Rheological Method for Determining the Molecular Weight of Collagen Gels by Using a Machine Learning Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Collagen Sample Dilution Process
2.2.2. Obtaining Collagen Samples of Different Molecular Weights
2.2.3. Rheological Tests
2.2.4. Application of Machine Learning Techniques
2.2.5. Statistical Analysis
2.2.6. Mw Measurements by Traditional Techniques
3. Results and Discussion
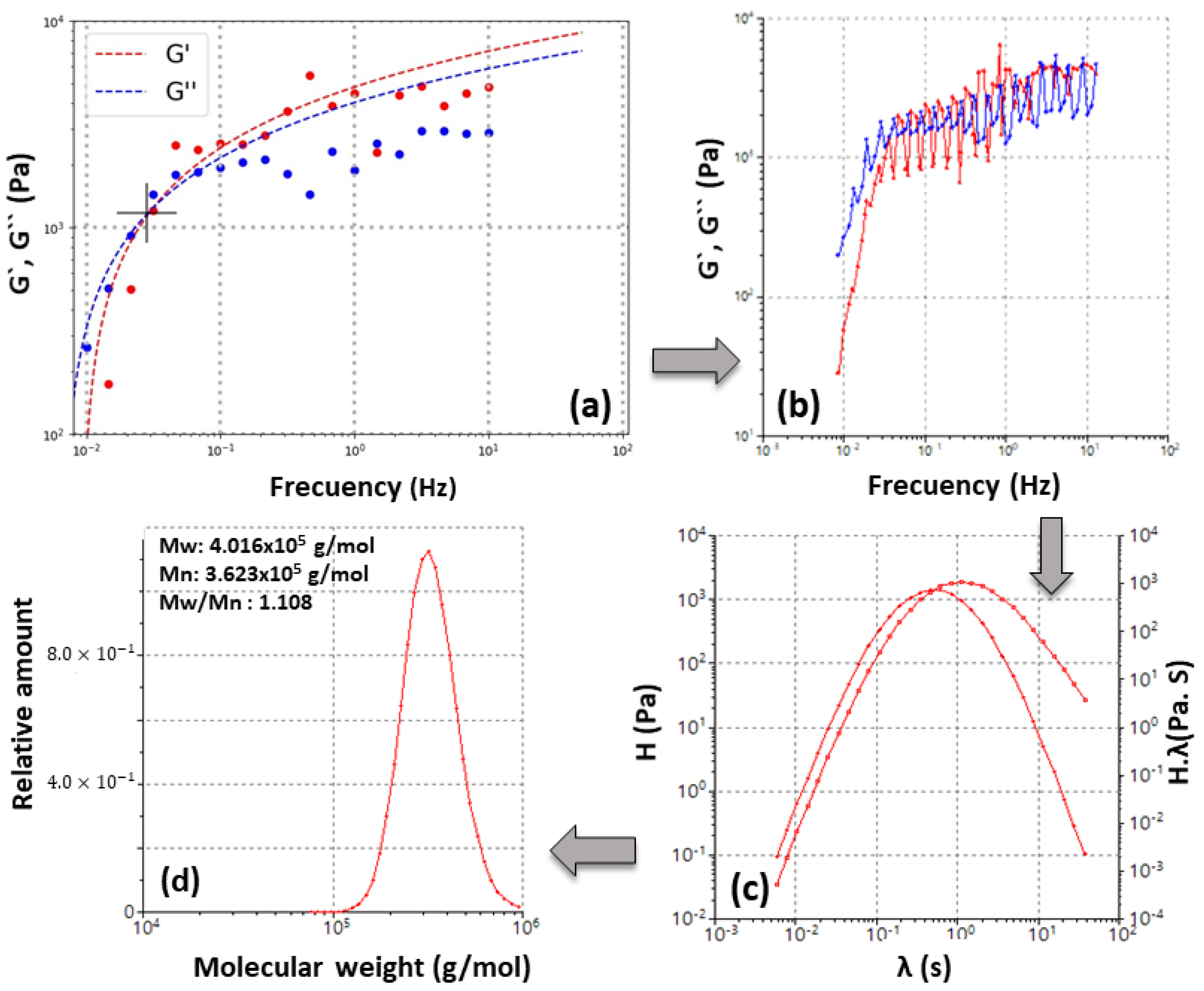
3.1. Mathematical Principle of the Rheological Method Used
3.1.1. Application of Machine Learning Techniques
3.1.2. Master Curve, Relaxation Spectrum, and Mw Gaussians Computation
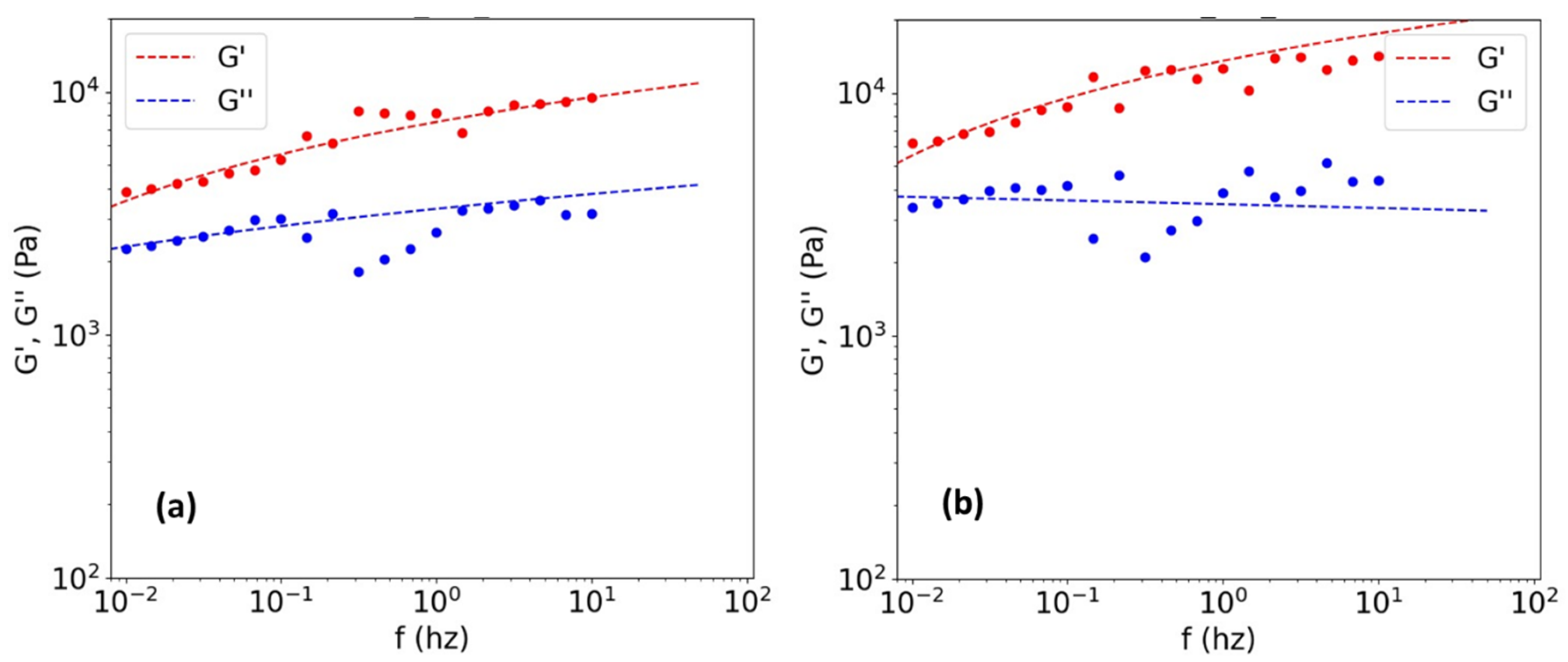
3.2. Effect of Collagen Concentration on the Rheology-Based Results
3.3. Validation of the Rheological Method
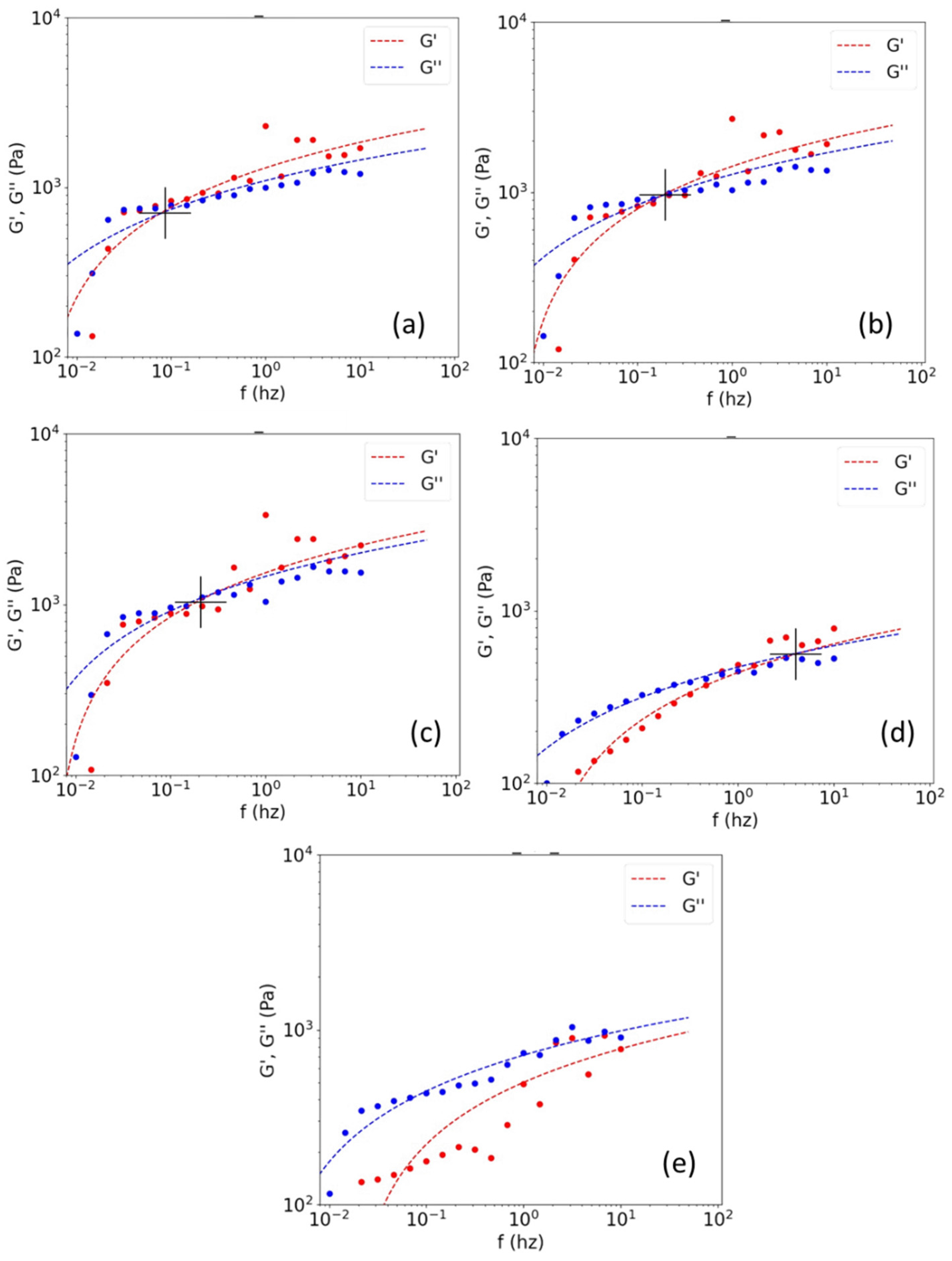
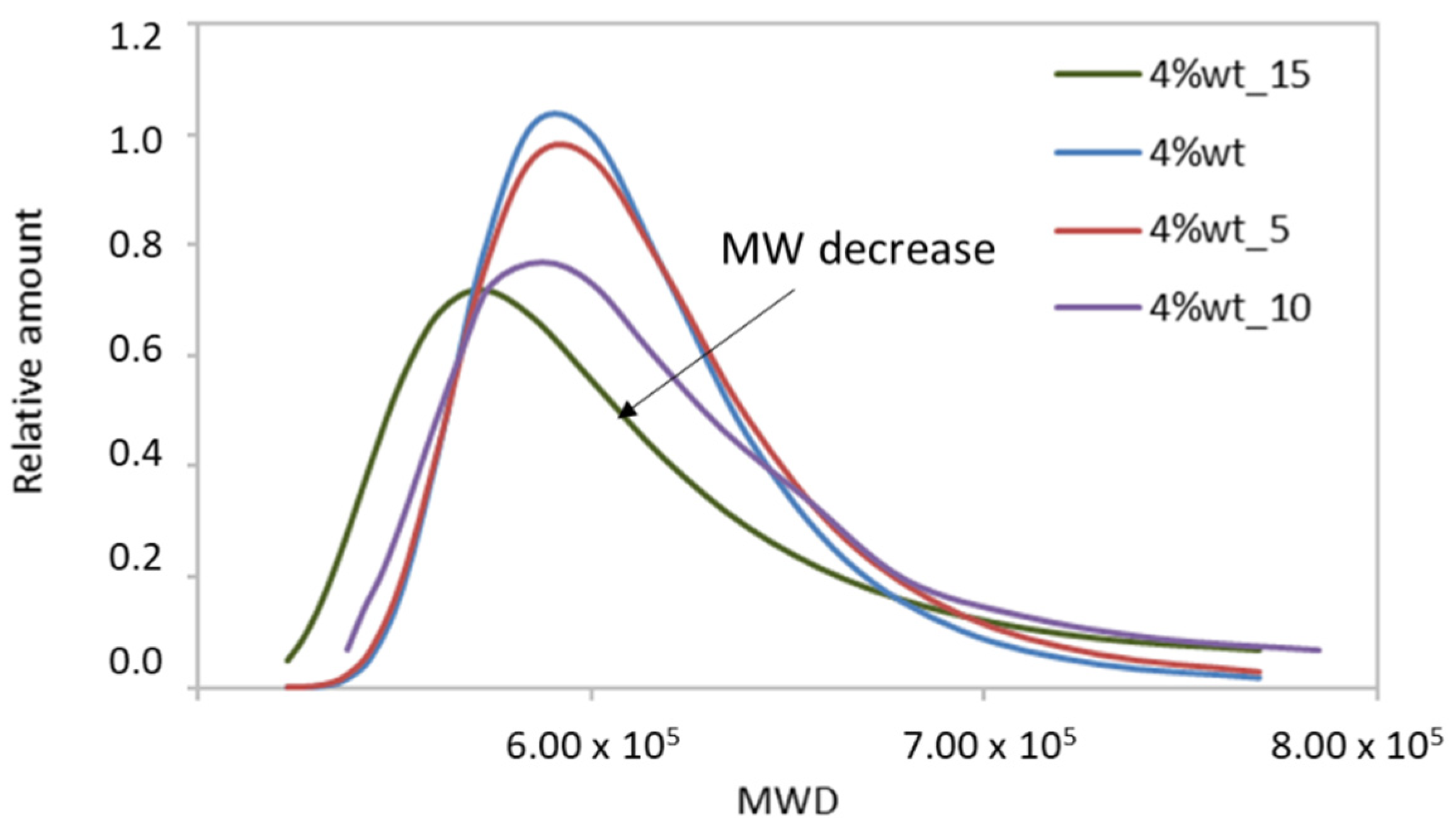
3.3.1. Application of the Method to Samples of Different Mw
3.3.2. Comparison with Traditional Measurement Techniques
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prashant, K.; Bhagwat, P.B.D. Collagen and collagenolytic proteases: A review. Biocatal. Agric. Biotechnol. 2018, 115, 60–66. [Google Scholar]
- Subhan, F.; Hussain, Z.; Tauseef, I.; Shehzad, A.; Wahid, F. A review on recent advances and applications of fish collagen. Crit. Rev. Food Sci. Nutr. 2021, 61, 1027–1037. [Google Scholar] [CrossRef]
- Sionkowska, A.; Skrzyński, S.; Śmiechowski, K.; Kołodziejczak, A. The review of versatile application of collagen. Polym. Adv. Technol. 2017, 28, 4–9. [Google Scholar] [CrossRef]
- Tiplea, R.E.; Lemnaru, G.M.; Trușcă, R.D.; Holban, A.; Kaya, M.G.A.; Dragu, L.D.; Ficai, D.; Ficai, A.; Bleotu, C. Antimicrobial films based on chitosan, collagen, and zno for skin tissue regeneration. Biointerface Res. Appl. Chem. 2021, 11, 11985–11995. [Google Scholar] [CrossRef]
- Tihăuan, B.-M.; Pircalabioru, G.G.; Axinie Bucos, M.; Marinaș, I.C.; Nicoară, A.-C.; Măruțescu, L.; Oprea, O.; Matei, E.; Maier, S.S. Crosslinked Collagenic Scaffold Behavior Evaluation by Physico-Chemical, Mechanical and Biological Assessments in an In Vitro Microenvironment. Polymers 2022, 14, 2430. [Google Scholar] [CrossRef]
- Sionkowska, A.; Adamiak, K.; Musial, K.; Gadomska, M. Collagen based materials in cosmetic applications: A review. Materials 2020, 13, 4217. [Google Scholar] [CrossRef]
- Jendricke, P.; Centner, C.; Zdzieblik, D.; Gollhofer, A.; König, D. Specific collagen peptides in combination with resistance training improve body composition and regional muscle strength in premenopausal women: A randomized controlled trial. Nutrients 2019, 11, 892. [Google Scholar] [CrossRef]
- Hashim, P.; Mohd Ridzwan, M.S.; Bakar, J.; Mat Hashim, D. Collagen in food and beverage industries. Int. Food Res. J. 2015, 22, 1–8. [Google Scholar]
- Le, A.; Morales-peñaloza, A.; Mart, M. Hydrolyzed Collagen—Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar]
- Klüver, E.; Baltzer, M.; Langer, A.; Meyer, M. Additive Manufacturing with Thermoplastic Collagen. Polymers 2022, 14, 974. [Google Scholar] [CrossRef]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Fan, H.; Chalamaiah, M.; Wu, J. Preparation of low-molecular-weight, collagen hydrolysates (peptides): Current progress, challenges, and future perspectives. Food Chem. 2019, 301, 125222. [Google Scholar] [CrossRef] [PubMed]
- Rich, A.; Crick, F.H.C. The molecular structure of collagen. J. Mol. Biol. 1961, 3, 483–506. [Google Scholar] [CrossRef]
- Shi, C.; Bi, C.; Ding, M.; Xie, J.; Xu, C.; Qiao, R.; Wang, X.; Zhong, J. Polymorphism and stability of nanostructures of three types of collagens from bovine flexor tendon, rat tail, and tilapia skin. Food Hydrocoll. 2019, 93, 253–260. [Google Scholar] [CrossRef]
- Xu, J.; Liu, F.; Goff, H.D.; Zhong, F.; Liu, F.; Goff, H.D.; Zhong, F. Effect of pre-treatment temperatures on the film-forming properties of collagen fiber dispersions. Food Hydrocoll. 2018, 107, 105326. [Google Scholar] [CrossRef]
- Bhuimbar, M.V.; Bhagwat, P.K.; Dandge, P.B. Extraction and characterization of acid soluble collagen from fish waste: Development of collagen-chitosan blend as food packaging film. J. Environ. Chem. Eng. 2019, 7, 102983. [Google Scholar] [CrossRef]
- Zhang, X.; Adachi, S.; Ura, K.; Takagi, Y. Properties of collagen extracted from Amur sturgeon Acipenser schrenckii and assessment of collagen fibrils in vitro. Int. J. Biol. Macromol. 2019, 137, 809–820. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, J.H.; Yun, S.Y.; Yoo, J.S.; Jun, C.H.; Chung, K.Y.; Suh, H. Reaction monitoring of succinylation of collagen with matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2000, 14, 2125–2128. [Google Scholar] [CrossRef]
- Kumar, R.; Sripriya, R.; Balaji, S.; Senthil Kumar, M.; Sehgal, P.K. Physical characterization of succinylated type I collagen by Raman spectra and MALDI-TOF/MS and in vitro evaluation for biomedical applications. J. Mol. Struct. 2011, 994, 117–124. [Google Scholar] [CrossRef]
- Cozza, N.; Bonani, W.; Motta, A.; Migliaresi, C. Evaluation of alternative sources of collagen fractions from Loligo vulgaris squid mantle. Int. J. Biol. Macromol. 2016, 87, 504–513. [Google Scholar] [CrossRef]
- Meyer, M.; Morgenstern, B. Characterization of gelatine and acid soluble collagen by size exclusion chromatography coupled with multi angle light scattering (SEC-MALS). Biomacromolecules 2003, 4, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Wu, S. Polymer molecular-weight distribution from dynamic melt viscoelasticity. Polym. Eng. Sci. 1985, 25, 122–128. [Google Scholar] [CrossRef]
- Tuminello, W.H. Molecular Weight and Molecular Weight Distribution From Dynamic Measurements of Polymer Melts. Polym. Eng. Sci. 1986, 26, 1339–1347. [Google Scholar] [CrossRef]
- Liu, Y.; Shaw, M.T.; Tuminello, W.H. Optimized data collection for determination of the MWD from the viscosity data of polymer melts. Polym. Eng. Sci. 1998, 38, 169–176. [Google Scholar] [CrossRef]
- Costello, B. Use of Rheology to Determine the Molecular Weight Distribution of Polymers. Annu. Trans. Nord. Rheol. Soc. 2005, 13, 61–64. [Google Scholar]
- Khak, M.; Ahmad Ramazani, S.A. Rheological measurement of molecular weight distribution of polymers. E-Polymers 2013, 13, 1–12. [Google Scholar] [CrossRef]
- Costello, B.; Smith, R.; Dallas, G. New Molecular Weight Distribution Software, Softpedia.
- Guide, U. HAAKE RheoWin V.4.86.0002; Thermo Fisher Scientific: Germany, 2014. [Google Scholar]
- Zhang, H. Rheological methods for determining molecular weight and molecular weight distribution. Mater. Sci. Technol. 2012, 6, 116–123. [Google Scholar] [CrossRef]
- Lai, G.; Li, Y.; Li, G. Effect of concentration and temperature on the rheological behavior of collagen solution. Int. J. Biol. Macromol. 2008, 42, 285–291. [Google Scholar] [CrossRef]
- Da Tan, J.; Ramalingam, B.; Wong, S.L.; Cheng, J.; Lim, Y.-F.; Chellappan, V.; Khan, S.A.; Kumar, J.; Hippalgaonkar, K. Machine Learning Predicts Conversion and Molecular Weight Distributions in Computer Controlled Polymerization. ChemRxiv 2022, 1–24. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, G.; Shi, B. Physicochemical properties of collagen, gelatin and collagen hydrolysate derived from bovine limed split wastes. J. Soc. Leather Technol. Chem. 2006, 90, 23–28. [Google Scholar]
- Nomura, Y.; Yamano, M.; Hayakawa, C.; Ishii, Y.; Shirai, K. Structural property and in vitro self-assembly of shark type I collagen. Biosci. Biotechnol. Biochem. 1997, 61, 1919–1923. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Mead, D.W. Determination of molecular weight distributions of linear flexible polymers from linear viscoelastic material functions. J. Rheol. 1994, 38, 1797–1827. [Google Scholar] [CrossRef]
- Maier, D.; Eckstein, A.; Friedrich, C.; Honerkamp, J. Evaluation of models combining rheological data with the molecular weight distribution. J. Rheol. 1998, 42, 1153–1173. [Google Scholar] [CrossRef]
- Friess, W.; Schlapp, M. Effects of processing conditions on the rheological behavior of collagen dispersions. Eur. J. Pharm. Biopharm. 2001, 51, 259–265. [Google Scholar] [CrossRef]
- Montgomery, D. Design and Analysis of Experiments, 5th ed.; John Wiley and Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Sotelo, C.G.; Comesaña, M.B.; Ariza, P.R.; Pérez-Martín, R.I. Characterization of Collagen from Different Discarded Fish Species of the West Coast of the Iberian Peninsula. J. Aquat. Food Prod. Technol. 2016, 25, 388–399. [Google Scholar] [CrossRef]
- Benjakul, S.; Nalinanon, S.; Shahidi, F. Fish Collagen. In Food Biochemistry and Food Processing, 2nd ed.; Wiley: Hoboken, NJ, USA, 2012; pp. 365–387. [Google Scholar] [CrossRef]



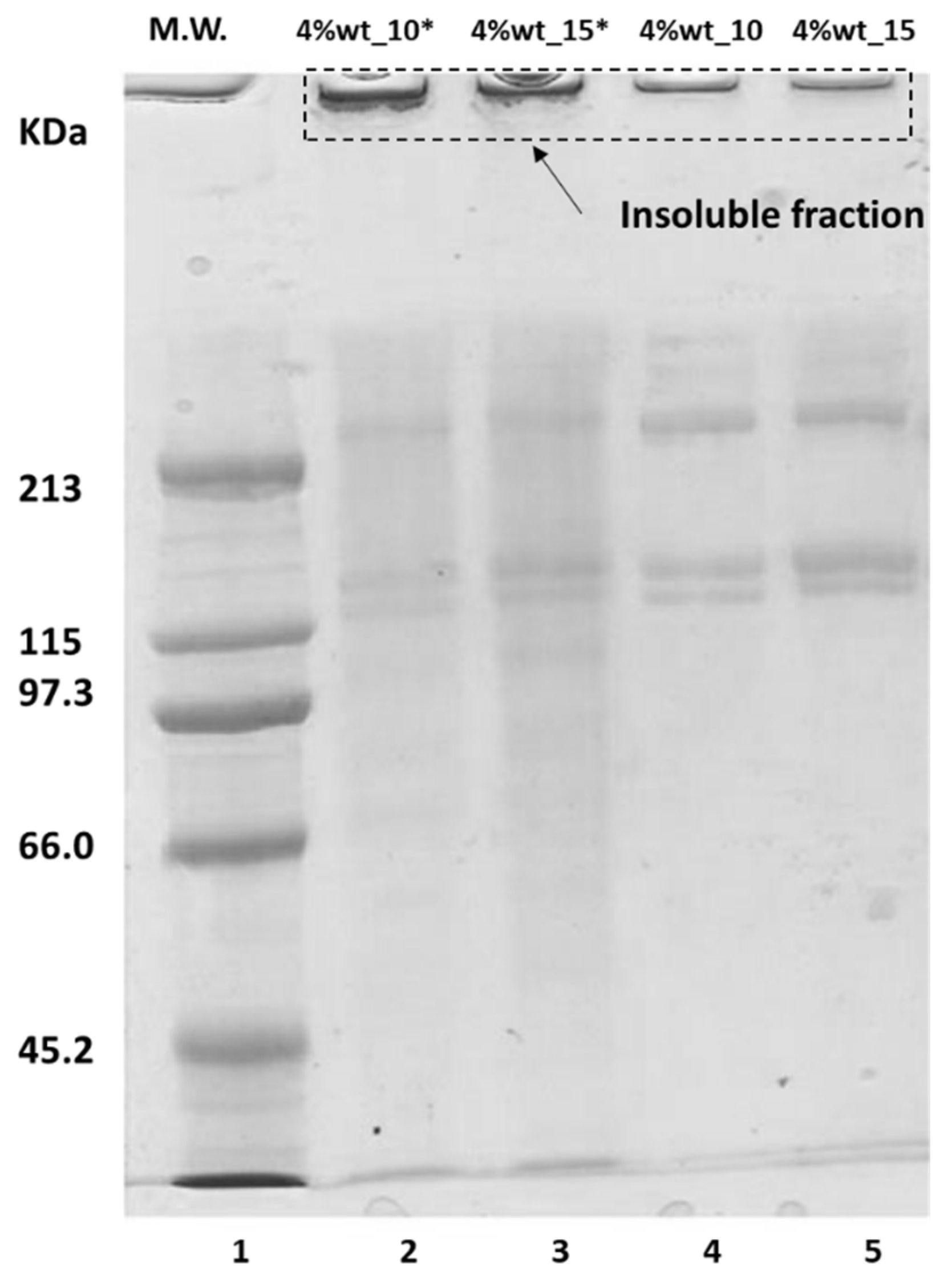
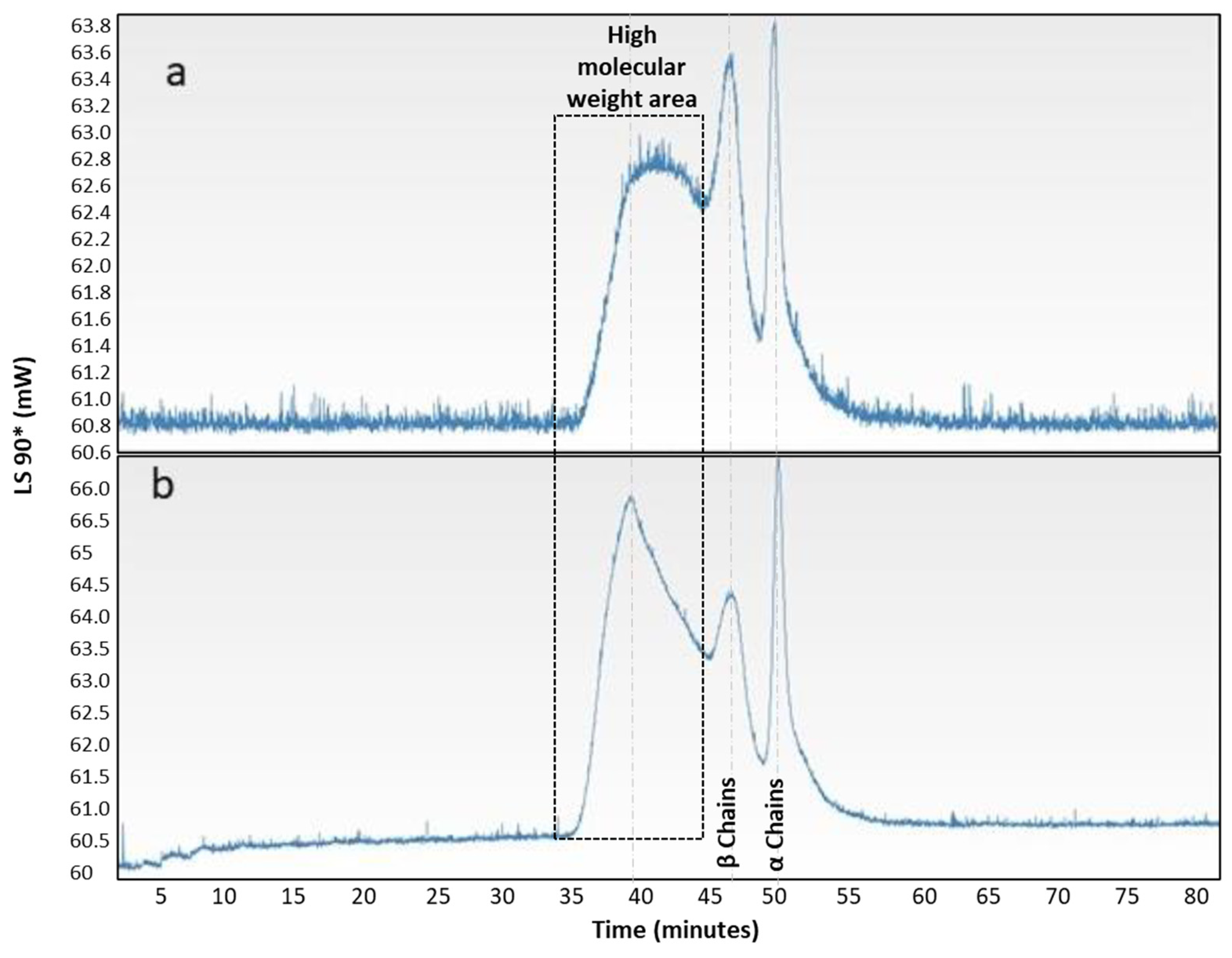
| Experimental Parameters | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stress (Pa) (CS)a | 10 | 50 | 100 | |||||||
| Strain or Deformation (CD)b | 0.01 | 0.05 | 0.5 | |||||||
| Temperature (°C) | 23 | 25 | 28 | 32 | 34 | 36 | 38 | 40 | ||
| Gap (mm) | 1 | 2 | ||||||||
| Sample (% wt) | Denaturation Temperature ± 1.50 (°C) |
|---|---|
| 1 | 41.94 |
| 2 | 40.82 |
| 3 | 38.41 |
| 4 | 38.15 |
| 5 | 38.12 |
| 15 | 37.20 |
| Sample b | 1% wt (Experimental) | 1% wt (Theoretical) | ||||||
| Exp. Cond. a | CS: 10 Pa, Gap: 2 mm | |||||||
| Temp. (°C) | 23 | 28 | 34 | 40 | 23 | 28 | 34 | 40 |
| Mw (g/mol) d | - c | - | 4.2 × 104 ± 4.0 × 103 | - | 1.7 × 105 ± 1.0 × 105 | 3.5 × 105 ± 1.0 × 104 | 5.7 × 105 ± 1.0 × 104 | 1369 ± 200 |
| Mn (g/mol) e | - | - | 3.5 × 104 ± 3.5 × 103 | - | 1.4 × 105 ± 1.5 × 105 | 3.0 × 105 ± 2.0 × 104 | 4.2 × 104 ± 1.0 × 104 | 1166 ± 420 |
| PDI f | - | - | 1.2 ± 0.7 | - | 1.2 ± 0.9 | 1.2 ± 0.6 | 1.4 ± 0.9 | 1.2 ± 0.9 |
| Sample | 2% wt (Experimental) | 2% wt (Theoretical) | ||||||
| Exp. Cond. | CD: 0.5, Gap: 2 mm | |||||||
| Temp. (°C) | 23 | 28 | 34 | 40 | 23 | 28 | 34 | 40 |
| Mw (g/mol) | - | 5.0 × 105 ± 4.0 × 104 | 5.5 × 105 ± 2.0 × 105 | - | 1.0 × 106 ± 4.0 × 105 | 5.5 × 105 ± 2.0 × 105 | 5.5 × 105 ± 2.0 × 105 | - |
| Mn (g/mol) | - | 3.1 × 105 ± 2.0 × 104 | 3.5 × 105 ± 7.0 × 103 | - | 5.8 × 105 ± 3.0 × 102 | 7.2 × 105 ± 1.0 × 105 | 3.5 × 105 ± 7.0 × 103 | - |
| PDI | - | 1.6 ± 0.8 | 1.6 ± 1.0 | - | 1.7 ± 0.7 | 1.9 ± 0.8 | 1.3 ± 1.1 | - |
| Sample | 3% wt (Experimental) | 3% wt (Theoretical) | ||||||
| Exp. Cond. | CD: 1.0, Gap: 2 mm | |||||||
| Temp. (°C) | 23 | 28 | 32 | 36 | 23 | 28 | 32 | 36 |
| Mw (g/mol) | 1.7 × 106 ± 4.0 × 103 | 7.3 × 105 ± 4.0 × 103 | 6.8 × 105 ± 1.5 × 102 | 5.2 × 105 ± 0.5 × 102 | 7.2 × 105 ± 1.5 × 102 | 8.5 × 105 ± 1.5 × 102 | 6.5 × 105 ± 1.0 × 102 | 4429 ± 120 |
| Mn (g/mol) | 9.4 × 105 ± 3.0 × 103 | 4.2 × 105 ± 1.0 × 102 | 3.7 × 105 ± 3.0 × 102 | 3.0 × 105 ± 1.0 × 103 | 3.7 × 105 ± 0.5 × 102 | 4.4 × 105 ± 4.0 × 102 | 3.4 × 105 ± 3.5 × 102 | 3163 ± 256 |
| PDI | 1.8 ± 0.2 | 1.7 ± 0.4 | 1.8 ± 0.3 | 1.7 ± 0.8 | 1.9 ± 0.1 | 1.9 ± 0.2 | 1.9 ± 0.4 | 1.4 ± 1.1 |
| Sample | 4% wt (Experimental) | 4% wt (Theoretical) | ||||||
| Exp. Cond. | CS: 300 Pa, Gap: 0.5 mm | |||||||
| Temp. (°C) | 23 | 28 | 32 | 36 | 23 | 28 | 32 | 36 |
| Mw (g/mol) | 5.2 × 105 ± 1.0 × 102 | 6.1 × 105 ± 9.0 × 101 | 6.9 × 105 ± 1.5 × 102 | - | 7.6 × 105 ± 1.5 × 102 | 6.7 × 105 ± 1.0 × 103 | 8.1 × 105 ± 1.0 × 103 | - |
| Mn (g/mol) | 2.4 × 105 ± 2.5 × 102 | 3.0 × 105 ± 3.0 × 102 | 3.6 × 105 ± 1.0 × 103 | - | 3.8 × 105 ± 1.5 × 103 | 3.7 × 105 ± 2.5 × 102 | 4.3 × 105 ± 1.0 × 103 | - |
| PDI | 2.1 ± 0.5 | 2.0 ± 0.2 | 1.9 ± 0.4 | - | 2.0 ± 0.1 | 1.8 ± 0.4 | 1.9 ± 0.3 | - |
| Sample | 5% wt (Experimental) | 5% wt (Theoretical) | ||||||
| Exp. Cond. | CS: 500 Pa, Gap: 1 mm | |||||||
| Temp. (°C) | 23 | 28 | 34 | 36 | 23 | 28 | 34 | 36 |
| Mw (g/mol) | 6.3 × 105 ± 1.5 × 102 | 6.8 × 105 ± 3.0 × 102 | 7.1 × 105 ± 1.0 × 102 | - | 7.6 × 105 ± 1.0 × 102 | 6.9 × 105 ± 0.9 × 102 | 7.2 × 105 ± 1.5 × 102 | - |
| Mn (g/mol) | 3.5 × 105 ± 1.0 × 103 | 3.6 × 105 ± 1.5 × 103 | 3.7 × 105 ± 2.0 × 103 | - | 3.6 × 105 ± 1.0 × 103 | 3.1 × 105 ± 5.0 × 102 | 3.6 × 105 ± 3.5 × 103 | - |
| PDI | 1.8 ± 0.5 | 1.9 ± 0.5 | 1.9 ± 0.2 | - | 2.1 ± 0.2 | 2.2 ± 0.5 | 2.0 ± 0.3 | - |
| Sample | 15% wt (Experimental) | 15% wt (Theoretical) | ||||||
| Exp. Cond. | CD: 0.5, Gap: 2 mm | |||||||
| Temp. (°C) | 23 | 28 | 34 | 36 | 23 | 28 | 34 | 36 |
| Mw (g/mol) | - | - | - | - | - | - | - | - |
| Mn (g/mol) | - | - | - | - | - | - | - | - |
| PDI | - | - | - | - | - | - | - | - |
| Run | Studied Population | p-Value | ||
|---|---|---|---|---|
| Concentration (% wt) | Temperature (°C) | Data | ||
| 1 | 1 | 34 | Experimental | - |
| 2 | 1 | 23, 28, 34, 40 | Theoretical | 5 × 10−18 |
| 3 | 1 | 23, 28, 34 | Theoretical | 7 × 10−12 |
| 4 | 2 | 28, 34 | Experimental | 3 × 10−17 |
| 5 | 2 | 23, 28, 34 | Theoretical | 2 × 10−10 |
| 6 | 3 | 23, 28, 32, 36 | Experimental | 6 × 10−13 |
| 7 | 3 | 23, 28, 32, 36 | Theoretical | 2 × 10−9 |
| 8 | 3 | 23, 28, 32 | Experimental | 4 × 10−8 |
| 9 | 3 | 23, 28, 32 | Theoretical | 8 × 10−6 |
| 10 | 4 | 23, 28, 32 | Experimental | 4 × 10−6 |
| 11 | 4 | 23, 28, 32 | Theoretical | 1 × 10−5 |
| 12 | 5 | 23, 28, 32 | Experimental | 2 × 10−4 |
| 13 | 5 | 23, 28, 32 | Theoretical | 7 × 10−3 |
| 14 | 5 | 23, 28 | Theoretical | 1 × 10−3 |
| 15 | 3, 4, 5 | 23 | Theoretical | 5 × 10−5 |
| 16 | 3, 4, 5 | 28 | Theoretical | 3 × 10−3 |
| 17 | 3, 4, 5 | 32 | Theoretical | 9 × 10−5 |
| 18 | 4, 5 | 28 | Theoretical | 0.02 |
| Exp. Cond. | CS: 300 Pa, Gap: 0.5 mm | |||||||
|---|---|---|---|---|---|---|---|---|
| Temp. (°C) | 23 | 28 | 32 | 36 | 23 | 28 | 32 | 36 |
| Sample | 4% wt (Theoretical) | 4% wt_5 (Theoretical) | ||||||
| Mw (g/mol) | 7.6 × 105 ± 1.5 × 102 | 6.7 × 105 ± 1.0 × 103 | 8.1 × 105 ± 1.0 × 103 | - | 6.2 × 105 ± 1.0 × 103 | 6.9 × 105 ± 3.0 × 102 | 6.5 × 105 ± 3.5 × 103 | - |
| Mn (g/mol) | 3.8 × 105 ± 1.5 × 103 | 3.7 × 105 ± 2.5 × 102 | 4.3 × 105 ± 1.0 × 103 | - | 2.8 × 105 ± 1.0 × 104 | 2.4 × 105 ± 6.5 × 102 | 2.8 × 105 ± 3.5 × 103 | - |
| PDI | 2.0 ± 0.1 | 1.8 ± 0.4 | 1.9 ± 0.3 | - | 2.2 ± 0.1 | 2.8 ± 0.6 | 2.3 ± 0.5 | - |
| Sample | 4% wt_10 (Theoretical) | 5% wt_15 (Theoretical) | ||||||
| Mw (g/mol) | 5.8 × 105 ± 1.5 × 102 | 6.1 × 105 ± 3.0 × 102 | 5.2 × 105 ± 1.0 × 102 | - | 5.5 × 105 ± 1.0 × 102 | 5.4 × 105 ± 0.9 × 102 | 5.7 × 105 ± 1.5 × 102 | - |
| Mn (g/mol) | 2.1 × 105 ± 3.0 × 103 | 2.0 × 105 ± 4.5 × 103 | 1.6 × 105 ± 1.0 × 104 | - | 1.6 × 105 ± 1.0 × 104 | 1.7 × 105 ± 2.0 × 103 | 1.9 × 105 ± 4.5 × 103 | - |
| PDI | 2.8 ± 0.4 | 3.1 ± 0.3 | 3.2 ± 0.5 | - | 3.4 ± 0.6 | 3.2 ± 0.1 | 3.0 ± 0.3 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez Carrero, K.C.; Velasco-Merino, C.; Asensio, M.; Guerrero, J.; Merino, J.C. Rheological Method for Determining the Molecular Weight of Collagen Gels by Using a Machine Learning Technique. Polymers 2022, 14, 3683. https://doi.org/10.3390/polym14173683
Núñez Carrero KC, Velasco-Merino C, Asensio M, Guerrero J, Merino JC. Rheological Method for Determining the Molecular Weight of Collagen Gels by Using a Machine Learning Technique. Polymers. 2022; 14(17):3683. https://doi.org/10.3390/polym14173683
Chicago/Turabian StyleNúñez Carrero, Karina C., Cristian Velasco-Merino, María Asensio, Julia Guerrero, and Juan Carlos Merino. 2022. "Rheological Method for Determining the Molecular Weight of Collagen Gels by Using a Machine Learning Technique" Polymers 14, no. 17: 3683. https://doi.org/10.3390/polym14173683





