The Effect of Topology on Block Copolymer Nanoparticles: Linear versus Star Block Copolymers in Toluene
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Linear and Star macro-CTAs
2.3. Preparation of the Block Copolymer Micelles through Self-Assembly in Toluene
2.4. Characterization
3. Results and Discussion
3.1. Synthesis of macro-CTAs with Linear and Star Structure
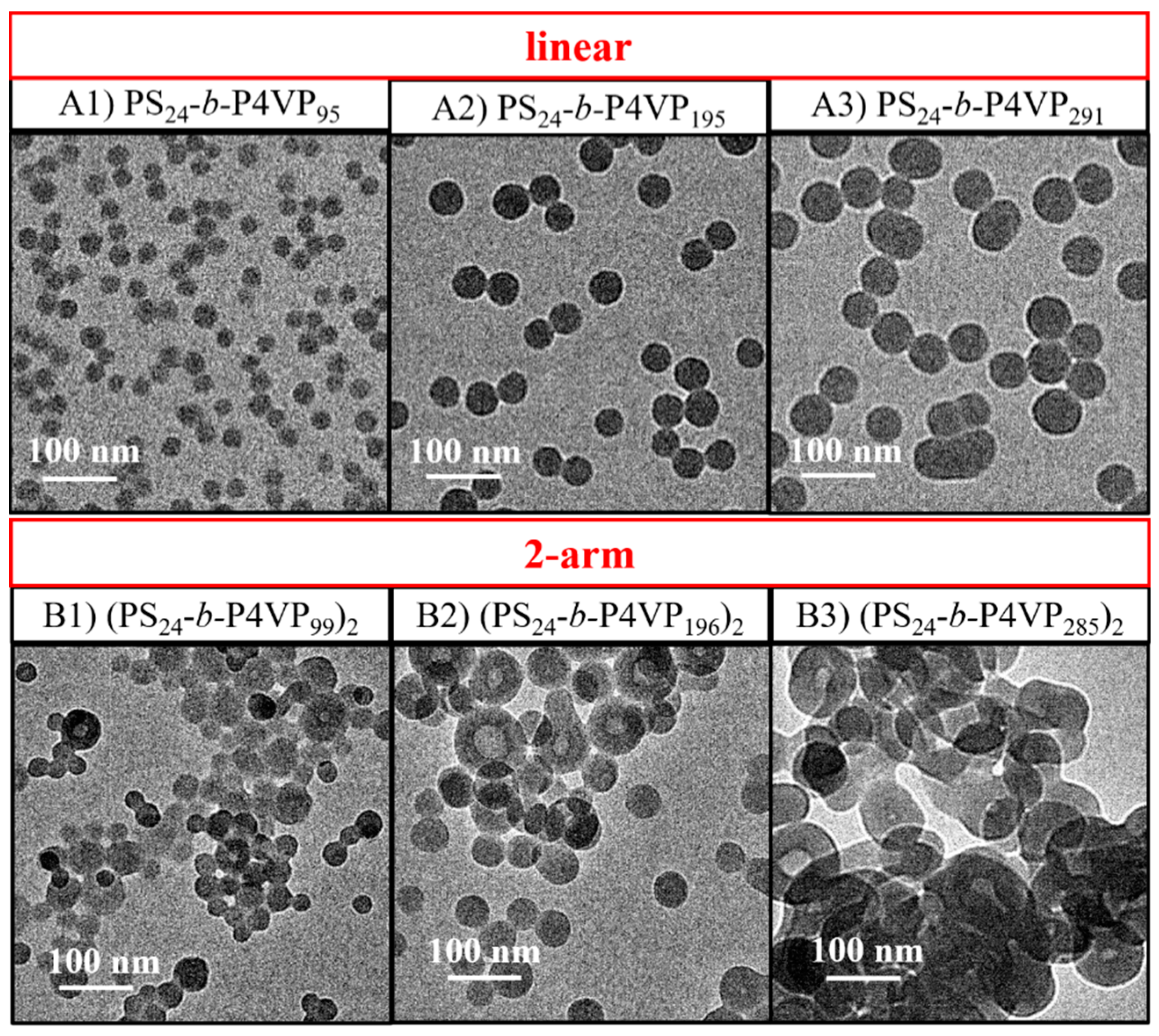
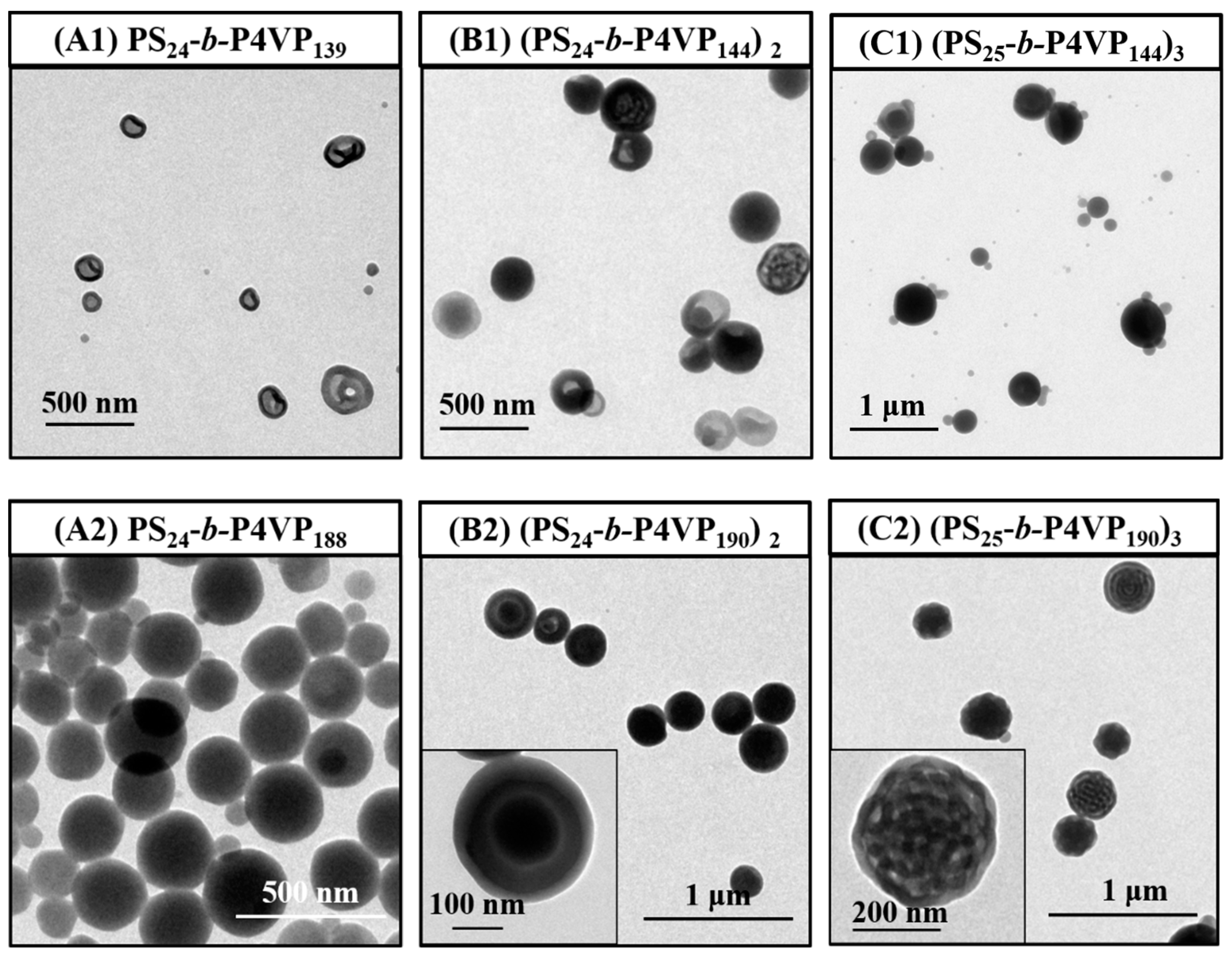
3.2. Effect of the Arm Number on BCP Nanoassemblies Prepared through PISA
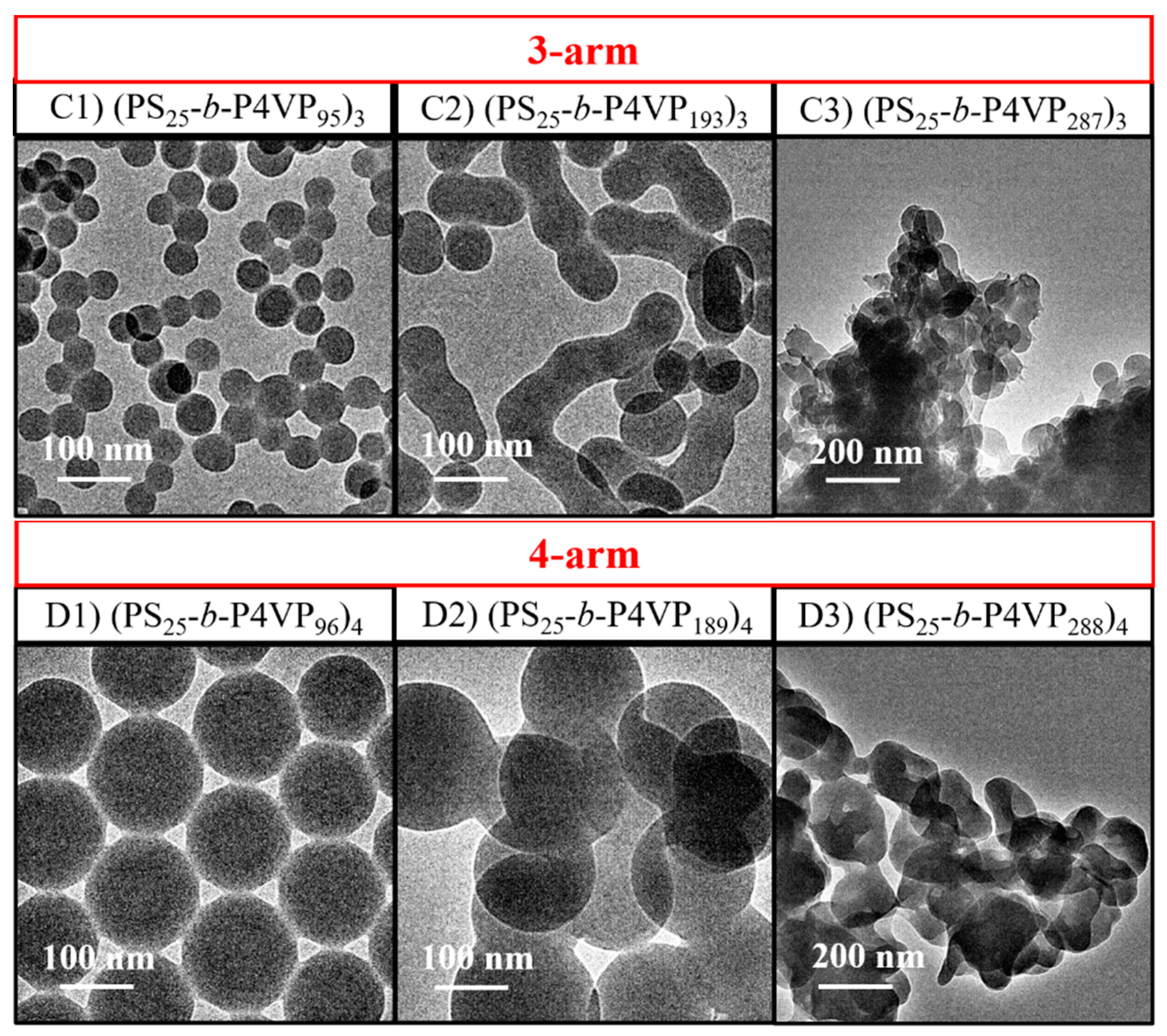
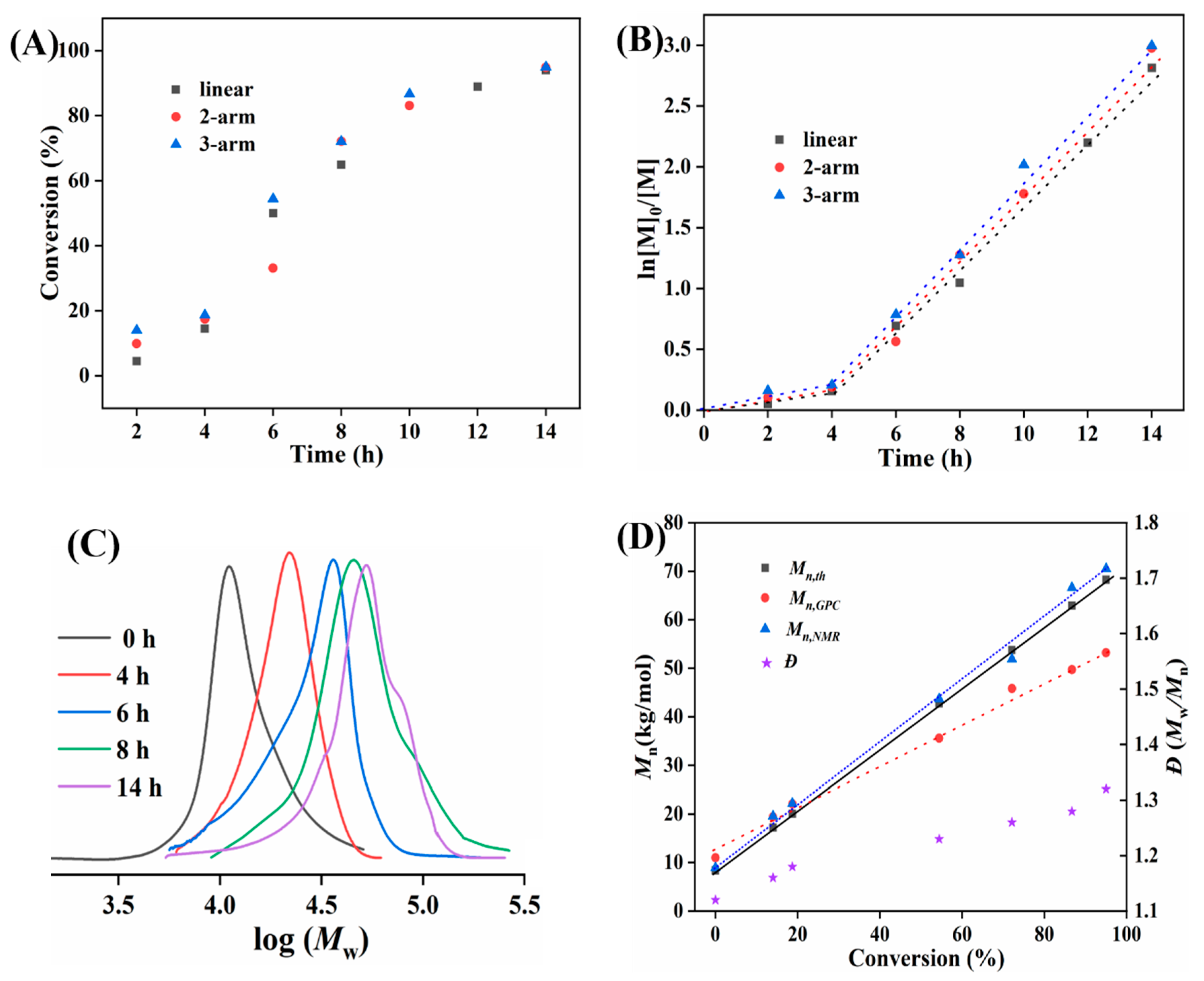
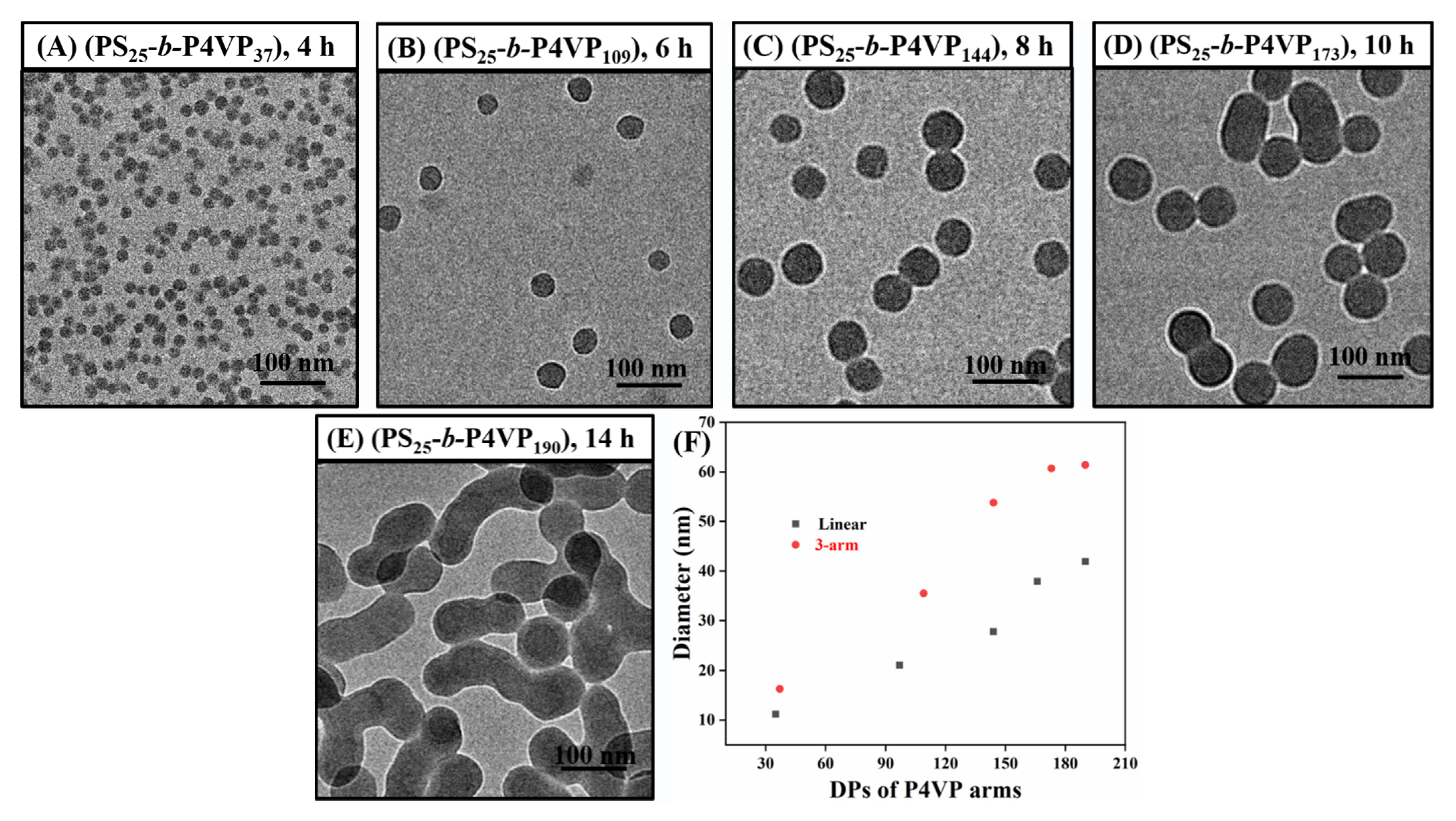
3.3. Effect of the Arm Number on Polymerization Kinetics and the Evolution of (PS-b-P4VP)n Nanoassemblies
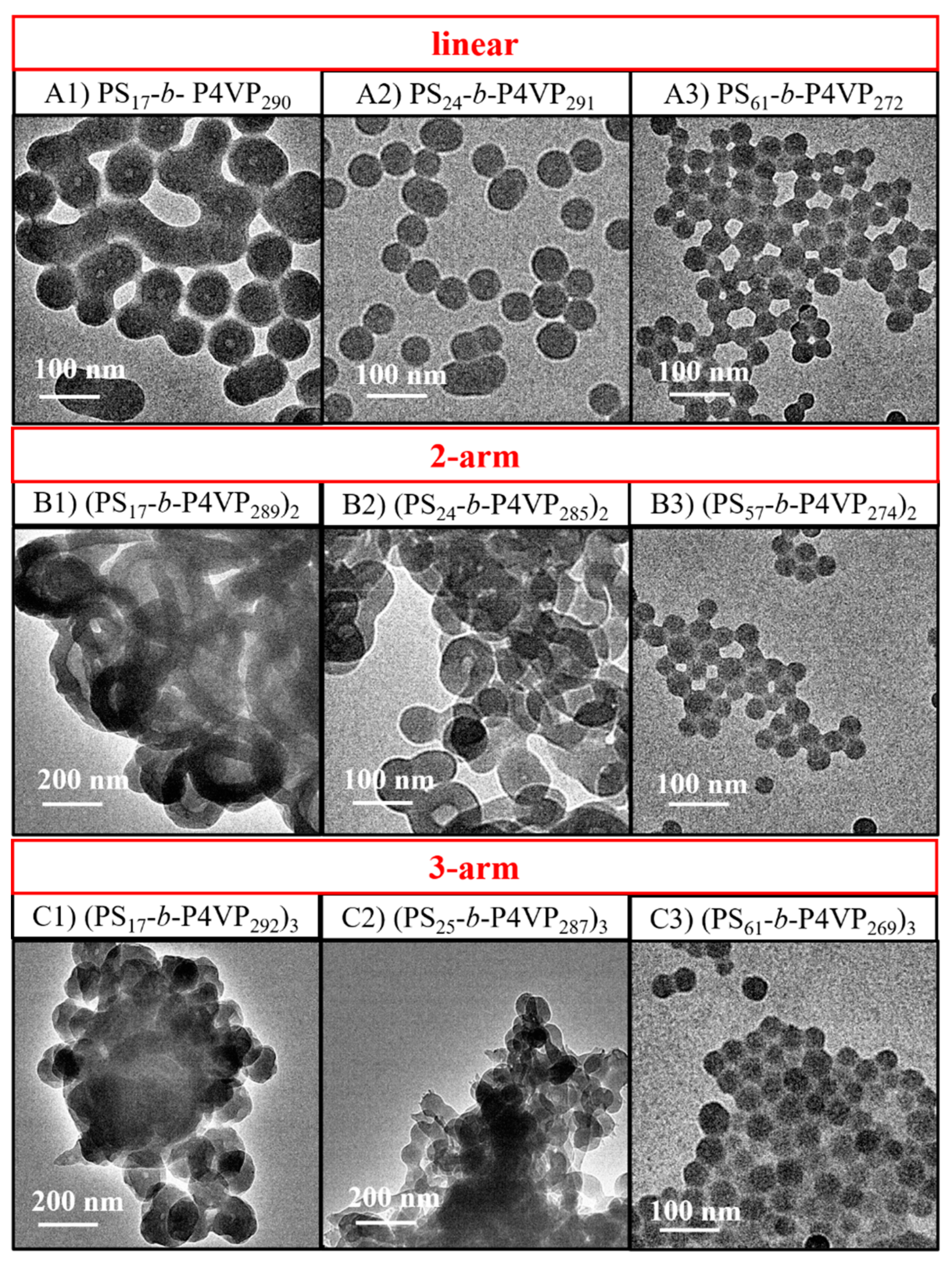
3.4. Effect of the Arm Number on BCP Nanoassemblies Prepared through General Self-Assembly in Toluene
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, J.; Chécot, F.; Gnanou, Y.; Lecommandoux, S. Toward ‘smart’ nano-objects by self-assembly of block copolymers in solution. Prog. Polym. Sci. 2005, 30, 691–724. [Google Scholar] [CrossRef]
- Lomas, H.; Canton, I.; MacNeil, S.; Du, J.; Armes, S.P.; Ryan, A.J.; Lewis, A.L.; Battaglia, G. Biomimetic pH Sensitive Polymersomes for Efficient DNA Encapsulation and Delivery. Adv. Mater. 2007, 19, 4238–4243. [Google Scholar] [CrossRef]
- Bockstaller, M.R.; Lapetnikov, Y.; Margel, S.; Thomas, E.L. Size-selective organization of enthalpic compatibilized nanocrystals in ternary block copolymer/particle mixtures. J. Am. Chem. Soc. 2003, 125, 5276–5277. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, F.; Cao, Z.; Wang, G.; Dang, Z.M. Photo, pH, and thermo triple-responsive spiropyran-based copolymer nanoparticles for controlled release. Chem. Commun. 2015, 51, 12633–12636. [Google Scholar] [CrossRef]
- Borchert, U.; Lipprandt, U.; Bilang, M.; Kimpfler, A.; Rank, A.; Peschka-Suss, R.; Schubert, R.; Lindner, P.; Forster, S. pH-induced release from P2VP-PEO block copolymer vesicles. Langmuir 2006, 22, 5843–5847. [Google Scholar] [CrossRef]
- Khan, H.; Cao, M.; Duan, W.; Ying, T.; Zhang, W. Synthesis of diblock copolymer nano-assemblies: Comparison between PISA and micellization. Polymer 2018, 150, 204–213. [Google Scholar] [CrossRef]
- Rodichkin, I.D.; Gumerov, R.A.; Potemkin, I.I. Self-assembly of miktoarm palm tree-like star copolymers in a selective solvent. J. Colloid Interface Sci. 2022, 606, 1966–1973. [Google Scholar] [CrossRef]
- Xiao, J.; He, Q.; Yang, M.; Li, H.; Qiu, X.; Wang, B.; Zhang, B.; Bu, W. Hierarchical self-assembly of miktoarm star copolymers with pathway complexity. Polym. Chem. 2021, 12, 1476–1486. [Google Scholar] [CrossRef]
- Liu, R.; Rong, Z.; Han, G.; Yang, X.; Zhang, W. Synthesis and self-assembly of star multiple block copolymer of poly(4-vinylpyridine)-block-polystyrene. Polymer 2021, 215, 123431. [Google Scholar] [CrossRef]
- D’Agosto, F.; Rieger, J.; Lansalot, M. RAFT-Mediated Polymerization-Induced Self-Assembly. Angew. Chem. Int. Ed. Engl. 2020, 59, 8368–8392. [Google Scholar] [CrossRef]
- Wang, X.; An, Z. New Insights into RAFT Dispersion Polymerization-Induced Self-Assembly: From Monomer Library, Morphological Control, and Stability to Driving Forces. Macromol. Rapid Commun. 2019, 40, e1800325. [Google Scholar] [CrossRef]
- Penfold, N.J.W.; Yeow, J.; Boyer, C.; Armes, S.P. Emerging Trends in Polymerization-Induced Self-Assembly. ACS Macro Lett 2019, 8, 1029–1054. [Google Scholar] [CrossRef]
- An, N.; Chen, X.; Yuan, J. Non-thermally initiated RAFT polymerization-induced self-assembly. Polym. Chem. 2021, 12, 3220–3232. [Google Scholar] [CrossRef]
- Liu, D.; Cai, W.; Zhang, L.; Boyer, C.; Tan, J. Efficient Photoinitiated Polymerization-Induced Self-Assembly with Oxygen Tolerance through Dual-Wavelength Type I Photoinitiation and Photoinduced Deoxygenation. Macromolecules 2020, 53, 1212–1223. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Huo, M.; Zeng, M.; Peng, L.; Feng, A.; Wang, X.; Yuan, J. Direct Synthesis of Polymer Nanotubes by Aqueous Dispersion Polymerization of a Cyclodextrin/Styrene Complex. Angew. Chem. Int. Ed. Engl. 2017, 56, 16541–16545. [Google Scholar] [CrossRef]
- Wang, X.; Man, S.; Zheng, J.; An, Z. Alkyl alpha-Hydroxymethyl Acrylate Monomers for Aqueous Dispersion Polymerization-Induced Self-Assembly. ACS Macro Lett. 2018, 7, 1461–1467. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, P.; Ding, Y.; Huang, L.; Wang, L.; Lu, X.; Cai, Y. Visible Light Initiated Thermoresponsive Aqueous Dispersion Polymerization-Induced Self-Assembly. Macromolecules 2019, 52, 1033–1041. [Google Scholar] [CrossRef]
- Luo, X.; Zhao, S.; Chen, Y.; Zhang, L.; Tan, J. Switching between Thermal Initiation and Photoinitiation Redirects RAFT-Mediated Polymerization-Induced Self-Assembly. Macromolecules 2021, 54, 2948–2959. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, C.; Gao, C.; Qu, Y.; Shi, K.; Zhang, W. Polymerization-induced self-assembly of block copolymer through dispersion RAFT polymerization in ionic liquid. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1517–1525. [Google Scholar] [CrossRef]
- Liu, H.; Gao, C.; Ding, Z.; Zhang, W. Synthesis of Polystyrene-block-Poly(4-vinylpyridine) Ellipsoids through Macro-RAFT-Agent-Mediated Dispersion Polymerization: The Solvent Effect on the Morphology of the In Situ Synthesized Block Copolymer Nanoobjects. Macromol. Chem. Phys. 2016, 217, 467–476. [Google Scholar] [CrossRef]
- Bezik, C.T.; Mysona, J.A.; Schneider, L.; Ramírez-Hernández, A.; Müller, M.; de Pablo, J.J. Is the “Bricks-and-Mortar” Mesophase Bicontinuous? Dynamic Simulations of Miktoarm Block Copolymer/Homopolymer Blends. Macromolecules 2022, 55, 745–758. [Google Scholar] [CrossRef]
- Velychkivska, N.; Sedláček, O.; Shatan, A.B.; Spasovová, M.; Filippov, S.K.; Chahal, M.K.; Janisova, L.; Brus, J.; Hanyková, L.; Hill, J.P.; et al. Phase Separation and pH-Dependent Behavior of Four-Arm Star-Shaped Porphyrin-PNIPAM4 Conjugates. Macromolecules 2022, 55, 2109–2122. [Google Scholar] [CrossRef]
- Kim, H.; Kang, B.-G.; Choi, J.; Sun, Z.; Yu, D.M.; Mays, J.; Russell, T.P. Morphological Behavior of A2B Block Copolymers in Thin Films. Macromolecules 2018, 51, 1181–1188. [Google Scholar] [CrossRef]
- Sheng, Y.J.; Nung, C.H.; Tsao, H.K. Morphologies of star-block copolymers in dilute solutions. J. Phys. Chem. B 2006, 110, 21643–21650. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wu, H.; Shen, Y. Dual-functional linear and star POSS-containing organic–inorganic hybrid block copolymers: Synthesis, self-assembly, and film property. J. Mater. Sci. 2022, 57, 7791–7803. [Google Scholar] [CrossRef]
- Yoon, K.; Kang, H.C.; Li, L.; Cho, H.; Park, M.-K.; Lee, E.; Bae, Y.H.; Huh, K.M. Amphiphilic poly(ethylene glycol)-poly(ε-caprolactone) AB2 miktoarm copolymers for self-assembled nanocarrier systems: Synthesis, characterization, and effects of morphology on antitumor activity. Polym. Chem. 2015, 6, 531–542. [Google Scholar] [CrossRef]
- Yang, J.; Dong, Q.; Liu, M.; Li, W. Universality and Specificity in the Self-Assembly of Cylinder-Forming Block Copolymers under Cylindrical Confinement. Macromolecules 2022, 55, 2171–2181. [Google Scholar] [CrossRef]
- Deng, R.; Wang, C.; Weck, M. Supramolecular Helical Miktoarm Star Polymers. ACS Macro. Lett. 2022, 11, 336–341. [Google Scholar] [CrossRef]
- Baulin, V.A. Topological Changes in Telechelic Micelles: Flowers versus Stars. Macromolecules 2022, 55, 517–522. [Google Scholar] [CrossRef]
- Ren, J.M.; McKenzie, T.G.; Fu, Q.; Wong, E.H.; Xu, J.; An, Z.; Shanmugam, S.; Davis, T.P.; Boyer, C.; Qiao, G.G. Star Polymers. Chem. Rev. 2016, 116, 6743–6836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, M.; Han, G.; Guo, T.; Ying, T.; Zhang, W. Topology Affecting Block Copolymer Nanoassemblies: Linear Block Copolymers versus Star Block Copolymers under PISA Conditions. Macromolecules 2018, 51, 5440–5449. [Google Scholar] [CrossRef]
- Qu, Y.; Chang, X.; Chen, S.; Zhang, W. In situ synthesis of thermoresponsive 4-arm star block copolymer nano-assemblies by dispersion RAFT polymerization. Polym. Chem. 2017, 8, 3485–3496. [Google Scholar] [CrossRef]
- Cao, M.; Nie, H.; Hou, Y.; Han, G.; Zhang, W. Synthesis of star thermoresponsive amphiphilic block copolymer nano-assemblies and the effect of topology on their thermoresponse. Polym. Chem. 2019, 10, 403–411. [Google Scholar] [CrossRef]
- Wang, X.; Figg, C.A.; Lv, X.; Yang, Y.; Sumerlin, B.S.; An, Z. Star Architecture Promoting Morphological Transitions during Polymerization-Induced Self-Assembly. ACS Macro Lett. 2017, 6, 337–342. [Google Scholar] [CrossRef]
- Zeng, R.; Chen, Y.; Zhang, L.; Tan, J. R-RAFT or Z-RAFT? Well-Defined Star Block Copolymer Nano-Objects Prepared by RAFT-Mediated Polymerization-Induced Self-Assembly. Macromolecules 2020, 53, 1557–1566. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, L.; Chen, Y.; Tan, J. Linear and Star Block Copolymer Nanoparticles Prepared by Heterogeneous RAFT Polymerization Using an omega, omega-Heterodifunctional Macro-RAFT Agent. ACS Macro Lett. 2022, 11, 910–918. [Google Scholar] [CrossRef]
- Thompson, K.L.; Mable, C.J.; Lane, J.A.; Derry, M.J.; Fielding, L.A.; Armes, S.P. Preparation of Pickering Double Emulsions Using Block Copolymer Worms. Langmuir 2015, 31, 4137–4144. [Google Scholar] [CrossRef]
- Docherty, P.J.; Girou, C.; Derry, M.J.; Armes, S.P. Epoxy-functional diblock copolymer spheres, worms and vesiclesviapolymerization-induced self-assembly in mineral oil. Polym. Chem. 2020, 11, 3332–3339. [Google Scholar] [CrossRef]
- Luo, X. A morphological transition of poly(ethylene glycol)-block-polystyrene with polymerization-induced self-assembly guided by using cosolvents. Eur. Polym. J. 2021, 158, 110639. [Google Scholar] [CrossRef]
- Dan, M.; Huo, F.; Zhang, X.; Wang, X.; Zhang, W. Dispersion RAFT polymerization of 4-vinylpyridine in toluene mediated with the macro-RAFT agent of polystyrene dithiobenzoate: Effect of the macro-RAFT agent chain length and growth of the block copolymer nano-objects. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 1573–1584. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, T.; Han, G.; Guo, T.; Zhang, W. Star Block Copolymer Nanoassemblies: Block Sequence is All-Important. Macromolecules 2018, 52, 718–728. [Google Scholar] [CrossRef]
- Cao, M.; Han, G.; Duan, W.; Zhang, W. Synthesis of multi-arm star thermo-responsive polymers and topology effects on phase transition. Polym. Chem. 2018, 9, 2625–2633. [Google Scholar] [CrossRef]
- Stenzel-Rosenbaum, M.; Davis, T.P.; Chen, V.; Fane, A.G. Star-polymer synthesis via radical reversible addition–fragmentation chain-transfer polymerization. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 2777–2783. [Google Scholar] [CrossRef]
- Pang, X.; Zhao, L.; Akinc, M.; Kim, J.K.; Lin, Z. Novel Amphiphilic Multi-Arm, Star-Like Block Copolymers as Unimolecular Micelles. Macromolecules 2011, 44, 3746–3752. [Google Scholar] [CrossRef]
- Whittaker, M.R.; Monteiro, M.J. Synthesis and aggregation behavior of four-arm star amphiphilic block copolymers in water. Langmuir 2006, 22, 9746–9752. [Google Scholar] [CrossRef]
- Skrabania, K.; Li, W.; Laschewsky, A. Synthesis of Double-Hydrophilic BAB Triblock Copolymers via RAFT Polymerisation and their Thermoresponsive Self-Assembly in Water. Macromol. Chem. Phys. 2008, 209, 1389–1403. [Google Scholar] [CrossRef]
- Papagiannopoulos, A.; Zhao, J.; Zhang, G.; Pispas, S.; Radulescu, A. Thermoresponsive aggregation of PS-PNIPAM-PS triblock copolymer: A combined study of light scattering and small angle neutron scattering. Eur. Polym. J. 2014, 56, 59–68. [Google Scholar] [CrossRef]
- Kim, S.H.; Jo, W.H. A Monte Carlo Simulation for the Micellization of ABA- and BAB-Type Triblock Copolymers in a Selective Solvent. Macromolecules 2001, 34, 7210–7218. [Google Scholar] [CrossRef]
- Lee, D.S.; Shim, M.S.; Kim, S.W.; Lee, H.; Park, I.; Chang, T. Novel Thermoreversible Gelation of Biodegradable PLGA-block-PEO-block-PLGA Triblock Copolymers in Aqueous Solution. Macromol. Rapid Commun. 2001, 22, 587–592. [Google Scholar] [CrossRef]
- Zhang, W.-J.; Hong, C.-Y.; Pan, C.-Y. Fabrication of Spaced Concentric Vesicles and Polymerizations in RAFT Dispersion Polymerization. Macromolecules 2014, 47, 1664–1671. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, S.; Khan, H.; Gao, C.; Zhou, H.; Zhang, W. One-pot preparation of BAB triblock copolymer nano-objects through bifunctional macromolecular RAFT agent mediated dispersion polymerization. Polym. Chem. 2016, 7, 1953–1962. [Google Scholar] [CrossRef]
- Dong, S.; Zhao, W.; Lucien, F.P.; Perrier, S.; Zetterlund, P.B. Polymerization induced self-assembly: Tuning of nano-object morphology by use of CO2. Polym. Chem. 2015, 6, 2249–2254. [Google Scholar] [CrossRef]
- Sahoo, S.; Gordievskaya, Y.D.; Bauri, K.; Gavrilov, A.A.; Kramarenko, E.Y.; De, P. Polymerization-Induced Self-Assembly (PISA) Generated Cholesterol-Based Block Copolymer Nano-Objects in a Nonpolar Solvent: Combined Experimental and Simulation Study. Macromolecules 2022, 55, 1139–1152. [Google Scholar] [CrossRef]
- Gao, P.; Cao, H.; Ding, Y.; Cai, M.; Cui, Z.; Lu, X.; Cai, Y. Synthesis of Hydrogen-Bonded Pore-Switchable Cylindrical Vesicles via Visible-Light-Mediated RAFT Room-Temperature Aqueous Dispersion Polymerization. ACS Macro Lett. 2016, 5, 1327–1331. [Google Scholar] [CrossRef]
- Ng, G.; Yeow, J.; Xu, J.; Boyer, C. Application of oxygen tolerant PET-RAFT to polymerization-induced self-assembly. Polym. Chem. 2017, 8, 2841–2851. [Google Scholar] [CrossRef]
- Pei, Y.; Lowe, A.B. Polymerization-induced self-assembly: Ethanolic RAFT dispersion polymerization of 2-phenylethyl methacrylate. Polym. Chem. 2014, 5, 2342–2351. [Google Scholar] [CrossRef]
- Kang, Y.; Pitto-Barry, A.; Maitland, A.; O’Reilly, R.K. RAFT dispersion polymerization: A method to tune the morphology of thymine-containing self-assemblies. Polym. Chem. 2015, 6, 4984–4992. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, X.; Zhu, Y.; Guo, R. Dispersion polymerization of styrene/acrylonitrile in polyether stabilized by macro-RAFT agents. Colloids Surf. A 2022, 647, 129166. [Google Scholar] [CrossRef]
- Dhiraj, H.S.; Ishizuka, F.; Elshaer, A.; Zetterlund, P.B.; Aldabbagh, F. RAFT dispersion polymerization induced self-assembly (PISA) of boronic acid-substituted acrylamides. Polym. Chem. 2022, 13, 3750–3755. [Google Scholar] [CrossRef]
- Li, J.-W.; Chen, M.; Zhou, J.-M.; Pan, C.-Y.; Zhang, W.-J.; Hong, C.-Y. RAFT dispersion copolymerization of styrene and N-methacryloxysuccinimide: Promoted morphology transition and post-polymerization cross-linking. Polymer 2021, 221, 123589. [Google Scholar] [CrossRef]
- McKenzie, B.E.; Friedrich, H.; Wirix, M.J.M.; de Visser, J.F.; Monaghan, O.R.; Bomans, P.H.H.; Nudelman, F.; Holder, S.J.; Sommerdijk, N.A.J.M. Controlling Internal Pore Sizes in Bicontinuous Polymeric Nanospheres. Angew. Chem. Int. Ed. 2015, 54, 2457–2461. [Google Scholar] [CrossRef] [PubMed]
- Barnhill, S.A.; Bell, N.C.; Patterson, J.P.; Olds, D.P.; Gianneschi, N.C. Phase Diagrams of Polynorbornene Amphiphilic Block Copolymers in Solution. Macromolecules 2015, 48, 1152–1161. [Google Scholar] [CrossRef]
- Gao, C.; Wu, J.; Zhou, H.; Qu, Y.; Li, B.; Zhang, W. Self-Assembled Blends of AB/BAB Block Copolymers Prepared through Dispersion RAFT Polymerization. Macromolecules 2016, 49, 4490–4500. [Google Scholar] [CrossRef]


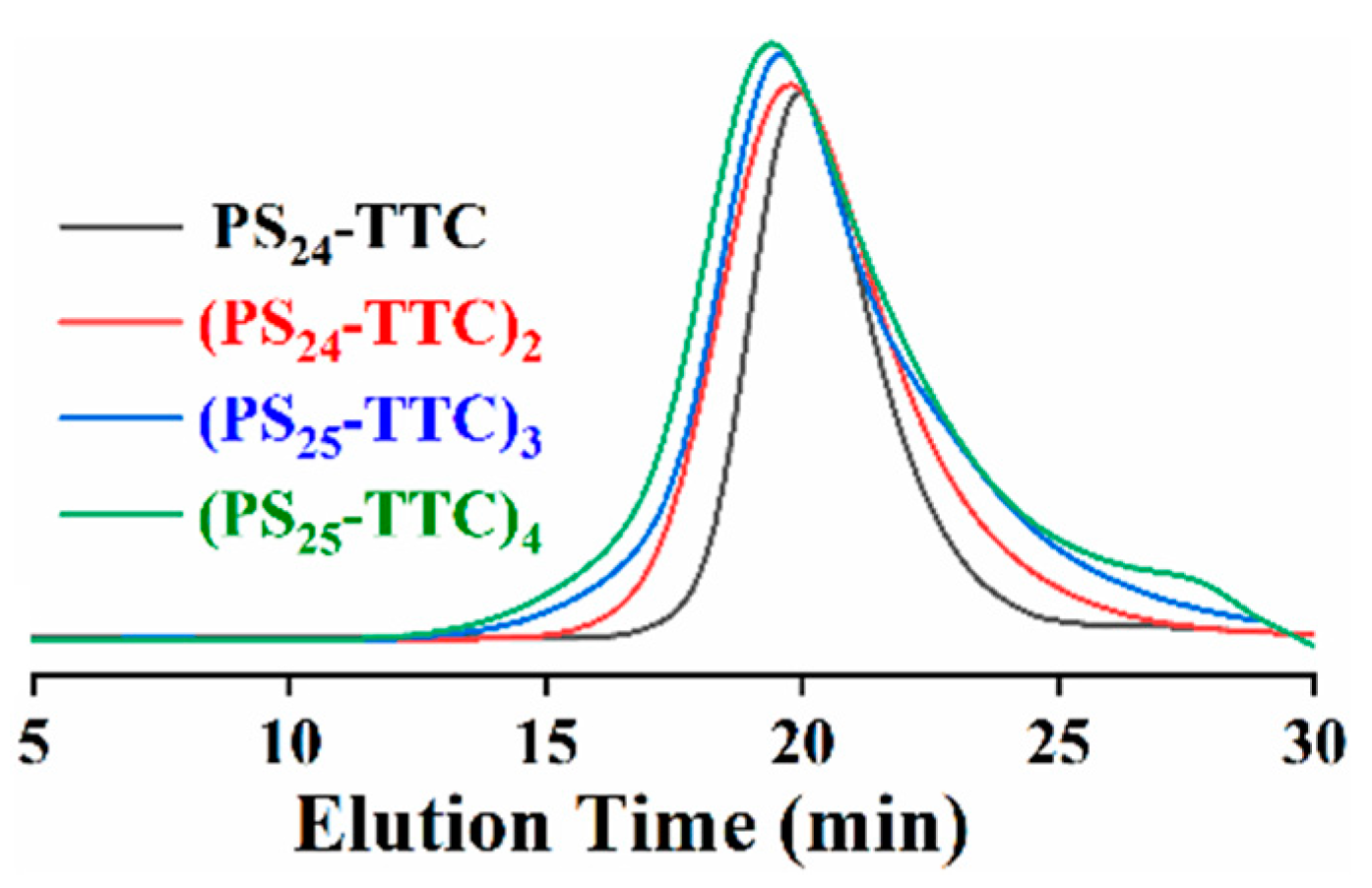

| Macro-CTA | [M]0:[CTA]0:[I]0 | Time (h) | Conv. a (%) | Mn (kg/mol) | Ð e | ||
|---|---|---|---|---|---|---|---|
| Mn,thb | Mn,GPCc | Mn,NMRd | |||||
| PS17-TTC | 100:5:1 | 32 | 85.0 | 2.02 | 3.1 | 2.1 | 1.11 |
| PS24-TTC | 150:5:1 | 32 | 80.0 | 2.75 | 3.2 | 3.1 | 1.11 |
| PS61-TTC | 500:5:1 | 32 | 61.0 | 6.60 | 6.4 | 6.5 | 1.14 |
| (PS17-TTC)2 | 200:5:2 | 32 | 85.0 | 3.97 | 4.4 | 4.1 | 1.12 |
| (PS24-TTC)2 | 300:5:2 | 32 | 80.4 | 5.43 | 5.5 | 5.38 | 1.13 |
| (PS57-TTC)2 | 1000:5:2 | 32 | 57.2 | 12.29 | 10.6 | 12.4 | 1.18 |
| (PS17-TTC)3 | 300:5:3 | 32 | 85.0 | 5.92 | 5.8 | 6.0 | 1.12 |
| (PS25-TTC)3 | 450:5:3 | 32 | 83.3 | 8.41 | 7.9 | 8.7 | 1.12 |
| (PS61-TTC)3 | 1500:5:3 | 32 | 59.8 | 19.64 | 21.3 | 19.5 | 1.22 |
| (PS17-TTC)4 | 400:5:4 | 32 | 85.0 | 7.86 | 7.8 | 7.7 | 1.18 |
| (PS25-TTC)4 | 600:5:4 | 32 | 83.3 | 11.19 | 10.9 | 11.0 | 1.20 |
| (PS60-TTC)4 | 2000:5:4 | 32 | 60.0 | 25.75 | 22.6 | 26.1 | 1.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, P.; Li, N.; Guo, C.; Li, S. The Effect of Topology on Block Copolymer Nanoparticles: Linear versus Star Block Copolymers in Toluene. Polymers 2022, 14, 3691. https://doi.org/10.3390/polym14173691
Zhang Y, Wang P, Li N, Guo C, Li S. The Effect of Topology on Block Copolymer Nanoparticles: Linear versus Star Block Copolymers in Toluene. Polymers. 2022; 14(17):3691. https://doi.org/10.3390/polym14173691
Chicago/Turabian StyleZhang, Yuan, Peng Wang, Nan Li, Chunyan Guo, and Sumin Li. 2022. "The Effect of Topology on Block Copolymer Nanoparticles: Linear versus Star Block Copolymers in Toluene" Polymers 14, no. 17: 3691. https://doi.org/10.3390/polym14173691




