Enhanced Mechanical Properties and Anti–Inflammation of Poly(L–Lactic Acid) by Stereocomplexes of PLLA/PDLA and Surface–Modified Magnesium Hydroxide Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of the Stereocomplex Microparticles (SC)
2.3. Synthesis and Characterization of the Surface–Modified Magnesium Hydroxide
2.4. Preparation and Characterization of the PLLA Composites
2.5. Mechanical Properties
2.6. Degradation Behavior
2.7. Antibacterials Assay
2.8. Cell Viability and Inflammation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Stereocomplex Microparticles
3.2. Characterization of the Surface–Modified Magnesium Hydroxide Nanoparticles
3.3. Characterization of the PLLA Composites
3.4. Mechanical Properties of the PLLA Composites
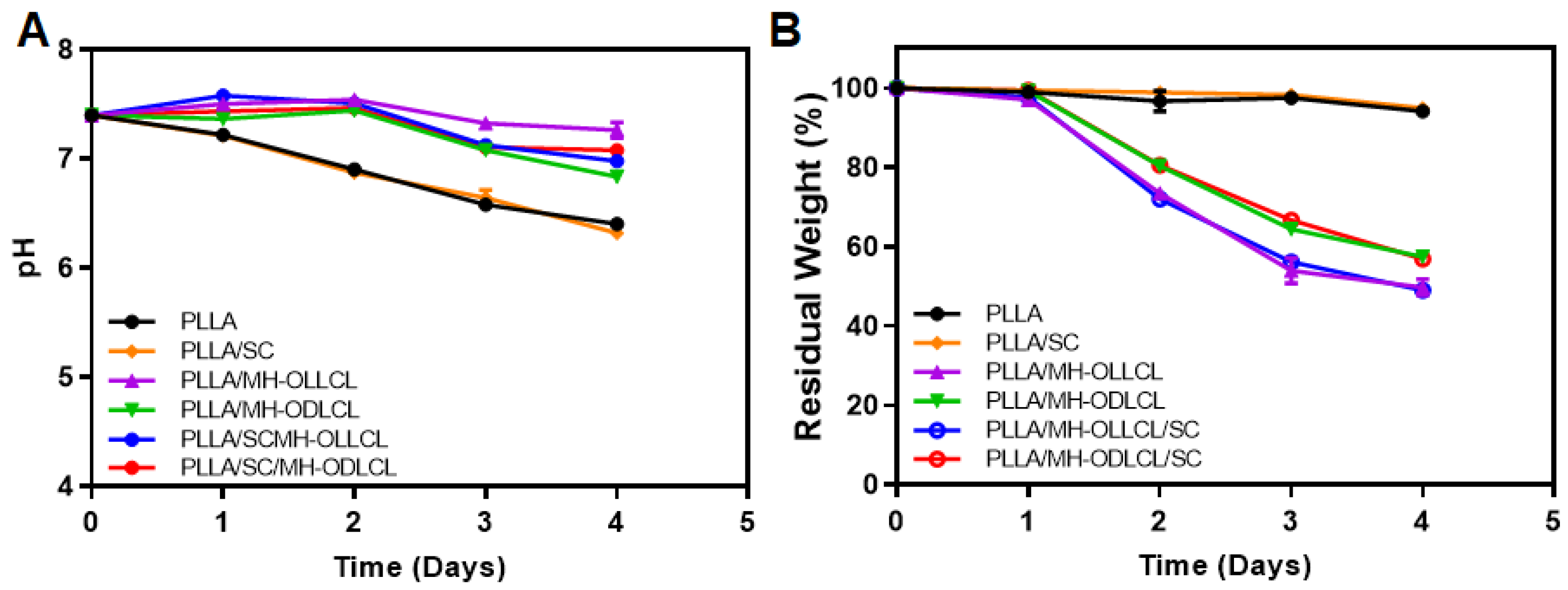
3.5. Degradation Behavior of the PLLA Composites
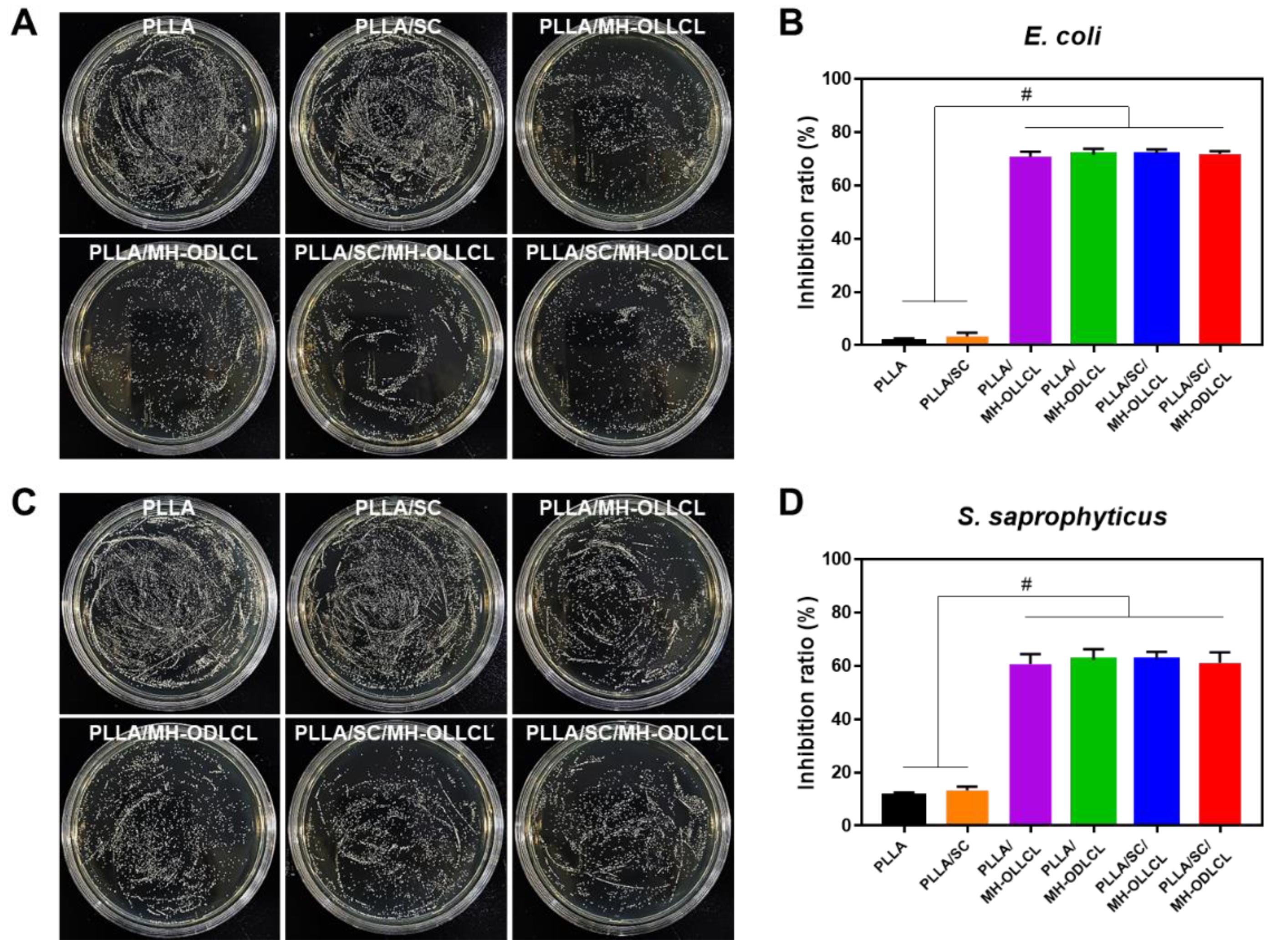
3.6. Biological Properties of the PLLA Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capuana, E.; Lopresti, F.; Ceraulo, M.; La Carrubba, V. Poly–L–Lactic Acid (PLLA)–Based Biomaterials for Regenerative Medicine: A Review on Processing and Applications. Polymers 2022, 14, 1153. [Google Scholar] [CrossRef] [PubMed]
- Arkaban, H.; Barani, M.; Akbarizadeh, M.R.; Pal Singh Chauhan, N.; Jadoun, S.; Dehghani Soltani, M.; Zarrintaj, P. Polyacrylic Acid Nanoplatforms: Antimicrobial, Tissue Engineering, and Cancer Theranostic Applications. Polymers 2022, 14, 1259. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, T.; Zhang, H.; Zhao, W.; Qu, L.; Chen, S.; Wu, S. Electrospun strong, bioactive, and bioabsorbable silk fibroin/poly (L–lactic–acid) nanoyarns for constructing advanced nanotextile tissue scaffolds. Mater. Today Bio 2022, 14, 100243. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Peng, S.; Chen, H.; Yu, X.; Zhao, X. Mechanical properties, rheological behaviors, and phase morphologies of high–toughness PLA/PBAT blends by in–situ reactive compatibilization. Compos. Part B 2019, 173, 107028. [Google Scholar] [CrossRef]
- Deng, L.; Xu, C.; Wang, X.; Wang, Z. Supertoughened Polylactide Binary Blend with High Heat Deflection Temperature Achieved by Thermal Annealing above the Glass Transition Temperature. ACS Sustain. Chem. Eng. 2018, 6, 480–490. [Google Scholar] [CrossRef]
- Eleuteri, M.; Bernal, M.; Milanesio, M.; Monticelli, O.; Fina, A. Stereocomplexation of Poly(Lactic Acid)s on Graphite Nanoplatelets: From Functionalized Nanoparticles to Self–assembled Nanostructures. Front. Chem. 2019, 7, 176. [Google Scholar] [CrossRef]
- Luo, F.; Fortenberry, A.; Ren, J.; Qiang, Z. Recent Progress in Enhancing Poly(Lactic Acid) Stereocomplex Formation for Material Property Improvement. Front. Chem. 2020, 8, 688. [Google Scholar] [CrossRef]
- Wan, Z.-Q.; Longo, J.M.; Liang, L.-X.; Chen, H.-Y.; Hou, G.-J.; Yang, S.; Zhang, W.-P.; Coates, G.W.; Lu, X.-B. Comprehensive Understanding of Polyester Stereocomplexation. J. Am. Chem. Soc. 2019, 141, 14780–14787. [Google Scholar] [CrossRef]
- Im, S.H.; Jung, Y.; Kim, S.H. In Situ Homologous Polymerization of l–Lactide Having a Stereocomplex Crystal. Macromolecules 2018, 51, 6303–6311. [Google Scholar] [CrossRef]
- Ko, K.-W.; Choi, B.; Kang, E.Y.; Shin, S.-W.; Baek, S.-W.; Han, D.K. The antagonistic effect of magnesium hydroxide particles on vascular endothelial activation induced by acidic PLGA degradation products. Biomater. Sci. 2021, 9, 892–907. [Google Scholar] [CrossRef]
- Yang, F.; Niu, X.; Gu, X.; Xu, C.; Wang, W.; Fan, Y. Biodegradable Magnesium–Incorporated Poly(l–lactic acid) Microspheres for Manipulation of Drug Release and Alleviation of Inflammatory Response. ACS Appl. Mater. Interfaces 2019, 11, 23546–23557. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Chun, S.Y.; Park, S.-B.; Kang, E.; Koh, W.-G.; Kwon, T.G.; Han, D.K.; Joung, Y.K. Scaffold–supported extracellular matrices preserved by magnesium hydroxide nanoparticles for renal tissue regeneration. Biomater. Sci. 2020, 8, 5427–5440. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Hu, Z.; Chen, J.; Qin, Y.; Wu, F.; Jin, T. Exenatide Microspheres for Monthly Controlled–Release Aided by Magnesium Hydroxide. Pharmaceutics 2021, 13, 816. [Google Scholar] [CrossRef] [PubMed]
- Go, E.J.; Kang, E.Y.; Lee, S.K.; Park, S.; Kim, J.H.; Park, W.; Kim, I.H.; Choi, B.; Han, D.K. An osteoconductive PLGA scaffold with bioactive β–TCP and anti–inflammatory Mg(OH)2 to improve in vivo bone regeneration. Biomater. Sci. 2020, 8, 937–948. [Google Scholar] [CrossRef]
- Jang, H.J.; Park, S.-B.; Bedair, T.M.; Oh, M.-K.; Ahn, D.-J.; Park, W.; Joung, Y.K.; Han, D.K. Effect of various shaped magnesium hydroxide particles on mechanical and biological properties of poly(lactic–co–glycolic acid) composites. J. Ind. Eng. Chem. 2018, 59, 266–276. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Y.; Chen, Z.; Pan, D.; Cheng, Y.; Liu, Z.; Lin, Z.; Guan, X. Investigation of Antibacterial Activity and Related Mechanism of a Series of Nano–Mg(OH)2. ACS Appl. Mater. Interfaces 2013, 5, 1137–1142. [Google Scholar] [CrossRef]
- Heydarian, M.; Schweinlin, M.; Schwarz, T.; Rawal, R.; Walles, H.; Metzger, M.; Rudel, T.; Kozjak-Pavlovic, V. Triple co–culture and perfusion bioreactor for studying the interaction between Neisseria gonorrhoeae and neutrophils: A novel 3D tissue model for bacterial infection and immunity. J. Tissue Eng. 2021, 12, 2041731420988802. [Google Scholar] [CrossRef]
- Kim, J.K.; Go, E.J.; Ko, K.W.; Oh, H.J.; Han, J.; Han, D.K.; Park, W. PLGA Microspheres Containing Hydrophobically Modified Magnesium Hydroxide Particles for Acid Neutralization–Mediated Anti–Inflammation. Tissue Eng. Regen. Med. 2021, 18, 613–622. [Google Scholar] [CrossRef]
- Shuai, C.; Yu, L.; Feng, P.; Peng, S.; Pan, H.; Bai, X. Construction of a stereocomplex between poly(d–lactide) grafted hydroxyapatite and poly(l–lactide): Toward a bioactive composite scaffold with enhanced interfacial bonding. J. Mater. Chem. B 2022, 10, 214–223. [Google Scholar] [CrossRef]
- Kamiya, H.; Iijima, M. Surface modification and characterization for dispersion stability of inorganic nanometer–scaled particles in liquid media. Sci. Technol. Adv. Mater. 2010, 11, 044304. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, B.; Yang, J.; Jia, J.; You, C.; Chen, M. Effects of modifying agents on surface modifications of magnesium oxide whiskers. Appl. Surf. Sci. 2016, 388, 370–375. [Google Scholar] [CrossRef]
- Kang, E.Y.; Park, S.-B.; Choi, B.; Baek, S.-W.; Ko, K.-W.; Rhim, W.-K.; Park, W.; Kim, I.-H.; Han, D.K. Enhanced mechanical and biological characteristics of PLLA composites through surface grafting of oligolactide on magnesium hydroxide nanoparticles. Biomater. Sci. 2020, 8, 2018–2030. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.-W.; Song, D.H.; Lee, H.I.; Kim, D.-S.; Heo, Y.; Kim, J.H.; Park, C.G.; Han, D.K. Poly(L–Lactic Acid) Composite with Surface–Modified Magnesium Hydroxide Nanoparticles by Biodegradable Oligomer for Augmented Mechanical and Biological Properties. Materials 2021, 14, 5869. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Wu, M.; Weng, Z.; Zhu, X.; Li, W.; Huang, P.; Wang, L.; Zheng, W.; Ohshima, M. Promoted formation of stereocomplex in enantiomeric poly(lactic acid)s induced by cellulose nanofibers. Carbohydr. Polym. 2022, 276, 118800. [Google Scholar] [CrossRef] [PubMed]
- Srithep, Y.; Akkaprasa, T.; Pholharn, D.; Morris, J.; Liu, S.-J.; Patrojanasophon, P.; Ngawhirunpat, T. Metronidazole–loaded polylactide stereocomplex electrospun nanofiber mats for treatment of periodontal disease. J. Drug Deliv. Sci. Technol. 2021, 64, 102582. [Google Scholar] [CrossRef]
- Park, H.-S.; Hong, C.-K. Relationship between the Stereocomplex Crystallization Behavior and Mechanical Properties of PLLA/PDLA Blends. Polymers 2021, 13, 1851. [Google Scholar] [CrossRef]
- Sun, C.; Zheng, Y.; Xu, S.; Ni, L.; Li, X.; Shan, G.; Bao, Y.; Pan, P. Role of Chain Entanglements in the Stereocomplex Crystallization between Poly(lactic acid) Enantiomers. ACS Macro Lett. 2021, 10, 1023–1028. [Google Scholar] [CrossRef]
- Gu, T.; Sun, D.-X.; Qi, X.-D.; Yang, J.-H.; Zhao, C.-S.; Lei, Y.-Z.; Wang, Y. Synchronously enhanced thermal conductivity and heat resistance in poly(l–lactide)/graphene nanoplatelets composites via constructing stereocomplex crystallites at interface. Compos. Part B 2021, 224, 109163. [Google Scholar] [CrossRef]
- Chen, J.; Rong, C.; Lin, T.; Chen, Y.; Wu, J.; You, J.; Wang, H.; Li, Y. Stable Co–Continuous PLA/PBAT Blends Compatibilized by Interfacial Stereocomplex Crystallites: Toward Full Biodegradable Polymer Blends with Simultaneously Enhanced Mechanical Properties and Crystallization Rates. Macromolecules 2021, 54, 2852–2861. [Google Scholar] [CrossRef]
- Liu, H.; Bai, D.; Du, S.; Li, X.; Bai, H.; Fu, Q. Stereocomplex Crystallization Induced Significant Improvement in Transparency and Stiffness–Toughness Performance of Core–Shell Rubber Nanoparticles Toughened Poly(l–lactide) Blends. Macromol. Mater. Eng. 2021, 306, 2100021. [Google Scholar] [CrossRef]
- Hsu, J.-P.; Nacu, A. Preparation of submicron–sized Mg(OH)2 particles through precipitation. Colloids Surf. A Physicochem. Eng. Asp. 2005, 262, 220–231. [Google Scholar] [CrossRef]
- Tashiro, K.; Kouno, N.; Wang, H.; Tsuji, H. Crystal Structure of Poly(lactic acid) Stereocomplex: Random Packing Model of PDLA and PLLA Chains As Studied by X-ray Diffraction Analysis. Macromolecules 2017, 50, 8048–8065. [Google Scholar] [CrossRef]
- Bedair, T.M.; Lee, C.K.; Kim, D.S.; Baek, S.W.; Bedair, H.M.; Joshi, H.P.; Choi, U.Y.; Park, K.H.; Park, W.; Han, I.; et al. Magnesium hydroxide–incorporated PLGA composite attenuates inflammation and promotes BMP2–induced bone formation in spinal fusion. J. Tissue. Eng. 2020, 11, 2041731420967591. [Google Scholar] [CrossRef]
- Arampatzis, A.S.; Kontogiannopoulos, K.N.; Theodoridis, K.; Aggelidou, E.; Rat, A.; Willems, A.; Tsivintzelis, I.; Papageorgiou, V.P.; Kritis, A.; Assimopoulou, A.N. Electrospun wound dressings containing bioactive natural products: Physico–chemical characterization and biological assessment. Biomater. Res. 2021, 25, 23. [Google Scholar] [CrossRef]
- Irfan, M.; Munir, H.; Ismail, H. Moringa oleifera gum based silver and zinc oxide nanoparticles: Green synthesis, characterization and their antibacterial potential against MRSA. Biomater. Res. 2021, 25, 17. [Google Scholar] [CrossRef]
- Halbus, A.F.; Horozov, T.S.; Paunov, V.N. Controlling the Antimicrobial Action of Surface Modified Magnesium Hydroxide Nanoparticles. Biomimetics 2019, 4, 41. [Google Scholar] [CrossRef]
- Balducci, G.; Bravo Diaz, L.; Gregory, D.H. Recent progress in the synthesis of nanostructured magnesium hydroxide. CrystEngComm 2017, 19, 6067–6084. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lee, J.-K.; Kim, J.H.; Lee, J.; Kim, D.S.; An, S.; Park, S.-B.; Kim, T.-H.; Rim, J.S.; Lee, S.; et al. Advanced PLGA hybrid scaffold with a bioactive PDRN/BMP2 nanocomplex for angiogenesis and bone regeneration using human fetal MSCs. Sci. Adv. 2021, 7, eabj1083. [Google Scholar] [CrossRef]
- Riemann, A.; Ihling, A.; Thomas, J.; Schneider, B.; Thews, O.; Gekle, M. Acidic environment activates inflammatory programs in fibroblasts via a cAMP–MAPK pathway. Biochim. Biophys. Acta. 2015, 1853, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Qiao, S.; Wu, D.; Li, Z.; Zhu, Y.; Zhan, F.; Lai, H.; Gu, Y. The combination of multi–functional ingredients–loaded hydrogels and three–dimensional printed porous titanium alloys for infective bone defect treatment. J. Tissue Eng. 2020, 11, 2041731420965797. [Google Scholar] [CrossRef] [PubMed]
- Onuma, Y.; Serruys, P.W. Bioresorbable scaffold: The advent of a new era in percutaneous coronary and peripheral revascularization? Circulation 2011, 123, 779–797. [Google Scholar] [CrossRef] [PubMed]
- Bartkowiak-Jowsa, M.; Będziński, R.; Kozłowska, A.; Filipiak, J.; Pezowicz, C. Mechanical, rheological, fatigue, and degradation behavior of PLLA, PGLA and PDGLA as materials for vascular implants. Meccanica 2013, 48, 721–731. [Google Scholar] [CrossRef] [Green Version]






| Tg (°C) | Tc (°C) | Tm,HC (°C) | ΔHm,HC (J/g) | Tm,SC (°C) | ΔHm,SC (J/g) | XC,HC (%) | XC,SC (%) | fSC (%) | |
|---|---|---|---|---|---|---|---|---|---|
| PLLA | 66.68 | 111.92 | 179.42 | 64.49 | – | – | 69.34 | – | – |
| PDLA | 66.62 | 104.62 | 179.88 | 63.08 | – | – | 67.83 | – | – |
| SC | 67.76 | 112.18 | 178.21 | 15.97 | 220.28 | 28.84 | 17.17 | 20.31 | 54.19 |
| Tg (°C) | Tc (°C) | Tm,HC (°C) | ΔHm,HC (J/g) | XC,HC (%) | |
|---|---|---|---|---|---|
| PLLA | 63.37 | – | 167.03 | 8.971 | 9.64 |
| PLLA/SC | 55.98 | 111.98 | 167.47 | 28.19 | 30.31 |
| PLLA/MH–OLLCL | 56.51 | 91.78 | 160.58 | 32.46 | 34.90 |
| PLLA/MH–ODLCL | 56.93 | 93.18 | 161.38 | 30.29 | 32.56 |
| PLLA/SC/MH–OLLCL | 55.81 | 91.32 | 160.45 | 27.95 | 30.05 |
| PLLA/SC/MH–ODLCL | 56.32 | 93.79 | 160.55 | 27.45 | 29.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, S.-W.; Kim, J.H.; Song, D.H.; Kim, D.-S.; Park, C.G.; Han, D.K. Enhanced Mechanical Properties and Anti–Inflammation of Poly(L–Lactic Acid) by Stereocomplexes of PLLA/PDLA and Surface–Modified Magnesium Hydroxide Nanoparticles. Polymers 2022, 14, 3790. https://doi.org/10.3390/polym14183790
Baek S-W, Kim JH, Song DH, Kim D-S, Park CG, Han DK. Enhanced Mechanical Properties and Anti–Inflammation of Poly(L–Lactic Acid) by Stereocomplexes of PLLA/PDLA and Surface–Modified Magnesium Hydroxide Nanoparticles. Polymers. 2022; 14(18):3790. https://doi.org/10.3390/polym14183790
Chicago/Turabian StyleBaek, Seung-Woon, Jun Hyuk Kim, Duck Hyun Song, Da-Seul Kim, Chun Gwon Park, and Dong Keun Han. 2022. "Enhanced Mechanical Properties and Anti–Inflammation of Poly(L–Lactic Acid) by Stereocomplexes of PLLA/PDLA and Surface–Modified Magnesium Hydroxide Nanoparticles" Polymers 14, no. 18: 3790. https://doi.org/10.3390/polym14183790
APA StyleBaek, S.-W., Kim, J. H., Song, D. H., Kim, D.-S., Park, C. G., & Han, D. K. (2022). Enhanced Mechanical Properties and Anti–Inflammation of Poly(L–Lactic Acid) by Stereocomplexes of PLLA/PDLA and Surface–Modified Magnesium Hydroxide Nanoparticles. Polymers, 14(18), 3790. https://doi.org/10.3390/polym14183790









