Intrinsically Negative Photosensitive Polyimides with Enhanced High-Temperature Dimensional Stability and Optical Transparency for Advanced Optical Applications via Simultaneous Incorporation of Trifluoromethyl and Benzanilide Units: Preparation and Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Methods
2.3. PI synthesis and Film Preparation
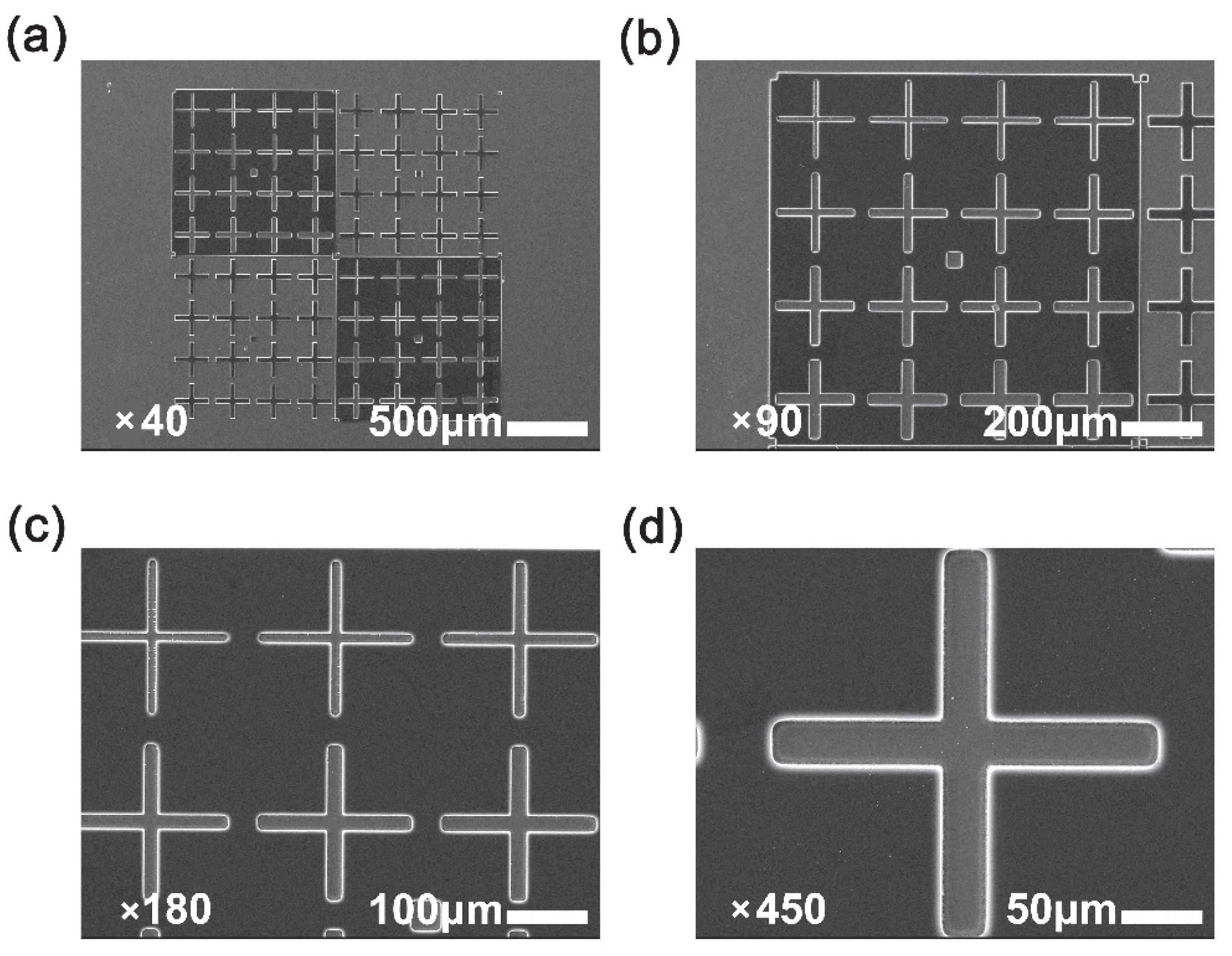
3. Results and Discussion
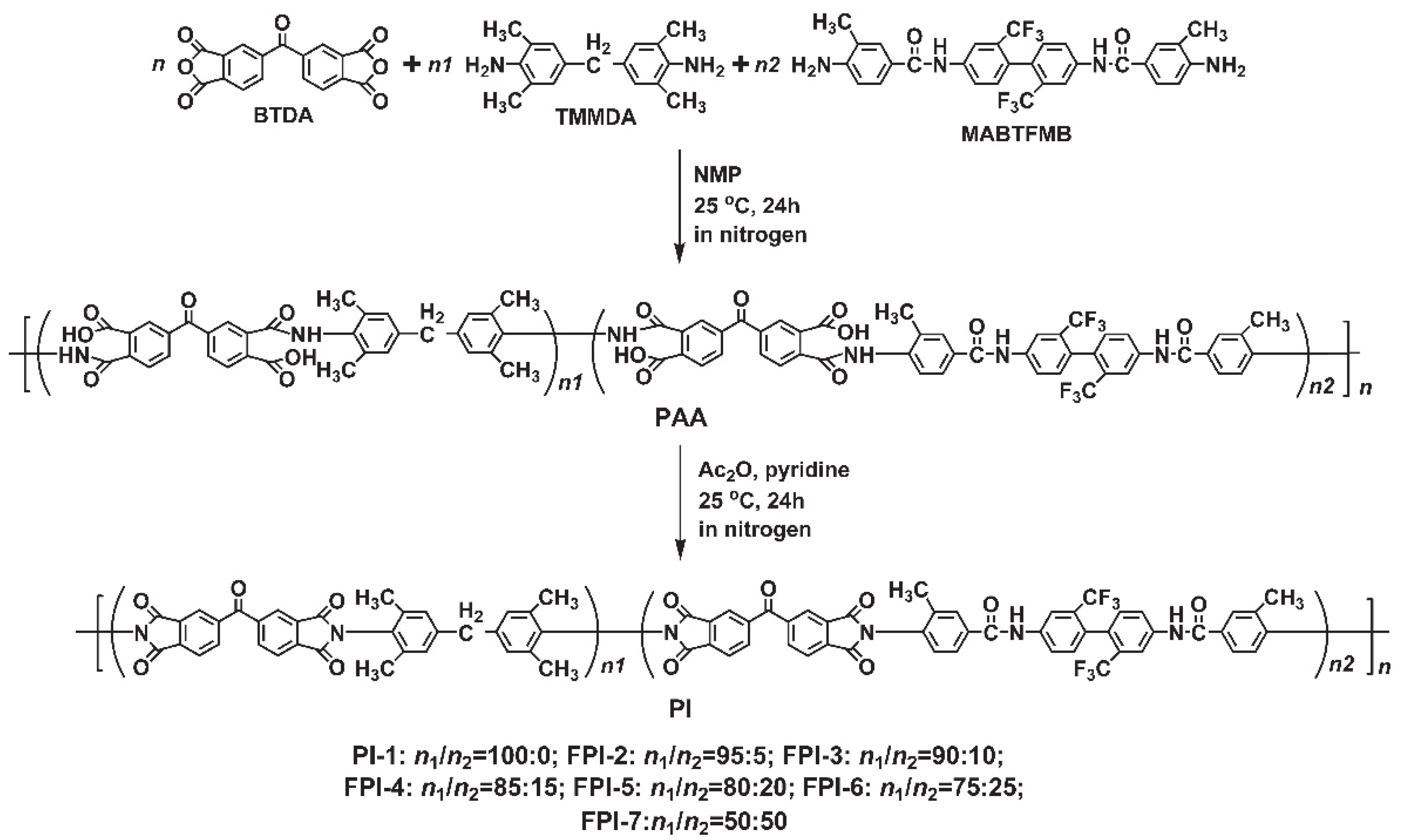
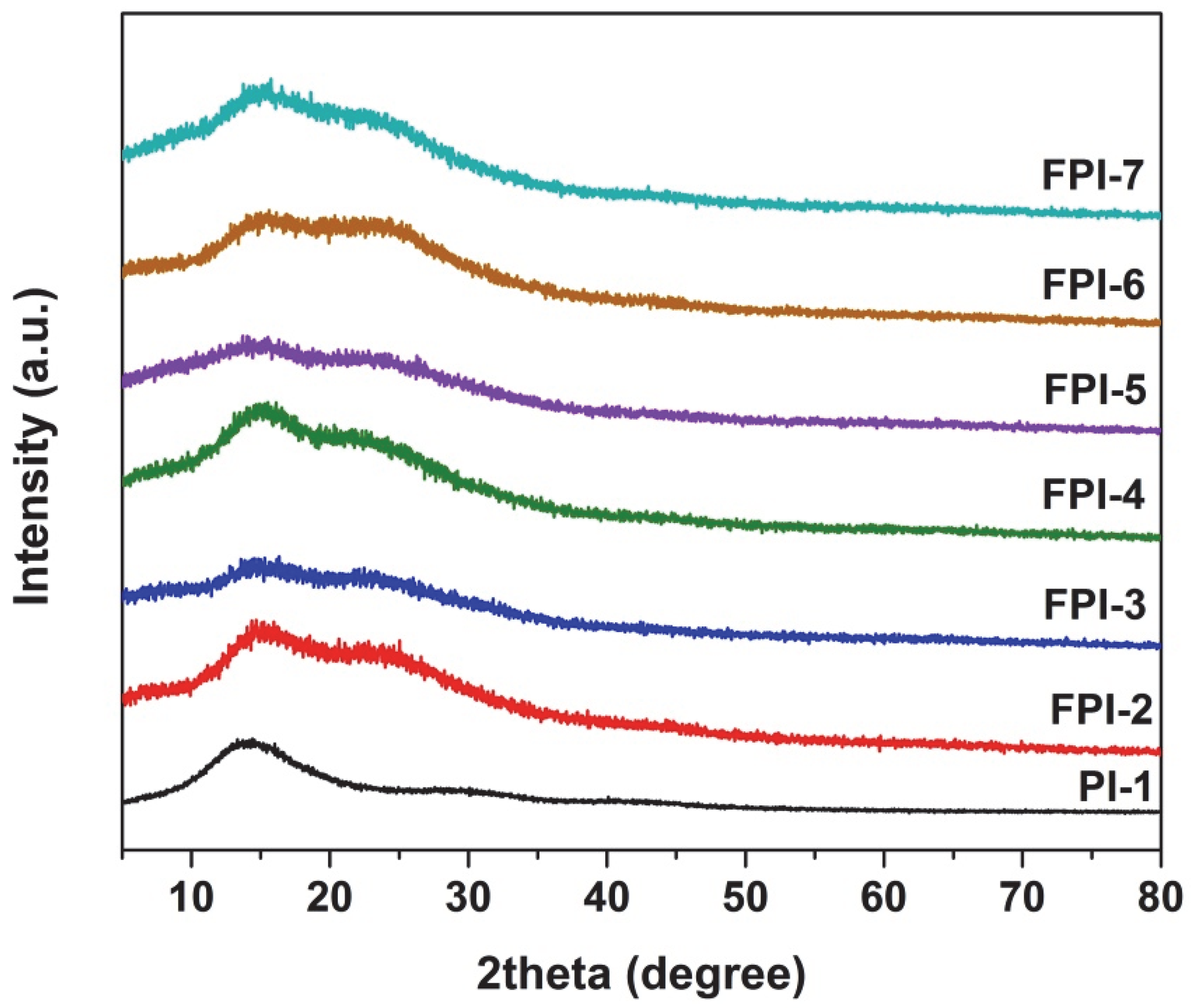
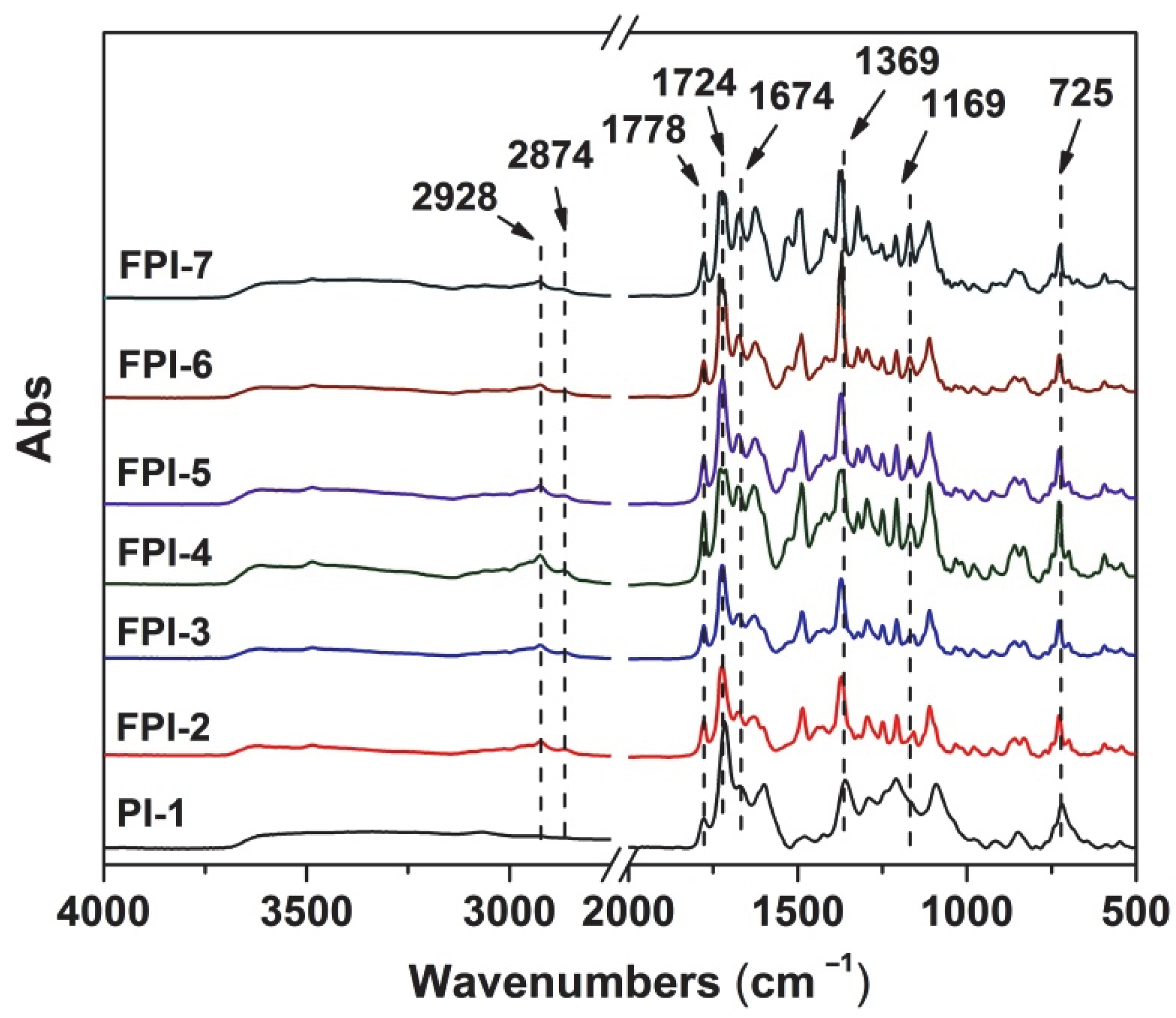
3.1. PI Synthesis and Film Preparation
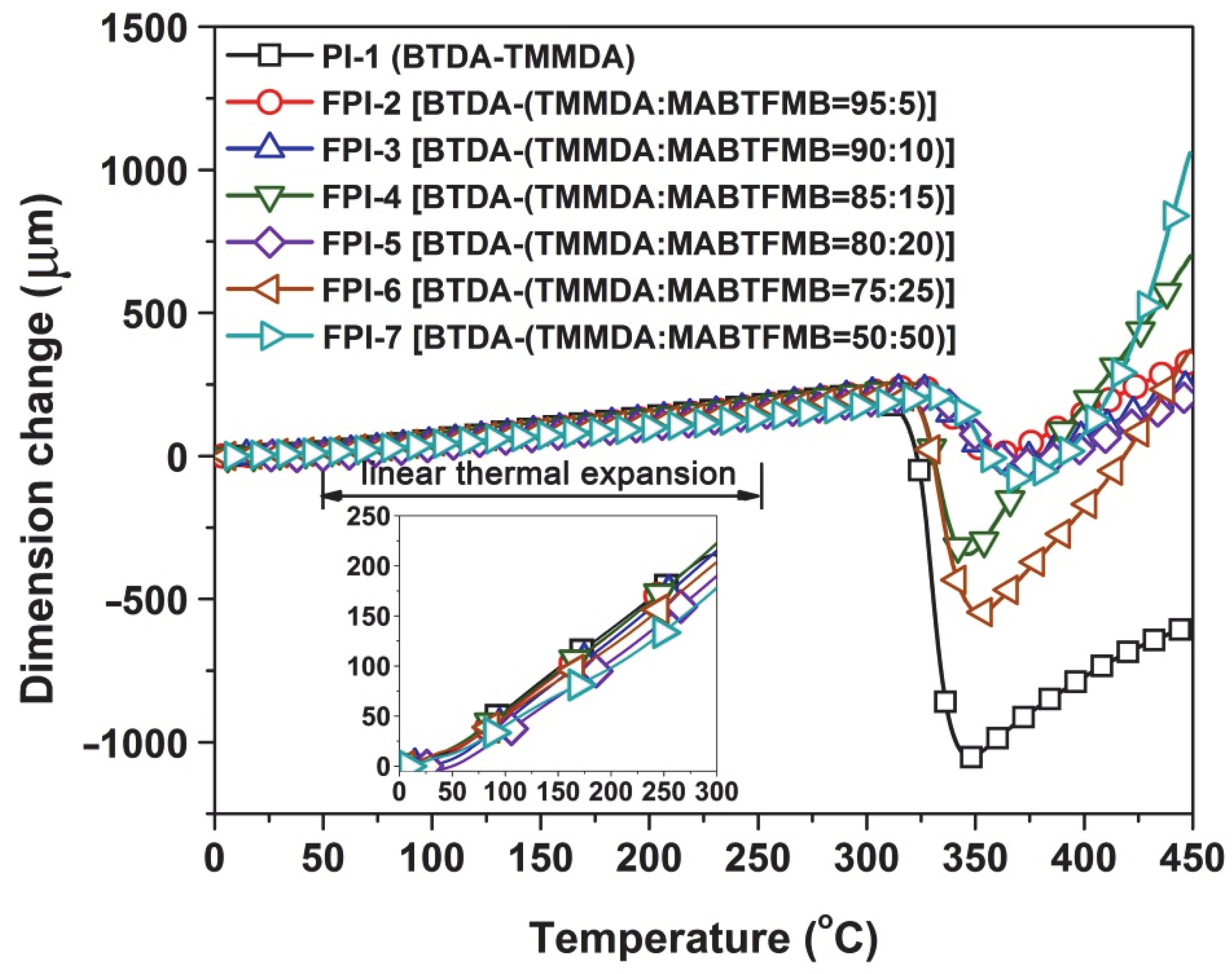
3.2. Thermal Properties
3.3. Optical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, M.C.; Higashihara, T.; Ueda, M. Recent progress in thermally stable and photosensitive polymers. Polym. J. 2018, 50, 57–76. [Google Scholar] [CrossRef]
- Tomikawa, M.; Okuda, R.; Ohnishi, H. Photosensitive polyimide for packaging applications. J. Photopolym. Sci. Technol. 2015, 28, 73–77. [Google Scholar] [CrossRef]
- Azuma, A.; Abe, S.; Sasaki, M. Effect of cross-linker and photosensitive polyimide to achieve full imidization and lower stress for good reliability. J. Photopolym. Sci. Technol. 2021, 34, 201–204. [Google Scholar] [CrossRef]
- Araki, H.; Kiuchi, Y.; Shimada, A.; Ogasawara, H.; Jukei, M.; Tomikawa, M. Low Df polyimide with photosensitivity for high frequency applications. J. Photopolym. Sci. Technol. 2020, 33, 165–170. [Google Scholar] [CrossRef]
- Onishi, H.; Kamemoto, S.; Yuba, T.; Tomikawa, M. Low-temperature curable positive-tone photosensitive polyimide coatings. J. Photopolym. Sci. Technol. 2012, 25, 341–344. [Google Scholar] [CrossRef]
- Masuda, Y.; Hashimoto, K.; Shoji, Y.; Koyama, Y.; Tomikawa, M.J. Redistribution layer preparation on glass panel for panel level fan out package by photosensitive polyimide. J. Photopolym. Sci. Technol. 2018, 31, 629–632. [Google Scholar] [CrossRef]
- Tseng, L.Y.; Lin, Y.C.; Kuo, C.C.; Chen, C.K.; Wang, C.E.; Kuo, C.C.; Ueda, M.; Chen, W.C. Alkaline-developable and negative-type photosensitive polyimide with high sensitivity and excellent mechanical properties using photo-base generator. J. Polym. Sci. 2020, 58, 2366–2375. [Google Scholar] [CrossRef]
- Yeh, Y.M.; Ueda, M.; Hsu, C.S. Alkaline-developable positive-type photosensitive polyimide with high mechanical strength and high resolution based on chain extendable poly(amic acid), thermally degradable cross-linker and photoacid generator. J. Polym. Sci. 2020, 58, 948–955. [Google Scholar] [CrossRef]
- Shoji, Y.; Hashimoto, K.; Koyama, Y.; Masuda, Y.; Araki, H.; Tomikawa, M. Low stress and low temperature curable photosensitive polyimide. J. Photopolym. Sci. Technol. 2021, 34, 195–199. [Google Scholar] [CrossRef]
- Yeh, Y.M.; Karapala, V.K.; Ueda, M.; Hsu, C.S. Low-temperature curable, alkaline-developable, and negative-type photosensitive polyimide with high resolution and mechanical properties based on chain extendable poly(amic acid) and photo-base generator. Polym. Adv. Technol. 2021, 32, 663–669. [Google Scholar] [CrossRef]
- Song, G.S.; Heo, Y.J.; Baek, J.J.; Lee, H.; Bae, G.Y.; Choi, K.H.; Koh, W.G.; Shin, G. The improved photosensitivity of photosensitive polyimides containing o-nitrobenzyl ether groups induced by the addition of photoacid generator. J. Polym. Sci. 2021, 59, 340–352. [Google Scholar] [CrossRef]
- Tseng, L.Y.; Lin, Y.C.; Kuo, C.C.; Kuo, C.C.; Ueda, M.; Chen, W.C. An ultra heat-resistant polyimide formulated with photo-base generator for alkaline-developable, negative-type photoresist. React. Funct. Polym. 2020, 157, 104760. [Google Scholar] [CrossRef]
- Do, S.J.; Seo, Y.B.; Kim, Y.H.; Won, J.C.; Ha, Y.M.; Kim, J. Novel positive working photosensitive polyimide-carbon black (PSPI-CB) composites containing triphenylene vinyl ether (TP-VE) monomers. Macromol. Res. 2021, 29, 164–171. [Google Scholar] [CrossRef]
- Lim, Y.R.; Park, H.; Bae, G.; Seo, M.K.; Yun, H.W.; Yoo, S.; Myung, S.; Lim, J.; Lee, S.S.; An, K.S.; et al. Resist- and etching-free patterning mediated by predefined photosensitive polyimide for two-dimensional semiconductor-based photodetectors. Adv. Mater. Interf. 2021, 8, 2001817. [Google Scholar] [CrossRef]
- Fan, J.; Zhu, T.; Wu, W.J.; Tang, S.H.; Liu, J.Q.; Tu, L.C. Low temperature photosensitive polyimide based insulating layer formation for microelectromechanical systems applications. J. Electron. Mater. 2015, 44, 4891–4897. [Google Scholar] [CrossRef]
- Windrich, F.; Kappert, E.J.; Malanin, M.; Eichhorn, K.J.; Hauβler, L.; Benes, N.E.; Voit, B. In-situ imidization analysis in microscale thin films of an ester-type photosensitive polyimide for microelectronic packaging applications. Eur. Polym. J. 2016, 84, 279–291. [Google Scholar] [CrossRef]
- Lin, A.A.; Sastri, V.R.; Tesoro, G.; Reiser, A.; Eachus, R. On the cross-linking mechanism of benzophenone-containing polyimides. Macromolecules 1985, 21, 1165–1169. [Google Scholar] [CrossRef]
- Rohde, O.; Smolka, P.; Falcigno, P.A. Novel auto-photosensitive polyimides with tailored properties. Polym. Eng. Sci. 1992, 32, 1623–1629. [Google Scholar] [CrossRef]
- Qian, Z.G.; Ge, Z.Y.; Li, Z.X.; He, M.H.; Liu, J.G.; Pang, Z.Z.; Fan, L.; Yang, S.Y. Synthesis and characterization of new inherent photoimageable polyimides based on fluorinated tetramethyl-substituted diphenylmethanediamines. Polymer 2002, 43, 6057–6063. [Google Scholar] [CrossRef]
- Jin, Q.; Yamashita, T.; Horie, K. Polyimides with alicyclic diamines. II. Hydrogen abstraction and photocrosslinking reactions of benzophenone-type polyimides. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 503–511. [Google Scholar] [CrossRef]
- Scaiano, J.C.; Netto-ferreira, J.C.; Becknell, A.F.; Small, R.D. The mechanism of photocure of inherently photosensitive polyimides containing a benzophenone group. Polym. Eng. Sci. 1989, 29, 942–944. [Google Scholar] [CrossRef]
- Liu, L.; Lu, Q.; Yin, J.; Qian, X.; Wang, W.; Zhu, Z.; Wang, Z. Photosensitive polyimide (PSPI) materials containing inorganic nanoparticles (I)PSPI/TiO2 hybrid materials by sol-gel process. Mater. Chem. Phys. 2002, 74, 210–213. [Google Scholar] [CrossRef]
- Sasaki, T. Low temperature curable polyimide for advanced package. Low temperature curable polyimide for advanced package. J. Photopolym. Sci. Technol. 2016, 29, 379–382. [Google Scholar] [CrossRef]
- Okuda, R.; Miyoshi, K.; Arai, N.; Tomikawa, M.; Ohbayashi, G. Low-temperature-curing type positive-tone photosensitive polyimide coatings for insulating layer in OLED displays. J. Photopolym. Sci. Technol. 2002, 15, 205–208. [Google Scholar] [CrossRef]
- An, Y.; Jia, Y.; Zhi, X.; Zhang, Y.; Qi, L.; Wu, L.; Jiang, G.; Liu, J. Preimidized auto-photosensitive polyimides containing methyl-substituted benzanilide units with increased high-temperature dimensional stability for advanced optical applications: Preparation and properties. J. Polym. Res. 2021, 28, 228. [Google Scholar] [CrossRef]
- Ozawa, H.; Ishiguro, E.; Kyoya, Y.; Kikuchi, Y.; Matsumoto, T. Colorless polyimides derived from an alicyclic tetracarboxylic dianhydride, CpODA. Polymers 2021, 13, 2824. [Google Scholar] [CrossRef]
- Hu, X.; Mu, H.; Wang, Y.; Wang, Z.; Yan, J. Colorless polyimides derived from isomeric dicyclohexyl-tetracarboxylic dianhydrides for optoelectronic applications. Polymer 2018, 134, 8–19. [Google Scholar] [CrossRef]
- Chang, J.H. Equibiaxially stretchable colorless and transparent polyimides for flexible display substrates. Rev. Adv. Mater. Sci. 2020, 59, 1–9. [Google Scholar] [CrossRef]
- Tsebriienko, T.; Popov, A.I. Effect of poly(titanium oxide) on the viscoelastic and thermophysical properties of interpenetrating polymer networks. Crystals 2021, 11, 794. [Google Scholar] [CrossRef]
- Seo, K.; Nam, K.H.; Lee, S.; Han, H. Low stress polyimide/silica nanocomposites as dielectrics for wafer level chip scale packaging. Mater. Lett. 2020, 163, 127204. [Google Scholar] [CrossRef]




| PI | BTDA (g, mol) | TMMDA (g, mol) | MABTFMB (g, mol) | NMP (g) | Ac2O (g, mol) | Pyridine (g, mol) |
|---|---|---|---|---|---|---|
| PI-1 | 9.6669, 0.03 | 7.6311, 0.03 | 0 | 69.2 | 15.3, 0.15 | 9.5, 0.12 |
| FPI-2 | 9.6669, 0.03 | 7.2495, 0.0285 | 0.8798, 0.0015 | 53.4 | 15.3, 0.15 | 9.5, 0.12 |
| FPI-3 | 9.6669, 0.03 | 6.8680, 0.0270 | 1.7596, 0.0030 | 54.9 | 15.3, 0.15 | 9.5, 0.12 |
| FPI-4 | 9.6669, 0.03 | 6.4864, 0.0255 | 2.6394, 0.0045 | 56.4 | 15.3, 0.15 | 9.5, 0.12 |
| FPI-5 | 9.6669, 0.03 | 6.1049, 0.0240 | 3.5192, 0.0060 | 57.9 | 15.3, 0.15 | 9.5, 0.12 |
| FPI-6 | 9.6669, 0.03 | 5.7233, 0.0225 | 4.3990, 0.0075 | 59.4 | 15.3, 0.15 | 9.5, 0.12 |
| FPI-7 | 9.6669, 0.03 | 3.8156, 0.0150 | 8.7980, 0.0150 | 66.8 | 15.3, 0.15 | 9.5, 0.12 |
| PI | [η]inh a (dL g−1) | Molecular Weight b | Solubility c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mn (×104 g mol−1) | Mw (×104 g mol−1) | PDI | NMP | DMAc | GBL | CPA | THF | ||
| PI-1 | 1.00 | 5.06 | 8.98 | 1.77 | ++ | ++ | − | ++ | ++ |
| FPI-2 | 0.96 | 4.89 | 9.19 | 1.88 | ++ | ++ | − | ++ | ++ |
| FPI-3 | 0.92 | 4.80 | 9.16 | 1.91 | ++ | ++ | − | ++ | + |
| FPI-4 | 0.91 | 4.57 | 8.83 | 1.93 | ++ | ++ | − | + | − |
| FPI-5 | 0.88 | 4.29 | 8.06 | 1.88 | ++ | ++ | − | − | − |
| FPI-6 | 0.86 | 4.26 | 7.95 | 1.87 | ++ | ++ | − | − | − |
| FPI-7 | 0.77 | 3.85 | 7.55 | 1.96 | ++ | ++ | − | − | − |
| PI | Thermal Properties a | Optical Properties b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tg (°C) | T5% (°C) | Rw750 (%) | CTE (×10−6/K) | λcut (nm) | T405 (%) | T436 (%) | L* | a* | b* | Haze (%) | |
| PI-1 | 347.6 | 530 | 72 | 56.5 | 359 | 38.1 | 60.9 | 93.68 | −2.21 | 16.92 | 4.31 |
| FPI-2 | 360.7 | 528 | 67 | 54.0 | 357 | 47.3 | 78.3 | 94.99 | −1.18 | 4.52 | 1.51 |
| FPI-3 | 367.6 | 509 | 65 | 53.9 | 359 | 48.1 | 78.6 | 94.97 | −1.12 | 4.61 | 1.38 |
| FPI-4 | 348.0 | 532 | 66 | 52.5 | 364 | 49.5 | 79.9 | 94.97 | −0.81 | 3.59 | 1.43 |
| FPI-5 | 348.7 | 527 | 66 | 48.5 | 364 | 51.3 | 80.1 | 95.07 | −1.04 | 4.03 | 0.45 |
| FPI-6 | 351.7 | 519 | 64 | 47.5 | 366 | 55.6 | 81.1 | 95.20 | −1.13 | 4.24 | 0.72 |
| FPI-7 | 370.6 | 522 | 64 | 40.7 | 367 | 58.2 | 81.3 | 97.92 | −0.29 | 1.27 | 1.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Wang, H.; Jia, J.; Pan, Z.; Ren, X.; Zhi, X.; Zhang, Y.; Du, X.; Wang, X.; Liu, J. Intrinsically Negative Photosensitive Polyimides with Enhanced High-Temperature Dimensional Stability and Optical Transparency for Advanced Optical Applications via Simultaneous Incorporation of Trifluoromethyl and Benzanilide Units: Preparation and Properties. Polymers 2022, 14, 3733. https://doi.org/10.3390/polym14183733
Gao Y, Wang H, Jia J, Pan Z, Ren X, Zhi X, Zhang Y, Du X, Wang X, Liu J. Intrinsically Negative Photosensitive Polyimides with Enhanced High-Temperature Dimensional Stability and Optical Transparency for Advanced Optical Applications via Simultaneous Incorporation of Trifluoromethyl and Benzanilide Units: Preparation and Properties. Polymers. 2022; 14(18):3733. https://doi.org/10.3390/polym14183733
Chicago/Turabian StyleGao, Yanshuang, Huasen Wang, Jie Jia, Zhen Pan, Xi Ren, Xinxin Zhi, Yan Zhang, Xuanzhe Du, Xiaolei Wang, and Jingang Liu. 2022. "Intrinsically Negative Photosensitive Polyimides with Enhanced High-Temperature Dimensional Stability and Optical Transparency for Advanced Optical Applications via Simultaneous Incorporation of Trifluoromethyl and Benzanilide Units: Preparation and Properties" Polymers 14, no. 18: 3733. https://doi.org/10.3390/polym14183733
APA StyleGao, Y., Wang, H., Jia, J., Pan, Z., Ren, X., Zhi, X., Zhang, Y., Du, X., Wang, X., & Liu, J. (2022). Intrinsically Negative Photosensitive Polyimides with Enhanced High-Temperature Dimensional Stability and Optical Transparency for Advanced Optical Applications via Simultaneous Incorporation of Trifluoromethyl and Benzanilide Units: Preparation and Properties. Polymers, 14(18), 3733. https://doi.org/10.3390/polym14183733







