Effect of Water-Resistant Properties of Kraft Paper (KP) Using Sulfur Hexafluoride (SF6) Plasma Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Kraft Paper for Plasma Treatment
2.2. Water Contact Angle and Water Absorption Time
2.3. Tensile Strength, Folding Endurance, and Cobb Test Analysis
2.4. Morphology and Structural Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Selection of Plasma Gas
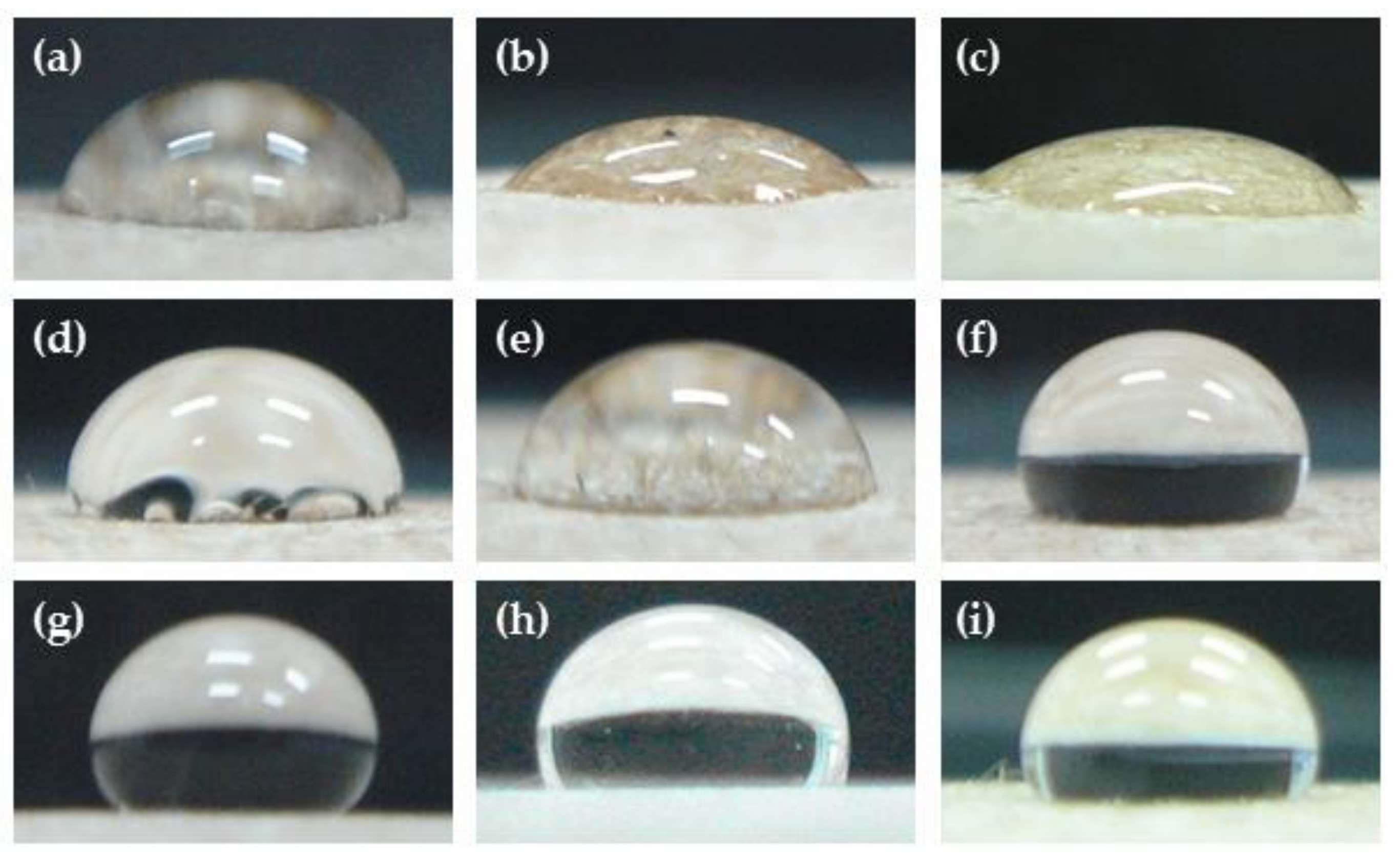
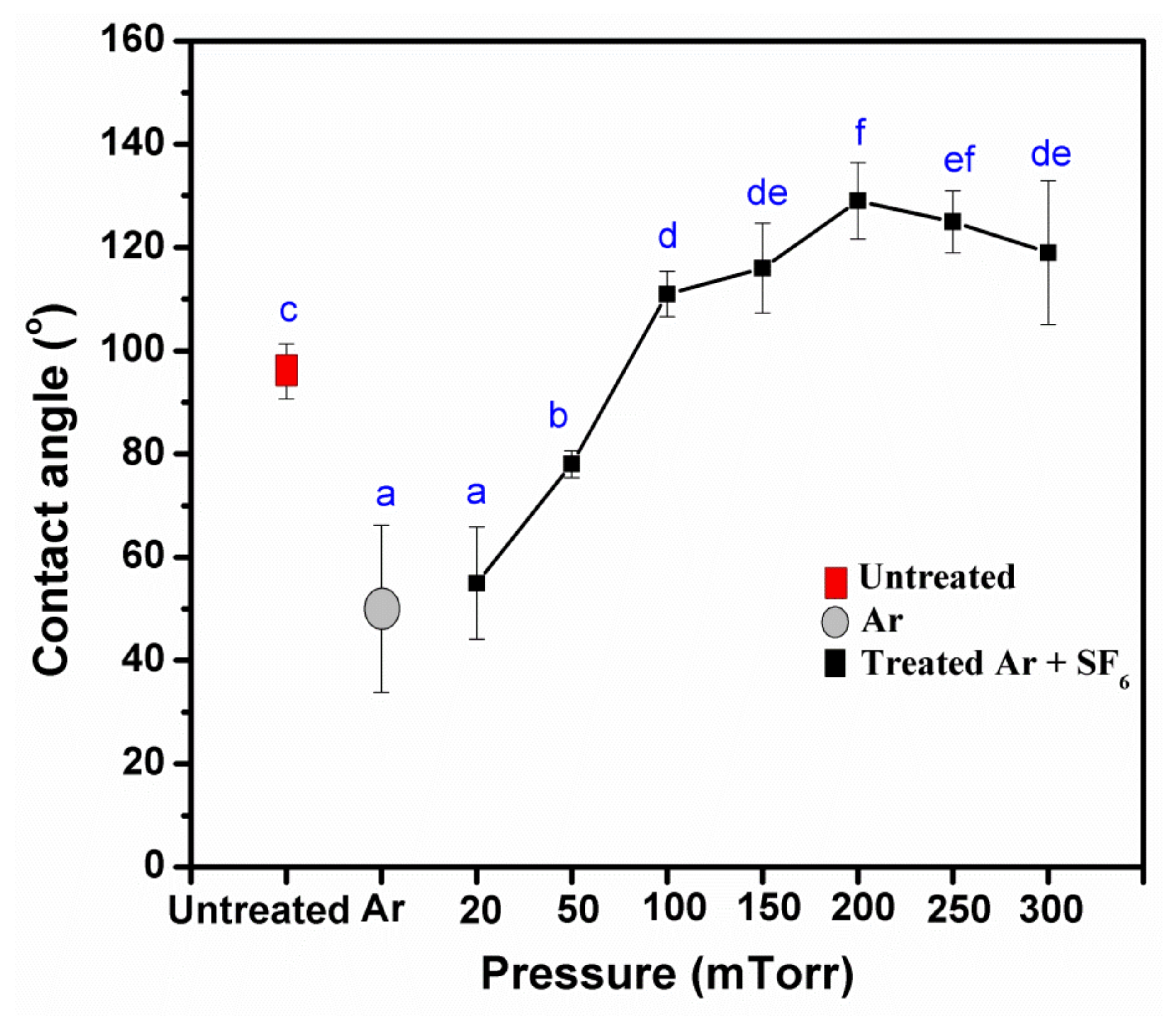
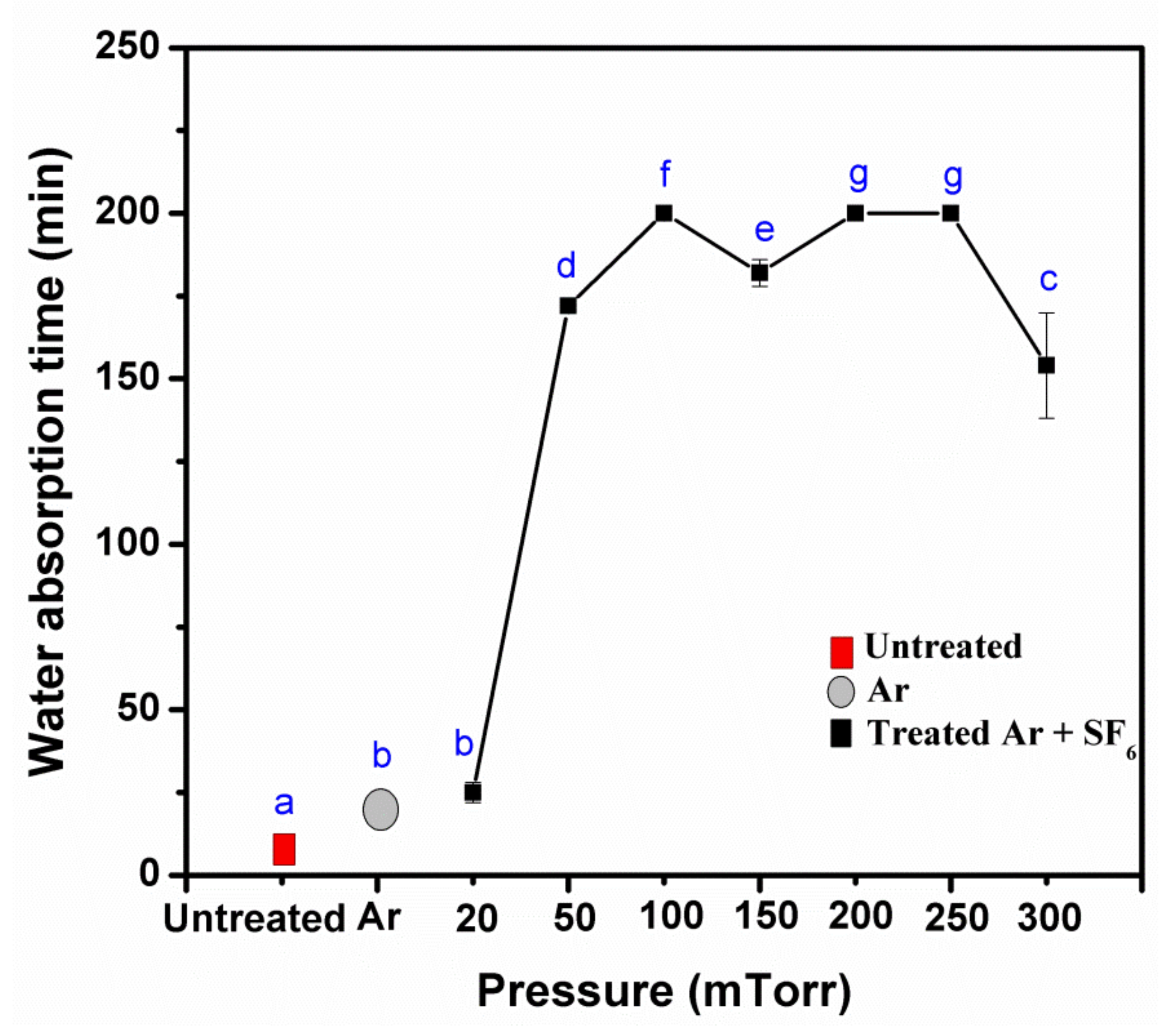
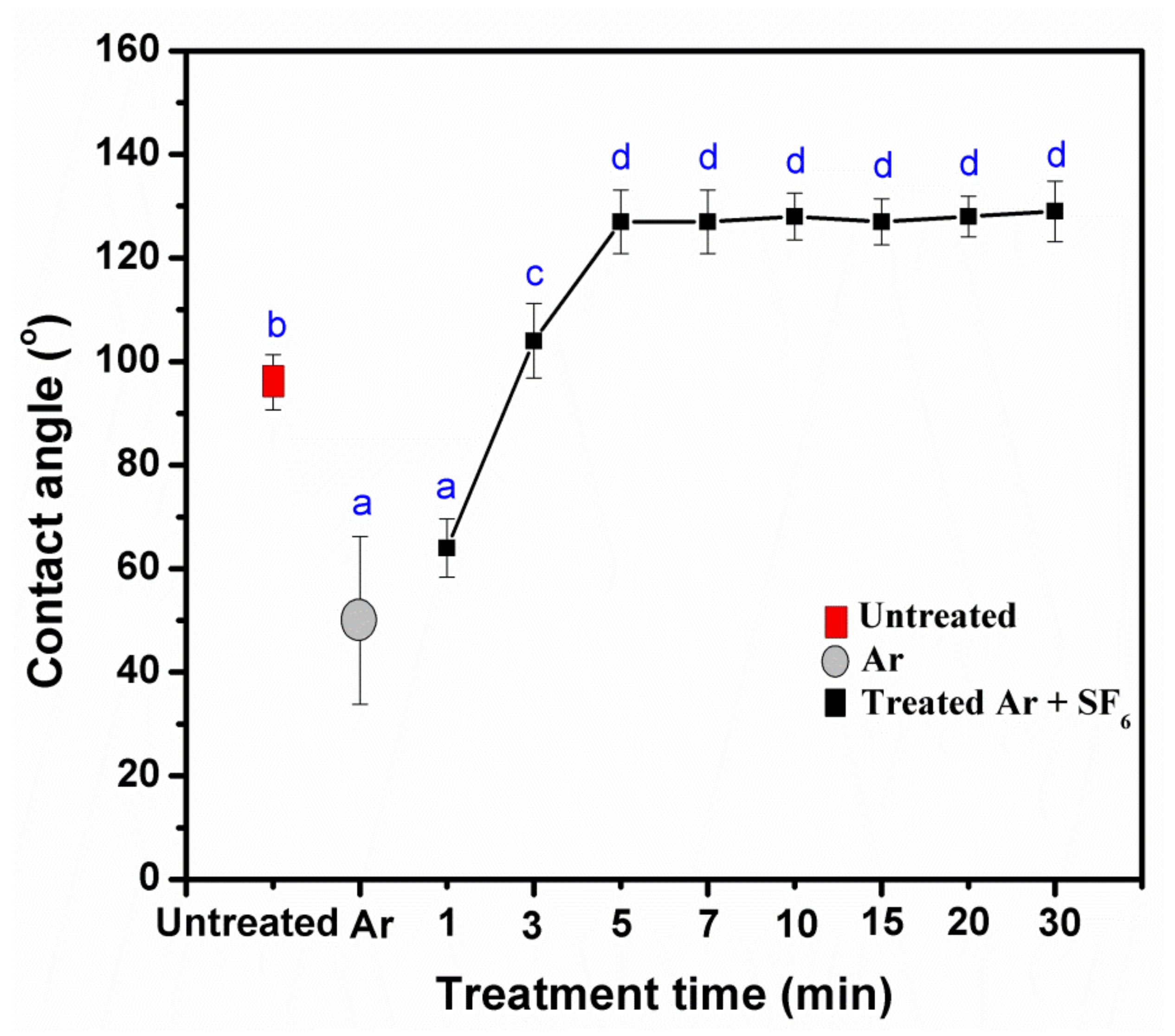
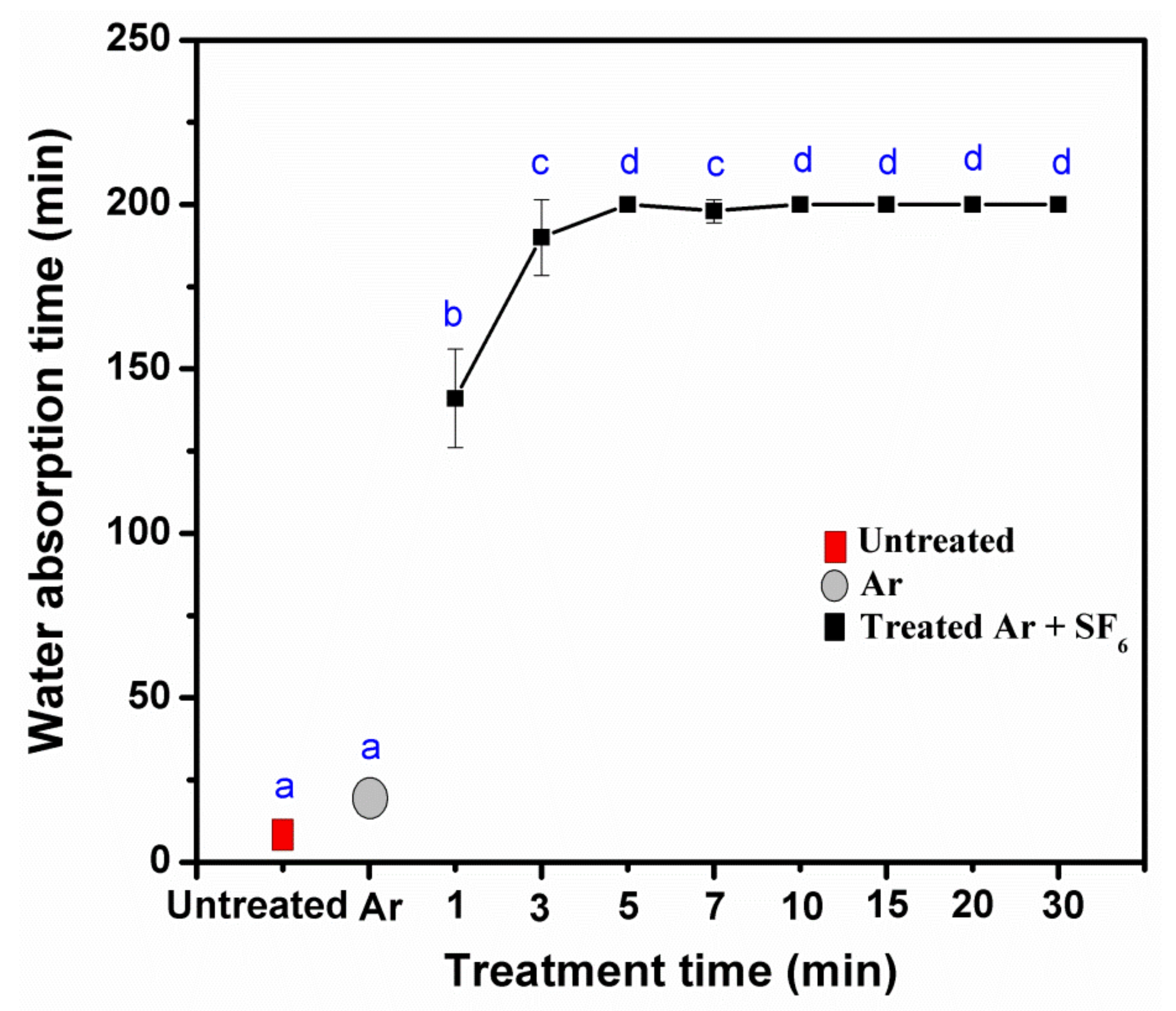
3.2. Effects of Pressure, Power, and Treatment of Times Impact the Water Resistance Properties
3.3. Physical Properties of Treated KP
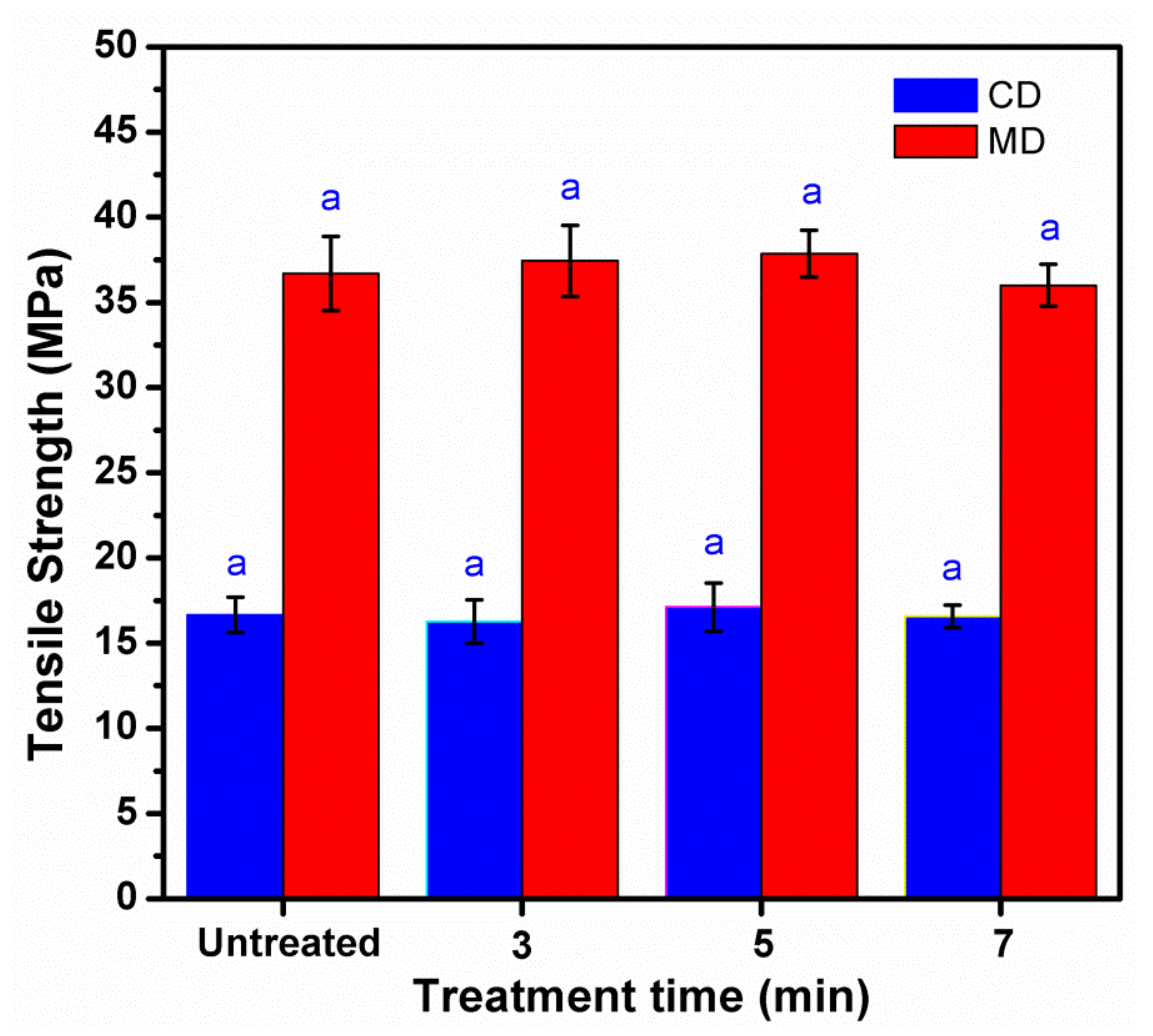
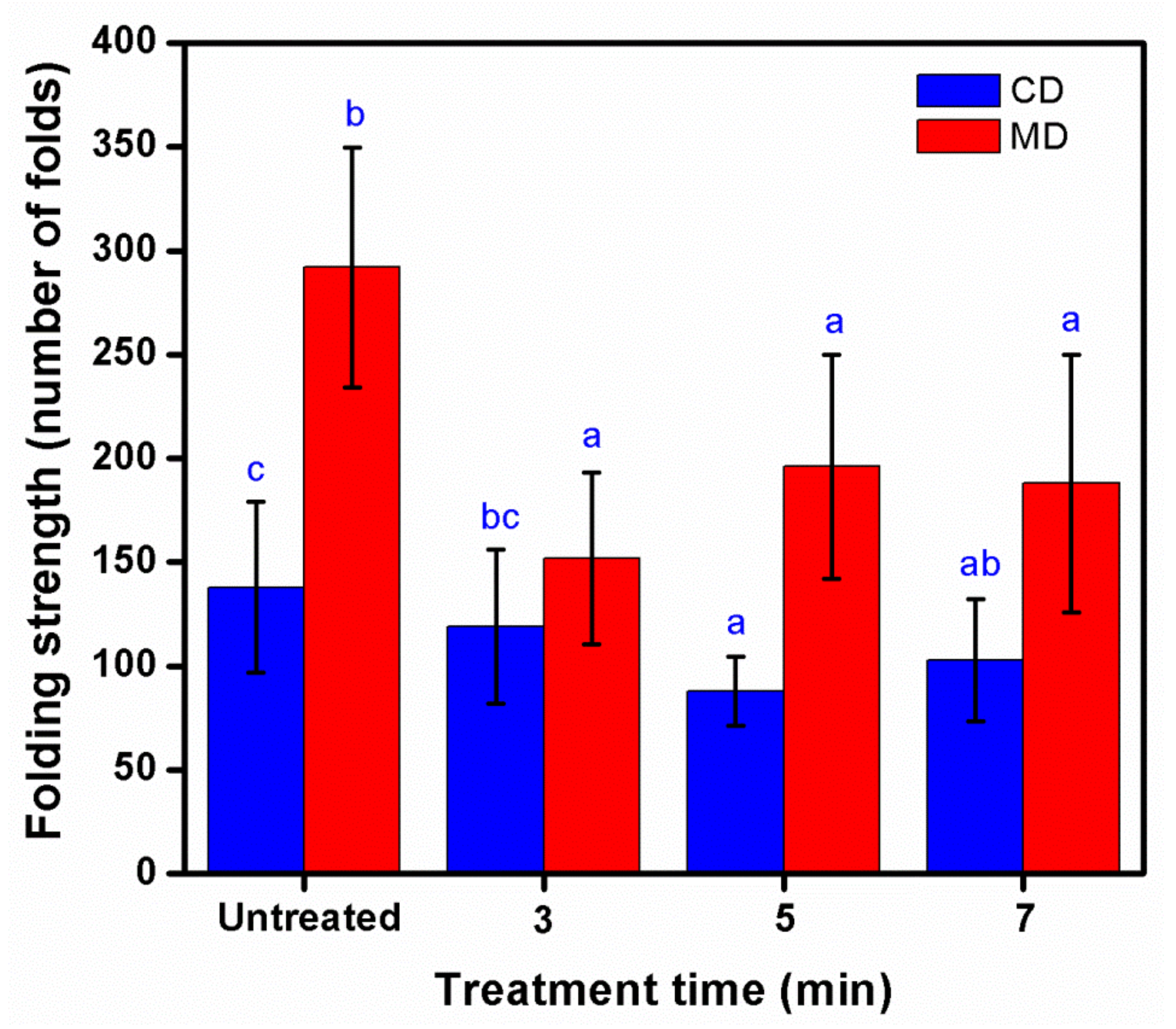
3.4. Mechanical Properties of Treated KP
3.5. Water Resistance Properties of Treated KP
3.6. Morphology of Treated KP
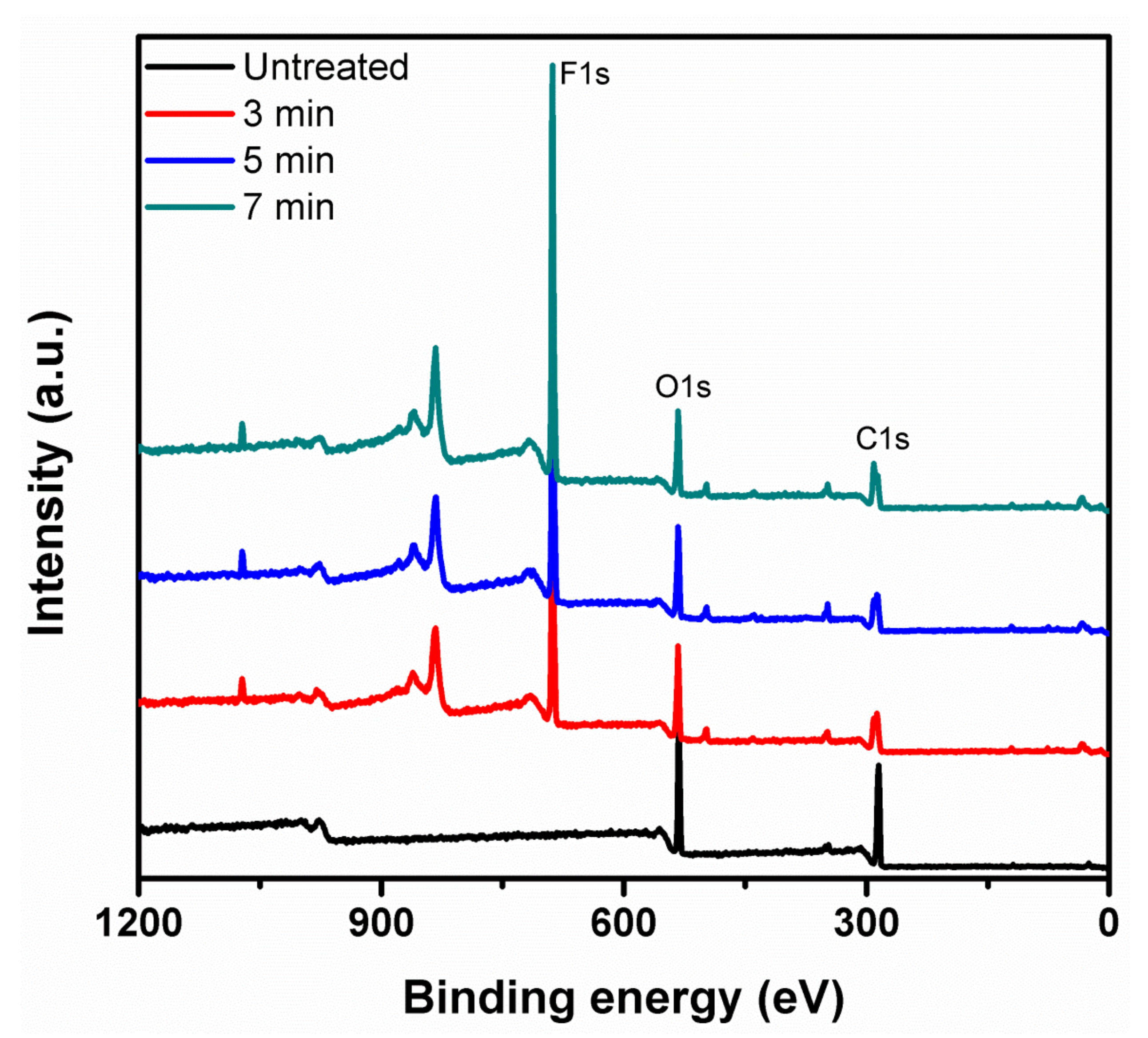
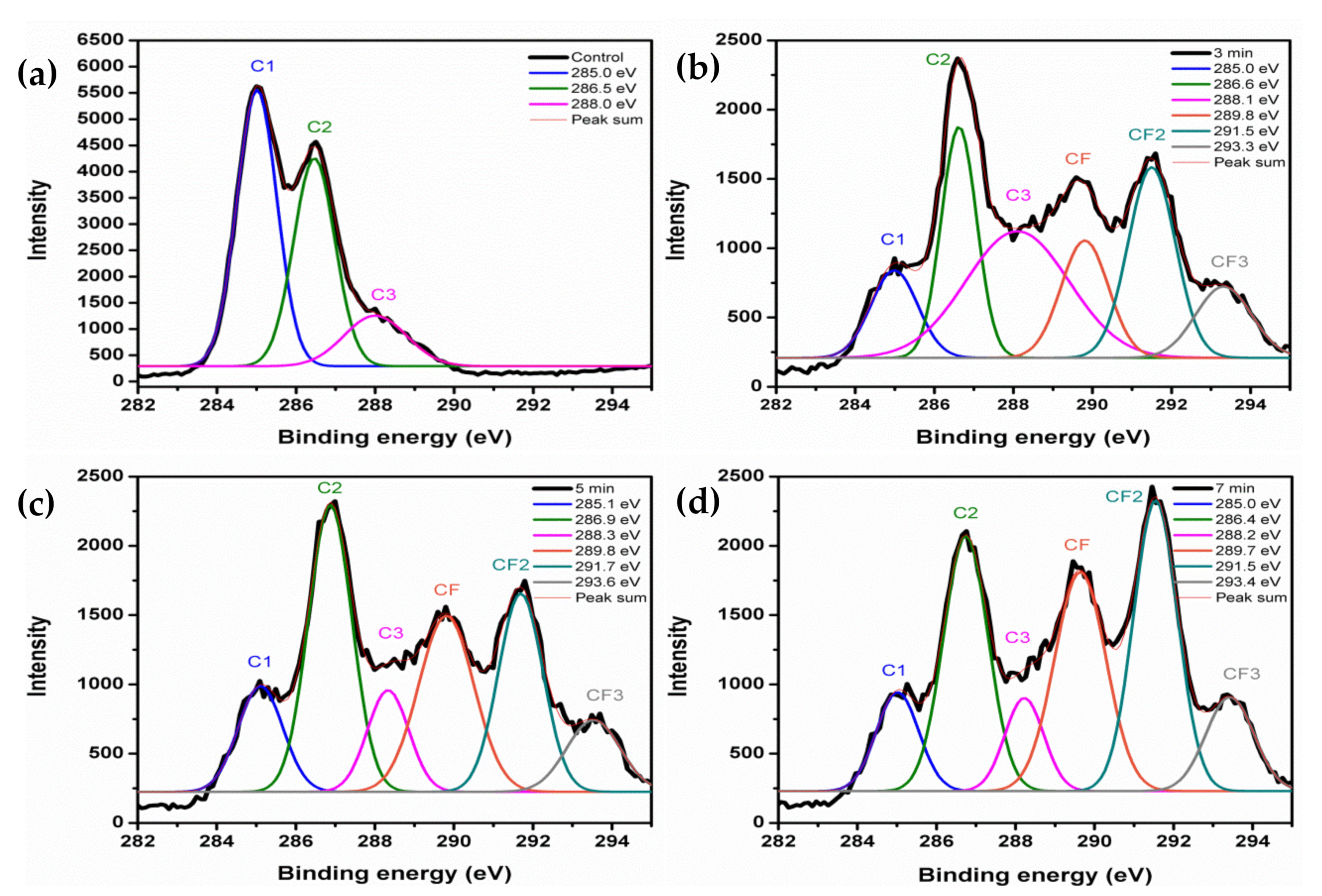
3.7. Chemical Composition of the Treated KP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Twede, D.; Selke, S. Cartons, Crates and Corrugated Board: Handbook of Paper and Wood Packaging Technology; DEStech Pub. Inc.: Lancaster, PA, USA, 2005. [Google Scholar]
- CASES, C. Why Sustainable Cardboard? Available online: https://capscases.co.uk/services/sustainable-cardboard-packaging/ (accessed on 7 July 2022).
- Fredi, G.; Dorigato, A. Recycling of bioplastic waste: A review. Adv. Ind. Eng. Polym. Res. 2021, 4, 159–177. [Google Scholar] [CrossRef]
- Corrugated Carton Box Flute & Carton Box Types. Available online: http://www.mabuchi.co.th/corrugated-carton-box-flute-carton-box-types/ (accessed on 25 July 2017).
- Frank, B. Corrugated box compression—A literature survey. Packag. Technol. Sci. 2014, 27, 105–128. [Google Scholar] [CrossRef]
- Netramai, S.; Kijchavengkul, T.; Kittipinyovath, P. Use of Paper in Food Packaging Applications. In Reference Module in Food Science; Smithers, G., Ed.; Elsevier B. V.: Amsterdam, The Netherlands, 2016; pp. 1–9. [Google Scholar]
- openPR. Thailand Folding Cartons and Corrugated Packaging Market 2020 Business Insights and Outlook—Sarnti Packaging Co., Ltd., Toppan Printing Co., Ltd, Bobst, SCG Packaging Pcl. Available online: https://www.openpr.com/news/2048773/thailand-folding-cartons-and-corrugated-packaging-market-2020 (accessed on 18 May 2020).
- Retaildive. Boxes: The Backbone of e-Commerce. Available online: https://www.retaildive.com/spons/boxes-the-backbone-of-e-commerce/596480/ (accessed on 15 March 2021).
- Lozano, J.; Saenz-Díez, J.; Martínez, E.; Jiménez, E.; Blanco, J. Methodology to improve machine changeover performance on food industry based on SMED. Int. J. Adv. Manuf. Technol. 2017, 90, 3607–3618. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.-l.; Sun, J. An overview of the reducing principle of design of corrugated box used in goods packaging. Procedia Environ. Sci. 2011, 10, 992–998. [Google Scholar] [CrossRef]
- Jo, J.-Y.; Min, C.-K.; Shin, J.-S. Manufacture of water-resistant corrugated board boxes for agricultural products in the cold chain system. J. Korea Tech. Assoc. Pulp Pap. Ind. 2012, 44, 29–34. [Google Scholar] [CrossRef]
- Jung, J.; Kasi, G.; Seo, J. Development of functional antimicrobial papers using chitosan/starch-silver nanoparticles. Int. J. Biol. Macromol. 2018, 112, 530–536. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Wongchiaya, P.; Boonyawan, D. Effect of sulphur hexafluoride (SF6) plasma on hydrophobicity of methylcellulose film. In Advanced Materials Research; Trans Tech Publications Ltd.: Zurich, Switzerland, 2010; pp. 214–218. [Google Scholar]
- Sheikhi, Z.; Mirmoghtadaie, L.; Khani, M.; Farhoodi, M.; Beikzadeh, S.; Abdolmaleki, K.; Kazemian-Bazkiaee, F.; Shokri, B.; Shojaee-Aliabadi, S. Physicochemical characterization of argon plasma-treated starch film. J. Agric. Sci. Technol. 2020, 22, 999–1008. [Google Scholar]
- Pankaj, S.K.; Keener, K.M. Cold plasma: Background, applications and current trends. Curr. Opin. Food Sci. 2017, 16, 49–52. [Google Scholar] [CrossRef]
- Chaiwat, W.; Wongsagonsup, R.; Tangpanichyanon, N.; Jariyaporn, T.; Deeyai, P.; Suphantharika, M.; Fuongfuchat, A.; Nisoa, M.; Dangtip, S. Argon plasma treatment of tapioca starch using a semi-continuous downer reactor. Food Bioprocess Technol. 2016, 9, 1125–1134. [Google Scholar] [CrossRef]
- Barani, H.; Calvimontes, A. Effects of oxygen plasma treatment on the physical and chemical properties of wool fiber surface. Plasma Chem. Plasma Processing 2014, 34, 1291–1302. [Google Scholar] [CrossRef]
- Caschera, D.; Mezzi, A.; Cerri, L.; de Caro, T.; Riccucci, C.; Ingo, G.M.; Padeletti, G.; Biasiucci, M.; Gigli, G.; Cortese, B. Effects of plasma treatments for improving extreme wettability behavior of cotton fabrics. Cellulose 2014, 21, 741–756. [Google Scholar] [CrossRef]
- Paosawatyanyong, B.; Supasai, T.; Pavarajarn, V.; Hodak, S. Hydrophobicity improvement of PET fabrics after SF6 plasma treatment. Int. Polym. Processing 2008, 23, 135–139. [Google Scholar] [CrossRef]
- Chaiwong, C.; Rachtanapun, P.; Wongchaiya, P.; Auras, R.; Boonyawan, D. Effect of plasma treatment on hydrophobicity and barrier property of polylactic acid. Surf. Coat. Technol. 2010, 204, 2933–2939. [Google Scholar] [CrossRef]
- Jinkarn, T.; Thawornwiriyanan, S.; Boonyawan, D.; Rachtanapun, P.; Sane, S. Effects of treatment time by sulfur hexafluoride (SF6) plasma on barrier and mechanical properties of paperboard. Packag. Technol. Sci. 2012, 25, 19–30. [Google Scholar] [CrossRef]
- Li, S.; Jinjin, D. Improvement of hydrophobic properties of silk and cotton by hexafluoropropene plasma treatment. Appl. Surf. Sci. 2007, 253, 5051–5055. [Google Scholar] [CrossRef]
- Hodak, S.K.; Supasai, T.; Paosawatyanyong, B.; Kamlangkla, K.; Pavarajarn, V. Enhancement of the hydrophobicity of silk fabrics by SF6 plasma. Appl. Surf. Sci. 2008, 254, 4744–4749. [Google Scholar] [CrossRef]
- Ho, K.K.C.; Lee, A.F.; Bismarck, A. Fluorination of carbon fibres in atmospheric plasma. Carbon 2007, 45, 775–784. [Google Scholar] [CrossRef]
- Hochart, F.; Levalois-Mitjaville, J.; De Jaeger, R.; Gengembre, L.; Grimblot, J. Plasma surface treatment of poly (acrylonitrile) films by fluorocarbon compounds. Appl. Surf. Sci. 1999, 142, 574–578. [Google Scholar] [CrossRef]
- Riccardi, C.; Barni, R.; Esena, P. Plasma treatment of silk. Solid State Phenom. 2005, 107, 125–128. [Google Scholar] [CrossRef]
- Ricciardi, M.R.; Papa, I.; Coppola, G.; Lopresto, V.; Sansone, L.; Antonucci, V. Effect of plasma treatment on the impact behavior of epoxy/basalt fiber-reinforced composites: A preliminary study. Polymers 2021, 13, 1293. [Google Scholar] [CrossRef]
- Thanakkasaranee, S.; Sadeghi, K.; Lim, I.-J.; Seo, J. Effects of incorporating calcined corals as natural antimicrobial agent into active packaging system for milk storage. Mater. Sci. Eng. C 2020, 111, 110781. [Google Scholar] [CrossRef]
- Park, S.; Thanakkasaranee, S.; Shin, H.; Ahn, K.; Sadeghi, K.; Lee, Y.; Tak, G.; Seo, J. Preparation and characterization of heat-resistant PET/bio-based polyester blends for hot-filled bottles. Polym. Test. 2020, 91, 106823. [Google Scholar] [CrossRef]
- Paosawatyanyong, B.; Kamlangkla, K.; Hodak, S. Hydrophobic and hydrophilic surface nano-modification of PET fabric by plasma process. J. Nanosci. Nanotechnol. 2010, 10, 7050–7054. [Google Scholar] [CrossRef] [PubMed]
- Pamidimukkala, K.M. X-ray Photoelectron Spectroscopy, Paper Surface Analysis By. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 1–17. [Google Scholar]
- Kuzmenko, V.; Wang, N.; Haque, M.; Naboka, O.; Flygare, M.; Svensson, K.; Gatenholm, P.; Liu, J.; Enoksson, P. Cellulose-derived carbon nanofibers/graphene composite electrodes for powerful compact supercapacitors. RSC Adv. 2017, 7, 45968–45977. [Google Scholar] [CrossRef] [Green Version]



| Gas | Radio Frequency (Watts) | Pressure (mTorr) | Contact Angle (°) * | Water Absorption Time (min) * |
|---|---|---|---|---|
| untreated | 0 | 0 | 96 ± 5.3 a | 8 ± 1.5 a |
| Ar | 50 | 100 | 50 ± 16.2 b | 19 ± 0.6 b |
| SF6 | 50 | 100 | 117 ± 2.0 c | >200 ± 0.0 c |
| SF6 + Ar | 50 | 100 | 115 ± 3.5 c | >200 ± 0.0 c |
| Radio Frequency (Watts) | Contact Angle (°) * | Water Absorption Time (min) * |
|---|---|---|
| 25 | 111.0 ± 10.8 a | 177.0 ± 14.3 a |
| 50 | 127.0 ± 6.1 b | >200.0 ± 0.0 b |
| 75 | n/d | n/d |
| Treatment Time (min) | Grammage (g/m2) * | Thickness (mm) * | Moisture (%) * |
|---|---|---|---|
| untreated | 126.6 ± 0.2 b | 0.200 ± 0.010 a | 7.77 ± 0.14 b |
| 3 | 124.1 ± 0.6 a | 0.200 ± 0.010 a | 7.20 ± 0.15 ab |
| 5 | 124.0 ± 0.2 a | 0.200 ± 0.010 a | 7.06 ± 0.11 a |
| 7 | 123.8 ± 0.8 a | 0.200 ± 0.010 a | 7.24 ± 0.17 ab |
| Treatment Time (min) | Cobb Test (g/m2) * |
|---|---|
| untreated | 124 ± 5.7 c |
| 3 | 34 ± 1.30 a |
| 5 | 28 ± 0.52 b |
| 7 | 29 ± 4.35 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rachtanapun, P.; Boonyawan, D.; Auras, R.A.; Kasi, G. Effect of Water-Resistant Properties of Kraft Paper (KP) Using Sulfur Hexafluoride (SF6) Plasma Coating. Polymers 2022, 14, 3796. https://doi.org/10.3390/polym14183796
Rachtanapun P, Boonyawan D, Auras RA, Kasi G. Effect of Water-Resistant Properties of Kraft Paper (KP) Using Sulfur Hexafluoride (SF6) Plasma Coating. Polymers. 2022; 14(18):3796. https://doi.org/10.3390/polym14183796
Chicago/Turabian StyleRachtanapun, Pornchai, Dheerawan Boonyawan, Rafael A. Auras, and Gopinath Kasi. 2022. "Effect of Water-Resistant Properties of Kraft Paper (KP) Using Sulfur Hexafluoride (SF6) Plasma Coating" Polymers 14, no. 18: 3796. https://doi.org/10.3390/polym14183796
APA StyleRachtanapun, P., Boonyawan, D., Auras, R. A., & Kasi, G. (2022). Effect of Water-Resistant Properties of Kraft Paper (KP) Using Sulfur Hexafluoride (SF6) Plasma Coating. Polymers, 14(18), 3796. https://doi.org/10.3390/polym14183796







