Structural and Mechanical Properties of Konjac Glucomannan Gels and Influence of Freezing-Thawing Treatments on Them
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample and Sample Preparation
2.2. Compression Measurements
2.3. Synchrotron SAXS/WAXS Measurements
2.4. Fourier Transform Infrared (FT-IR) Spectroscopy
2.5. Scanning Electron Microscopy Observation
3. Results and Discussion
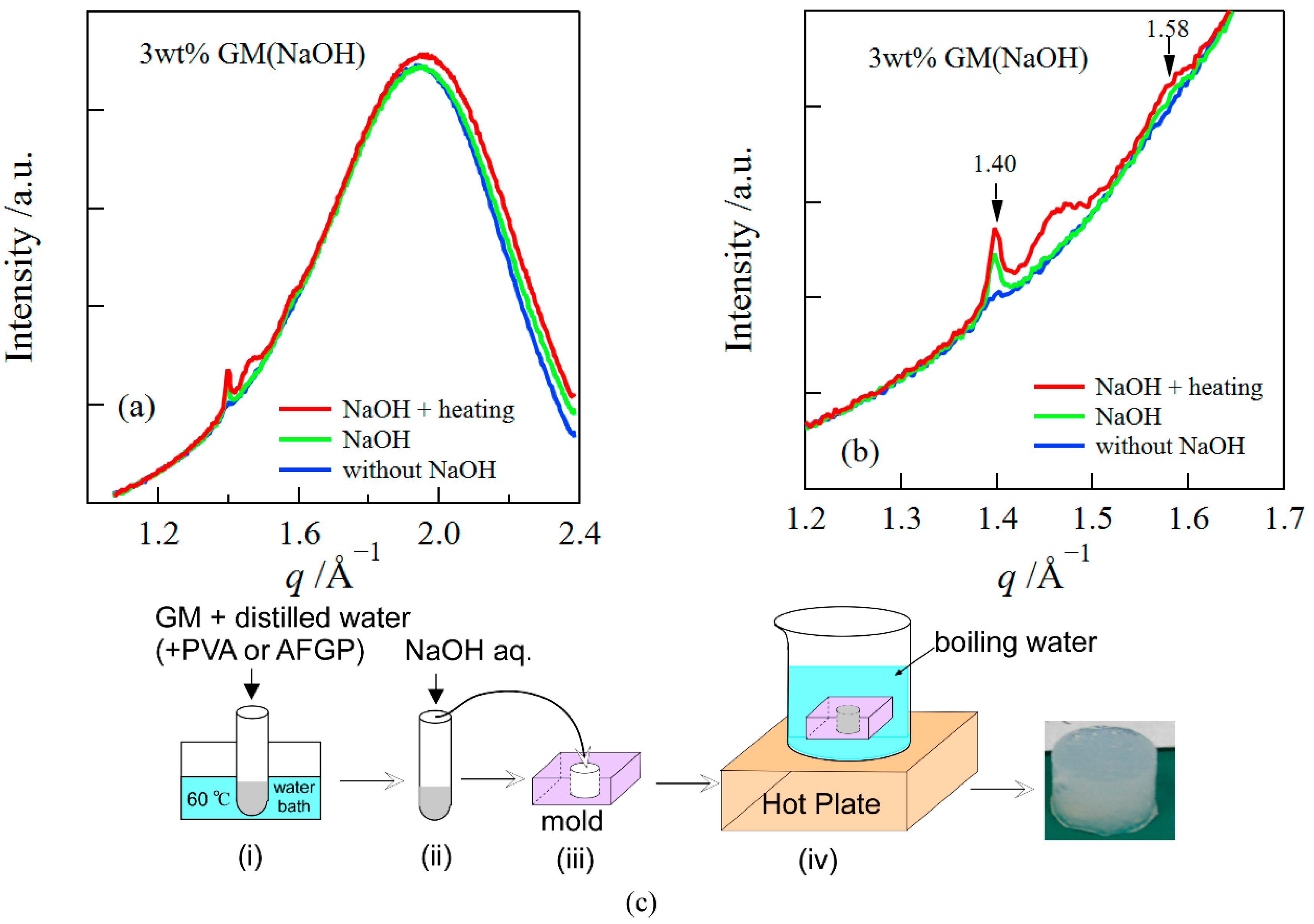
3.1. Structure of GM Gels
3.2. Influence of FT Treatment on Mechanical and Structural Properties
3.3. Influence of Additives on Mechanical and Structural Properties of Freeze-Thawed Gels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dave, V.; McCarthy, S.P. Review of Konjac Glucomannan. J. Environ. Polym. Degrad. 1997, 5, 237–241. [Google Scholar]
- Katsuraya, K.; Okuyama, K.; Hatanaka, K.; Oshima, R.; Sato, T.; Matsuzaki, K. Constitution of konjac glucomannan: Chemical analysis and 13C NMR spectroscopy. Carbohydr. Polym. 2003, 53, 183–189. [Google Scholar] [CrossRef]
- Maekaji, K. The Mechanism of Gelation of Konjac Mannan. Agric. Biol. Chem. 1974, 38, 315–321. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, X.; Cheng, Y.; Zhao, G.; Zhou, Y. Gelation mechanism of alkali induced heat-set konjac glucomannan gel. Trends Food Sci. Technol. 2021, 116, 244–254. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, R.; Perkins, W.S.; Cheng, Y. Morphology evolution and gelation mechanism of alkali induced konjac glucomannan hydrogel. Food Chem. 2018, 269, 80–88. [Google Scholar] [CrossRef]
- Zhang, T.; de Vries, R.; Xu, X.; Xue, Y.; Xue, C. Microstructural changes during alkali- and heat induced gelation of konjac glucomannan. Food Hydrocoll. 2021, 114, 106552. [Google Scholar] [CrossRef]
- Takeno, H. Synchrotron Small-Angle X-Ray Scattering and Small-Angle Neutron Scattering Studies of Nanomaterials. In X-ray and Neutron Techniques for Nanomaterials Characterization; Kumar, C.S.S.R., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 717–760. [Google Scholar]
- Takeno, H.; Inoguchi, H.; Hsieh, W.-C. Mechanical and structural properties of cellulose nanofiber/poly(vinyl alcohol) hydrogels cross-linked by a freezing/thawing method and borax. Cellulose 2020, 27, 4373–4387. [Google Scholar] [CrossRef]
- Takeno, H.; Inoguchi, H.; Hsieh, W.-C. Mechanically robust ionic liquid gels composed of cellulose nanofiber and poly(vinyl alcohol). Mater. Today Commun. 2022, 31, 103495. [Google Scholar] [CrossRef]
- Takeno, H.; Yanagita, M.; Motegi, Y.; Kondo, S. Relationship between Helical Aggregates and Polymorphs in a 12-Hydroxystearic Acid Gel: Their Thermal Stability and Formation Kinetics. Colloid. Polym. Sci. 2015, 293, 199–207. [Google Scholar] [CrossRef]
- Kang, T.; You, Y.; Jun, S. Supercooling preservation technology in food and biological samples: A review focused on electric and magnetic field applications. Food Sci. Biotechnol. 2020, 29, 303–321. [Google Scholar] [CrossRef]
- Genevro, G.M.; de Moraes, M.A.; Beppu, M.M. Freezing influence on physical properties of glucomannan hydrogels. Int. J. Biol. Macromol. 2019, 128, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Colmenero, F.; Cofrades, S.; Herrero, A.M.; Solas, M.T.; Ruiz-Capillas, C. Konjac gel for use as potential fat analogue for healthier meat product development: Effect of chilled and frozen storage. Food Hydrocoll. 2013, 30, 351–357. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and morphology of freeze/thawed PVA hydrogels. Macromolecules 2000, 33, 2472–2479. [Google Scholar] [CrossRef]
- Millon, L.E.; Nieh, M.P.; Hutter, J.L.; Wan, W.K. SANS characterization of an anisotropic poly(vinyl alcohol) hydrogel with vascular applications. Macromolecules 2007, 40, 3655–3662. [Google Scholar] [CrossRef]
- Holloway, J.L.; Lowman, A.L.; Palmese, G.R. The role of crystallization and phase separation in the formation of physically cross-linked PVA hydrogels. Soft Matter 2013, 9, 826–833. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Wu, J. Physically crosslinked hydrogels from polysaccharides prepared by freeze–thaw technique. React. Funct. Polym. 2013, 73, 923–928. [Google Scholar] [CrossRef]
- Sosnowska, K.; Wróblewska, M.; Basa, A. Does the Freeze–Thaw Technique Affect the Properties of the Alginate/Chitosan Glutamate Gels with Posaconazole as a Model Antifungal Drug? Int. J. Mol. Sci. 2022, 23, 6775. [Google Scholar] [CrossRef]
- Graham, B.; Fayter, A.E.R.; Houston, J.E.; Evans, R.C.; Gibson, M.I. Facially Amphipathic Glycopolymers Inhibit Ice Recrystallization. J. Am. Chem. Soc. 2018, 140, 5682–5685. [Google Scholar] [CrossRef]
- Naullage, P.M.; Qiu, Y.; Molinero, V. What Controls the Limit of Supercooling and Superheating of Pinned Ice Surfaces? J. Phys. Chem. Lett. 2018, 9, 1712–1720. [Google Scholar] [CrossRef]
- Budke, C.; Dreyer, A.; Jaeger, J.; Gimpel, K.; Berkemeier, T.; Bonin, A.S.; Nagel, L.; Plattner, C.; DeVries, A.L.; Sewald, N.; et al. Quantitative Efficacy Classification of Ice Recrystallization Inhibition Agents. Cryst. Growth Des. 2014, 14, 4285–4294. [Google Scholar] [CrossRef]
- Deller, R.C.; Vatish, M.; Mitchell, D.A.; Gibson, M.I. Synthetic polymers enable non-vitreous cellular cryopreservation by reducing ice crystal growth during thawing. Nat. Commun. 2014, 5, 3244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inada, T.; Koyama, T.; Goto, F.; Seto, T. Ice Nucleation in Emulsified Aqueous Solutions of Antifreeze Protein Type III and Poly(vinyl alcohol). J. Phys. Chem. B 2011, 115, 7914–7922. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Inoue, T. Efforts toward commercialization of antifreeze proteins. Synthesiology 2021, 12, 85–92. [Google Scholar] [CrossRef]
- Shimizu, N.; Yatabe, K.; Nagatani, Y.; Saijyo, S.; Kosuge, T.; Igarashi, N. Software Development for Analysis of Small—Angle X ray Scattering Data. AIP Conf. Proc. 2016, 1741, 050017. [Google Scholar]
- Zhang, F.; Ilavsky, J.; Long, G.G.; Quintana, J.P.G.; Allen, A.J.; Jemian, P.R. Glassy Carbon as an Absolute Intensity Calibration Standard for Small-Angle Scattering. Metall. Mater. Trans. A 2010, 41A, 1151–1158. [Google Scholar] [CrossRef]
- Yui, T.; Ogawa, K.; Sarko, A. Molecular and crystal structure of konjac glucomannan in the mannan II polymorphic form. Carbohydr. Res. 1992, 229, 41–55. [Google Scholar] [CrossRef]
- Millane, R.P.; Hendrixson, T.L. Crystal Structures of mannan and glucomannans. Carbohydr. Polym. 1994, 25, 245–251. [Google Scholar] [CrossRef]
- Shibayama, M.; Kurokawa, H.; Nomura, S.; Muthukumar, M.; Stein, R.S.; Roy, S. Small-Angle Neutron-Scattering from Poly(Vinyl Alcohol)-Borate Gels. Polymer 1992, 33, 2883–2890. [Google Scholar] [CrossRef]
- Fayter, A.; Huband, S.; Gibson, M.I. X-ray diffraction to probe the kinetics of ice recrystallization inhibition. Analyst 2020, 145, 3666–3677. [Google Scholar] [CrossRef]
- Malkin, T.L.; Murray, B.J.; Brukhno, A.V.; Anwar, J.; Salzmann, C.G. Structure of ice crystallized from supercooled water. Proc. Natl. Acad. Sci. USA 2012, 109, 1041–1045. [Google Scholar] [CrossRef]
- Wiener, C.G.; Tyagi, M.; Liu, Y.; Weiss, R.A.; Vogt, B.D. Supramolecular Hydrophobic Aggregates in Hydrogels Partially Inhibit Ice Formation. J. Phys. Chem. B 2016, 120, 5543–5552. [Google Scholar] [CrossRef] [PubMed]
- Beaucage, G. Small-angle scattering from polymeric mass fractals of arbitrary mass-fractal dimension. J. Appl. Crystallogr. 1996, 29, 134–146. [Google Scholar] [CrossRef] [Green Version]
- Sukumaran, S.K.; Beaucage, G.; Mark, J.E.; Viers, B. Neutron scattering from equilibrium-swollen networks. Eur. Phys. J. E 2005, 18, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.S.; Benoît, H. Polymers and Neutron Scattering; Clarendon Press: Oxford, UK; New York, NY, USA; Oxford University Press: Oxford, UK; New York, NY, USA, 1994; p. xix. 436p. [Google Scholar]
- Glatter, O.; Kratky, O. Small Angle X-ray Scattering; Academic Press: London, UK, 1982. [Google Scholar]
- Hassan, C.M.; Peppas, N.A. Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. Adv. Polym. Sci. 2000, 153, 37–65. [Google Scholar]









| Sample | T/°C | Rg,1/Å | p1 | Rg,2/Å | p2 |
|---|---|---|---|---|---|
| GM | 20 (before FT) | 470 ± 84 | 2.3 ± 0.1 | 35 ± 6 | 2.3 ± 1.4 |
| 25 (after FT) | 379 ± 143 | 2.0 ± 0.2 | 40 ± 9 | 2.6 ± 1.3 | |
| GM/PVA | 25 (before FT) | 480 ± 81 | 2.4 ± 0.1 | 32 ± 7 | 2.2 ± 1.3 |
| 25 (after FT) | 377 ± 144 | 2.0 ± 0.2 | 47 ± 10 | 2.7 ± 0.8 | |
| GM/AFGP | 25 (before FT) | 477 ± 94 | 2.4 ± 0.1 | 32 ± 9 | 2.4 ± 1.5 |
| 20 (after FT) | 383 ± 173 | 2.0 ± 0.2 | 49 ± 15 | 2.9 ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeno, H.; Hashimoto, R.; Lu, Y.; Hsieh, W.-C. Structural and Mechanical Properties of Konjac Glucomannan Gels and Influence of Freezing-Thawing Treatments on Them. Polymers 2022, 14, 3703. https://doi.org/10.3390/polym14183703
Takeno H, Hashimoto R, Lu Y, Hsieh W-C. Structural and Mechanical Properties of Konjac Glucomannan Gels and Influence of Freezing-Thawing Treatments on Them. Polymers. 2022; 14(18):3703. https://doi.org/10.3390/polym14183703
Chicago/Turabian StyleTakeno, Hiroyuki, Ryuki Hashimoto, Yunqiao Lu, and Wen-Chuan Hsieh. 2022. "Structural and Mechanical Properties of Konjac Glucomannan Gels and Influence of Freezing-Thawing Treatments on Them" Polymers 14, no. 18: 3703. https://doi.org/10.3390/polym14183703





