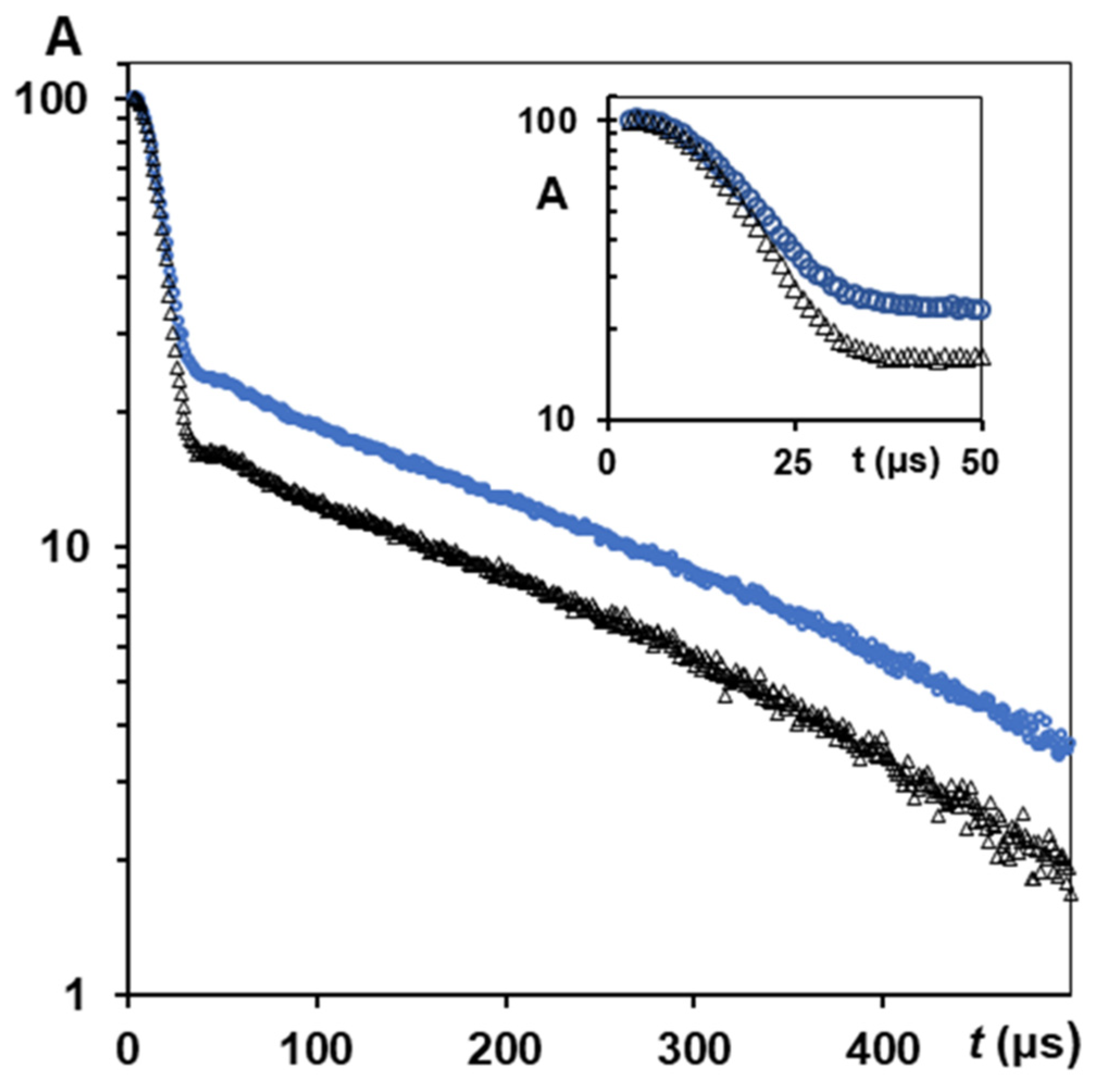
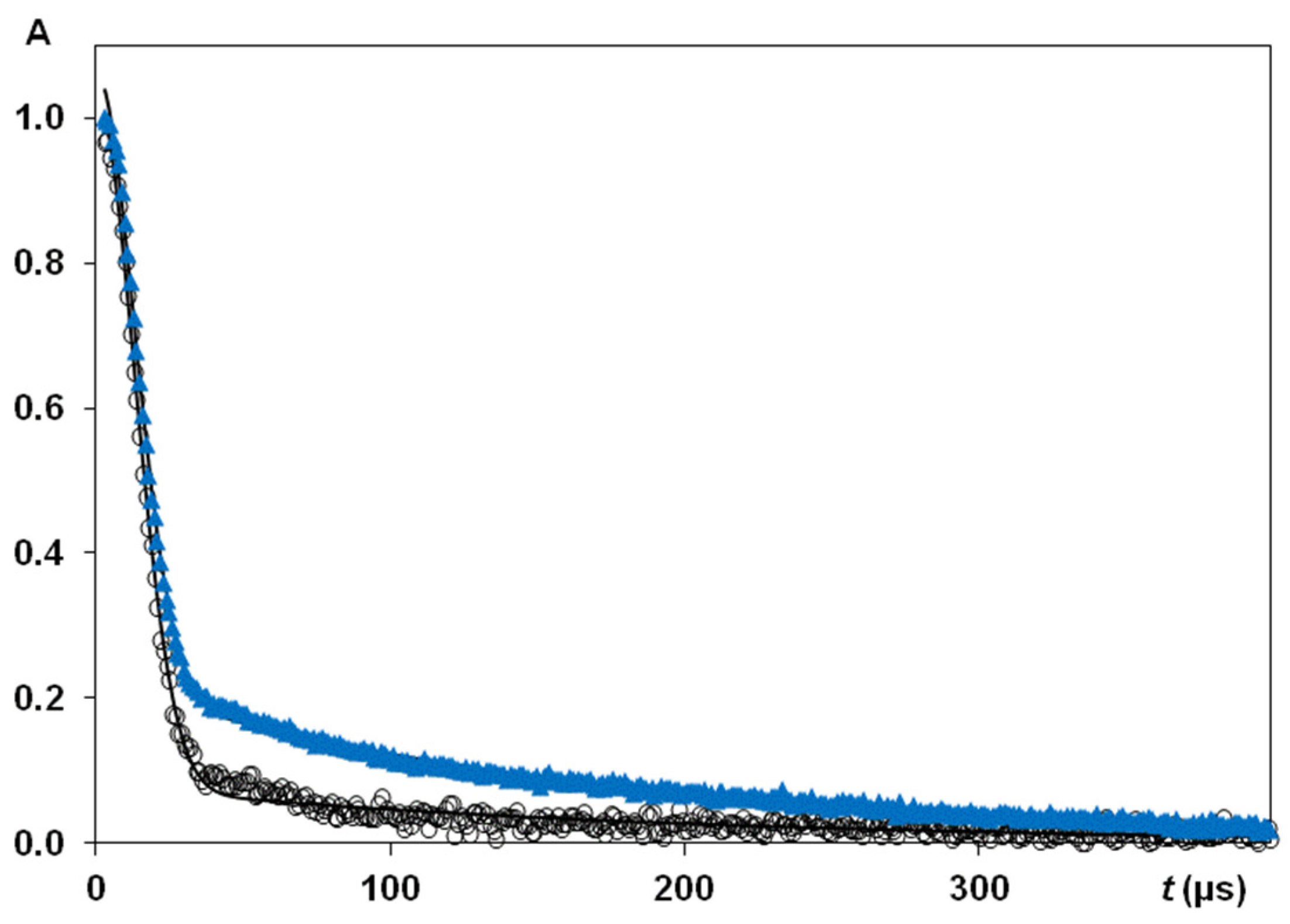
3.2.5. CPMG Study of Natural Silk Fibres B. mori Spin Exchange
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Dedication
References
- Chen, S.; Liu, M.; Huang, H.; Cheng, L.; Zhao, H.-P. Mechanical properties of Bombyx mori silkworm silk fibre and its corresponding silk fibroin filament: A comparative study. Mater. Des. 2019, 181, 108077. [Google Scholar] [CrossRef]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2012, 65, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G.; Scheibel, T.R. Composite materials based on silk proteins. Prog. Polym. Sci. 2010, 35, 1093–1115. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.W.; Loh, X.J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.D.; Zhang, Y.W.; et al. Structure, mechanical properties, and application of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Lawrence, B.D.; Wharram, S.; Kluge, J.A.; Leisk, G.G.; Omenetto, F.G.; Rosenblatt, M.I.; Kaplan, D.L. Effect of hydration on silk film material properties. Macromol. Biosci. 2010, 10, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.; Wong Po Foo, C.T.; Rossetti, F.; Texter, M.; Vunjak-Novakovic, G.; Kaplan, D.L.; Merkle, H.P.; Meinel, L. Silk fibroin as an organic polymer for controlled drug deliver. J. Control. Release 2006, 111, 219–227. [Google Scholar] [CrossRef]
- Guo, C. Protein secondary structure in Bombyx mori silk investigated by solid-state NMR. In Nuclear Magnetic Resonance (NMR) Spectroscopy Characterization of Nanomaterials and Biopolymers; Arizona State University: Tempe, AZ, USA, 2017; Chapter 6; pp. 157–185. [Google Scholar]
- Chen, X.; Shao, Z.; Marinkovic, N.S.; Miller, L.M.; Zhou, P.; Chance, M.R. Conformation transition kinetics of regenerated Bombyx mori silk fibroin membrane monitored by time-resolved FTIR spectroscopy. Biophys. Chem. 2001, 89, 25–34. [Google Scholar] [CrossRef]
- Asakura, T.; Isobe, K.; Kametani, S.; Ukpebor, O.T.; Silverstein, M.C.; Boutis, G.S. Characterization of water in hydrated Bombyx mori silk fibroin fibre and films by 2H NMR relaxation and 13C solid state NMR. Acta Biomater. 2017, 50, 322–333. [Google Scholar] [CrossRef]
- Wang, D.; Liu, H.; Fan, Y. Silk fibroin for vascular regeneration. Microsc. Res. Tech. 2017, 80, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Rodin, V.V.; Knight, D.P. Water self-diffusion in natural silk fibres as determined by the pulsed magnetic field gradient method. Biophysics 2003, 48, 404–410. [Google Scholar]
- Plaza, G.R.; Corsini, P.; Perez-Rigueiro, J.; Marsano, E.; Guinea, G.V.; Elices, M. Effect of water on Bombyx mori regenerated silk fibres and its application in modifying their mechanical properties. J. Appl. Polym. Sci. 2008, 109, 1793–1801. [Google Scholar] [CrossRef]
- Rodin, V.V. Natural silk Bombyx mori with low water content. In Magnetic Resonance in Studying Natural and Synthetic Materials, 1st ed.; Rodin, V.V., Ed.; Bentham Science Publisher: Sharjah, United Arab Emirates, 2018; pp. 26–35. 244p, ISBN 978-1-68108-630-9. [Google Scholar]
- Rodin, V.V.; Reznichenko, G.M.; Vasina, E.L. Properties of natural silk fibres: Deformation study and NMR-data. Biophysics 2004, 49, 918–926. [Google Scholar]
- Rodin, V.V. Collagen tissues with different degree of cross-links and natural silk as studied by 1H DQF NMR. In Magnetic Resonance in Studying Natural and Synthetic Materials, 1st ed.; Rodin, V.V., Ed.; Bentham Science Publisher: Sharjah, United Arab Emirates, 2018; Chapter 4; pp. 68–81. 244p, ISBN 978-1-68108-630-9. [Google Scholar]
- Asakura, T.; Yao, J. 13C CP/MAS NMR study on structural heterogeneity in Bombyx mori silk fiber and their generation by stretching. Protein Sci. 2002, 11, 2706–2713. [Google Scholar] [CrossRef] [PubMed]
- Rodin, V.V.; Knight, D.P. Natural materials by NMR data: Cross-relaxation in macromolecules of natural silk. Mater. Sci. 2003, 10, 16–21. [Google Scholar]
- Rodin, V.V.; Knight, D.P. Molecular mobility in natural polymers: Bombyx mori silk with low water content as studied by 1H DQF NMR. Biophysics 2004, 49, 730–737. [Google Scholar]
- Jelinski, L.W.; Blye, A.; Liivak, O. Orientation, structure, wet-spinning and molecular basis for supercontraction of spider dragline silk. Intern. J. Biol. Macromol. 1999, 24, 197–201. [Google Scholar] [CrossRef]
- Tsukada, M.; Freddi, G.; Nagura, M.; Ishikawa, H.; Kasai, N. Structural changes of silk fibres induced by heat treatment. J. Appl. Polym. Sci. 1992, 46, 1945–1953. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.; Cebe, P. Effect of water on the thermal properties of silk fibroin. Thermochim. Acta 2007, 461, 137–144. [Google Scholar] [CrossRef]
- Motta, A.; Fambri, L.; Migliaresi, C. Regenerated silk fibroin films: Thermal and dynamic mechanical analysis. Macromol. Chem. Phys. 2002, 203, 1658–1665. [Google Scholar] [CrossRef]
- Sohn, S.; Strey, H.H.; Gido, S.P. Phase behaviour and hydration of silk fibroin. Biomacromolecules 2004, 5, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Wu, P.; Chen, X.; Shao, Z. The effect of water on the conformation transition of Bombyx mori silk fibroin. Vib. Spectrosc. 2009, 51, 105–109. [Google Scholar] [CrossRef]
- Yazawa, K.; Ishida, K.; Masunaga, H.; Hikima, T.; Numata, K. Influence of water content on the β-sheet formation, thermal stability, water removal, and mechanical properties of silk materials. Biomacromolecules 2016, 17, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Rodin, V.V.; Foucat, L.; Renou, J.P. Natural polymers according to NMR data: Cross-relaxation in hydrated collagen macromolecules from two connective tissues. Biophysics 2004, 49, 608–616. [Google Scholar]
- Fechete, R.; Demco, D.E.; Eliav, U.; Blumich, B.; Navon, G. Self-diffusion anisotropy of water in sheep Achilles tendon. NMR Biomed. 2005, 18, 577–586. [Google Scholar] [CrossRef]
- Peto, S.; Gillis, P.; Henri, V.P. Structure and dynamics of water in tendon from NMR relaxation measurements. Biophys. J. 1990, 57, 71–84. [Google Scholar] [CrossRef]
- Takamiya, H.; Kusaka, Y.; Seo, Y.; Noguchi, M.; Ikoma, K.; Morimoto, T.; Hirasawa, Y. Characteristics of proton NMR T2 relaxation of water in the normal and regenerating tendon. Jpn. J. Physiol. 2000, 50, 569–576. [Google Scholar] [CrossRef]
- Rodin, V.V. Methods of magnetic resonance in studying natural biomaterials. In Encyclopedia of Physical Organic Chemistry; Wang, Z., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; Chapter 53; Volume 4, pp. 2861–2908. [Google Scholar]
- Rodin, V.V.; Nikerov, V.A. NMR relaxation and PFG NMR studies of water dynamics in oriented collagen fibres with different degree of cross-linking. Curr. Tissue Eng. 2014, 3, 47–61. [Google Scholar] [CrossRef]
- Han, S.; Gemmell, S.; Helmer, K.; Grigg, P.; Wellen, J.; Hoffman, A.; Sotak, C. Changes in ADC caused by tensile loading of rabbit Achilles tendon: Evidence for water transport. J. Magn. Reson. 2000, 144, 217–227. [Google Scholar] [CrossRef]
- Fechete, R.; Demco, D.E.; Blumich, B.; Eliav, U.; Navon, G. Anisotropy of collagen fibre orientation in sheep tendon by 1H double-quantum-filtered NMR signals. J. Magn. Reson. 2003, 162, 166–175. [Google Scholar] [CrossRef]
- Rodin, V.V. Magnetic resonance in studying cells, biotechnology dispersions, fibers and collagen based tissues for biomedical engineering. Chapter in the book. In Biological, Physical and Technical Basics of Cell Engineering; Artmann, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Henkelman, R.M.; Stanisz, G.J.; Kim, J.K.; Bronskill, M.J. Anisotropy of NMR properties of tissues. Magn. Reson. Med. 1994, 32, 592–601. [Google Scholar] [CrossRef]
- Eliav, U.; Navon, J. Multiple Quantum MRS. In Handbook of Magnetic Resonance Spectroscopy In Vivo: MRS Theory, Practice and Applications; Bottomley, P.A., Griffiths, J.R., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; ISBN 978-1-118-99766-6. [Google Scholar]
- Wang, N.; Xia, Y. Anisotropic analysis of multi-component T2 and T1ρ relaxations in Achilles tendon by NMR spectroscopy and microscopic MRI. J. Magn. Reson. Imaging 2013, 38, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Hänninen, N.; Rautiainen, J.; Rieppo, L.; Saarakkala, S.; Nissi, M.J. Orientation anisotropy of quantitative MRI relaxation parameters in ordered tissue. Sci. Rep. 2017, 7, 9606. [Google Scholar] [CrossRef] [PubMed]
- Gere, J.M.; Timoshenko, S.P. Mechanics of Materials, 2nd ed.; Brooks/Cole Engineering Division: Monterey, CA, USA, 1984. [Google Scholar]
- Rodin, V.V.; Sakharov, B.V.; Izmailova, V.N.; Knight, D.P. Study of the collagen spicule structure by NMR relaxation and electron microscopy. Russ. J. Biotechnol. 2001, 1, 257–265. [Google Scholar]
- Rodin, V.V.; Sakharov, B.V.; Gerasimov, V.N.; Knight, D.P. NMR-relaxation in natural polymers (collagen spicules). Intern. Polym. Sci. Technol. 2001, 28, 70–78. [Google Scholar] [CrossRef]
- Belton, P.; Colquhoun, I.; Grant, A.; Wellner, N.; Field, J.; Shewry, P.; Tatham, A. FTIR and NMR studies on the hydration of a high-Mr subunit of glutenin. Int. J. Biol. Macromol. 1995, 17, 74–80. [Google Scholar] [CrossRef]
- Wellner, N.; Belton, P.S.; Tatham, A.S. Fourier transform IR spectroscopic study of hydration-induced structure changes in the solid state of ω-gliadins. Biochem. J. 1996, 319, 741–747. [Google Scholar] [CrossRef]
- Asakura, T.; Kuzuhara, A.; Tabeta, R.; Saito, H. Conformation Characterization of Bombyx mori Silk Fibroin in the Solid State by High-Frequency 13C Cross Polarization-Magic Angle Spinning NMR, X-ray Diffraction, and Infrared Spectroscopy. Macromolecules 1985, 18, 1841–1845. [Google Scholar] [CrossRef]
- Yoshimizu, H.; Asakura, T. The structure of Bombyx mori silk fibroin membrane swollen by water studied with ESR, 13C-NMR, and FTIR spectroscopies. J. Appl. Polym. Sci. 1990, 40, 1745–1756. [Google Scholar] [CrossRef]
- Chen, X.; Knight, D.P.; Shao, Z.; Volrath, F. Regenerated Bombyx mori silk solutions studied with rheometry and FTIR. Polymer 2001, 42, 9969–9974. [Google Scholar] [CrossRef]
- Rodin, V.V. Basics principles of NMR and experimental techniques. In Magnetic Resonance in Studying Natural and Synthetic Materials, 1st ed.; Rodin, V.V., Ed.; Bentham Science Publisher: Sharjah, United Arab Emirates, 2018; Chapter 1; pp. 1–25, 200. 244p, ISBN 978-1-68108-630-9. [Google Scholar]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef]
- Estilaei, M.; MacKay, A.; Roberts, C.; Mayo, J. 1H NMR measurements of wet/dry ratio and T1, T2 distribution in lung. J. Magn. Reson. 1997, 124, 410–419. [Google Scholar] [CrossRef]
- Fedotov, V.D.; Shnaider, K.H. Structure and Dynamics of Polymers: Investigations with NMR Method; Nauka: Moscow, Russia, 1992. [Google Scholar]
- Rot, G.-K.; Keller, F.; Schnaider, X. Radiospectroscopy of Polymers; Mir: Moscow, Russia, 1987. [Google Scholar]
- MacKey, A.; Bloom, M.; Tepfer, M.; Taylor, I.E.P. Broadline proton magnetic resonance study of cellulose, pectin, and bean cell walls. Biopolymers 1982, 21, 1521–1534. [Google Scholar] [CrossRef]
- Flibotte, S.; Menon, R.S.; MacKay, A.; Hailey, J.R.T. Proton magnetic resonance of western red cedar. Wood Fiber Sci. 1990, 22, 362–376. [Google Scholar]
- Nicasy, R.; Huinink, H.; Erich, B.; Olaf, A. NMR Profiling of Reaction and Transport in Thin Layers:A Review. Polymers 2022, 14, 798. [Google Scholar] [CrossRef] [PubMed]
- Woessner, D.E.; Zimmerman, J.R. Nuclear transfer and anisotropic motional spin phenomena: Relaxation time temperature dependence studies of water adsorbed on silica gel. J. Phys. Chem. 1963, 67, 1590–1600. [Google Scholar] [CrossRef]
- Watanabe, T.; Murase, N.; Staemmler, M.; Gersonde, K. Multiexponential proton relaxation processes of compartmentalized water in gels. Magn. Reson. Med. 1992, 27, 118–134. [Google Scholar] [CrossRef]
- Luz, Z.; Meiboom, S. Nuclear magnetic resonance study of the protolysis of trimethylammonium ion in aqueous solution—Order of the reaction with respect to solvent. J. Chem. Phys. 1963, 39, 366. [Google Scholar] [CrossRef]
- Belton, P.S. NMR studies of protein hydration. Prog. Biophys. Molec. Biol. 1994, 6, 61–79. [Google Scholar]
- Hanus, F.; Gillis, P. Relaxation of water adsorbed on the surface of silica powder. J. Magn. Reson. 1984, 59, 437–445. [Google Scholar] [CrossRef]
- Carver, J.P.; Richards, R.E. A general two-site solution for the chemical exchange produced dependence of T2 upon the Carr-Purcell pulse separation. J. Magn. Reson. 1972, 6, 89. [Google Scholar] [CrossRef]
- Kovrigin, E.L.; Kempf, J.G.; Grey, M.J.; Loria, J.P. Faithful estimation of dynamics parameters from CPMG relaxation dispersion measurements. J. Magn. Reson. 2006, 180, 93–104. [Google Scholar] [CrossRef]
- Baldwin, A.J. An exact solution for R2eff in CPMG experiments in the case of two site chemical exchange. J. Magn. Reson. 2014, 244, 114–124. [Google Scholar] [CrossRef]
- Migchelsen, C.; Berendsen, H.J.C. Proton exchange and molecular orientation of water in hydrated collagen fibers. An NMR study of H2O and D2O. J. Chem. Phys. 1973, 59, 296–305. [Google Scholar] [CrossRef]
- Berendsen, H.J.C. Nuclear magnetic resonance study of collagen hydration. J. Chem. Phys. 1962, 36, 3297–3305. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Migchelsen, C. Hydration structure of fibrous macromolecules. Ann. N. Y. Acad. Sci. 1965, 125, 365–379. [Google Scholar] [CrossRef]
- Hazlewood, C.F.; Chang, D.C.; Nichols, B.L.; Woessner, D.E. Nuclear magnetic resonance transverse relaxation times of water protons in skeletal muscle. Biophys. J. 1974, 14, 583–606. [Google Scholar] [CrossRef]
- Kasturi, S.R.; Chang, D.C.; Hazlewood, C.F. Study of anisotropy in nuclear magnetic resonance relaxation times of water protons in skeletal muscle. Biophys. J. 1980, 30, 369–381. [Google Scholar] [CrossRef] [Green Version]
- Chavez, F.V.; Halle, B. Molecular basis of water proton relaxation in gels and tissue. Magn. Reson. Med. 2006, 56, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Peschier, L.J.C.; Bouwstra, J.A.; de Blayser, J.; Junginger, H.E.; Leyte, J.C. Cross-relaxation effects in pulsed-field-gradient stimulated-echo measurement on water in a macromolecular matrix. J. Mag. Reson. 1996, 110, 150–157. [Google Scholar] [CrossRef]





| T2g (μs) | A2g (%) | T2e (μs) | A2e (%) | |
|---|---|---|---|---|
| Randomly oriented fibres, HL = 0.11 | 12.4 | 82 | 133 | 18 |
| Randomly oriented fibres, HL = 0.32 | 8.5 | 52 | 277 | 48 |
| Fibres after D2O exchange, HL = 0.11 | 12.9 | 68 | 193 | 32 |
| Silk B. mori cocoon, HL = 0.10 | 13.4 | 73 | 167 | 27 |
| Silk B. mori cocoon, HL = 0.24 | 16.4 | 55 | 297 | 45 |
| N | T2e (μs) | SD | A2e (%) | R2e = 1.35 | T2g (μs) | SD | A2g (%) | R2g = 1.14 | |
|---|---|---|---|---|---|---|---|---|---|
| Orientation 90° | 6 | 212.5 | 7.4 | 20.2 | 14.4 | 0.9 | 79.7 | ||
| Orientation 0° | 9 | 157.4 | 14.3 | 19.2 | 12.6 | 1.1 | 80.8 |
| HL | T2e (μs) | A2e (%) | T2g (μs) | A2g (%) | T1e (ms) | A1e (%) | T1s (s) | A1e (%) | M2(× 109 s−2) | T1me (ms) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Orientation 0° | 0.085 | 150.3 | 20 | 12.8 | 80 | -- | -- | -- | -- | -- | -- |
| Orientation 0° | 0.11 | 162.7 | 18 | 11.7 | 81 | 802 | 95 | 1.29 | 5 | 6.9 | 745 |
| Orientation 0° | 0.35 | 429.0 | 55 | 9.5 | 45 | -- | -- | -- | -- | 6.1 | 451 |
| Orientation 90° | 0.085 | 215.2 | 19 | 14.1 | 81 | -- | -- | -- | -- | -- | -- |
| Orientation 90° | 0.11 | 260.1 | 26 | 12.1 | 74 | 464 | 74 | 1.26 | 25 | 6.4 | 603 |
| Orientation 90° | 0.42 | 557.5 | 50 | 10.3 | 50 | 362 | 82 | 0.93 | 16 | 4.74 | 314 |
| Orientation 90° | 1.03 | 597.0 | 58 | 11.0 | 41 | 326 | -- | -- | -- | 4.12 | 326 |
| N | T2e (μs) | SD | A2e (%) | R2e = 1.2 | T2g (μs) | SD | A2g (%) | R2g = 1.11 | M2 (× 109 s−2) | SD | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Orientation 90° | 4 | 267.0 | 28.1 | 27.5 | 13.4 | 0.8 | 72.5 | 5.7 | 0.1 | ||
| Orientation 0° | 4 | 222.1 | 7.4 | 29.2 | 12.1 | 2.1 | 70.7 | 6.1 | 0.1 |
| T2e (μs) | A2e (%) | T2g (μs) | A2g (%) | T2e* (μs) | A2e* (%) | T2g* (μs) | A2g* (%) | |
|---|---|---|---|---|---|---|---|---|
| Oriented fibres, 90° | 212.5 | 20.2 | 14.4 | 79.7 | 267.0 | 27.5 | 13.4 | 72.5 |
| Oriented fibres, 0° | 157.4 | 19.2 | 12.6 | 80.8 | 222.0 | 29.2 | 12.1 | 70.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodin, V.V.; Belton, P.S. Changes in Natural Silk Fibres by Hydration, Tensile Loading and Heating as Studied by 1H NMR: Anisotropy in NMR Relaxation Times. Polymers 2022, 14, 3665. https://doi.org/10.3390/polym14173665
Rodin VV, Belton PS. Changes in Natural Silk Fibres by Hydration, Tensile Loading and Heating as Studied by 1H NMR: Anisotropy in NMR Relaxation Times. Polymers. 2022; 14(17):3665. https://doi.org/10.3390/polym14173665
Chicago/Turabian StyleRodin, Victor V., and Peter S. Belton. 2022. "Changes in Natural Silk Fibres by Hydration, Tensile Loading and Heating as Studied by 1H NMR: Anisotropy in NMR Relaxation Times" Polymers 14, no. 17: 3665. https://doi.org/10.3390/polym14173665