Design of Robust FEP Porous Ultrafiltration Membranes by Electrospinning-Sintered Technology
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Chemicals
2.2. Fabrication of Ultrafine Fibrous FEP Porous Membranes
2.2.1. Preparation of Electro-Spinning Solution
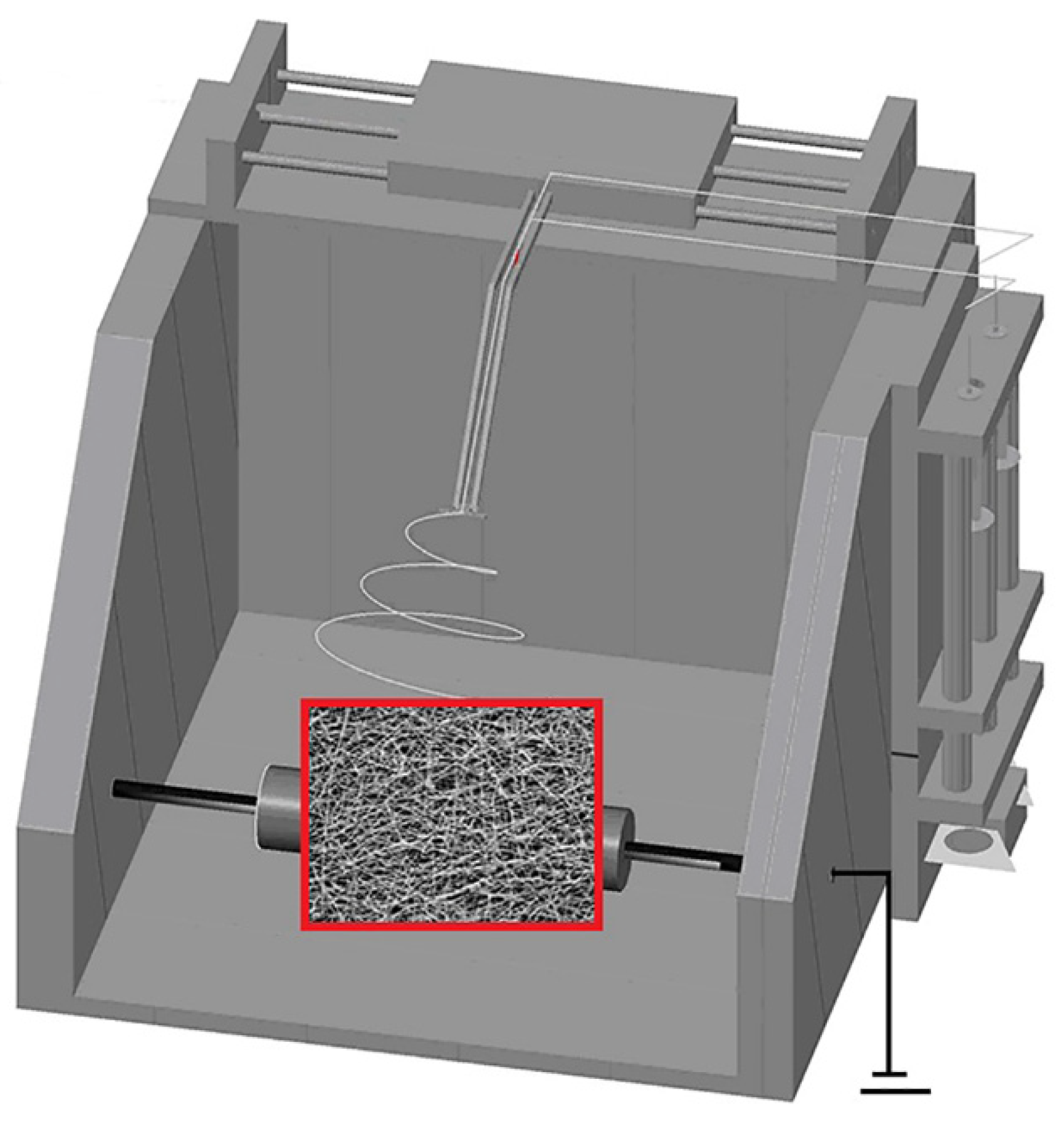
2.2.2. Preparation of Nascent Ultrafine Fibrous FEP/PVA Membranes
2.2.3. Sintering Process of Nascent Ultrafine Fibrous FEP/PVA Membranes
2.3. Membrane Properties and Characterization
2.3.1. Morphology of Ultrafine Fibrous FEP Porous Membranes
2.3.2. Differential Scanning Calorimeter (DSC)
2.3.3. Water Contact Angle (WCA)
2.3.4. Liquid Entry Pressure (LEP)
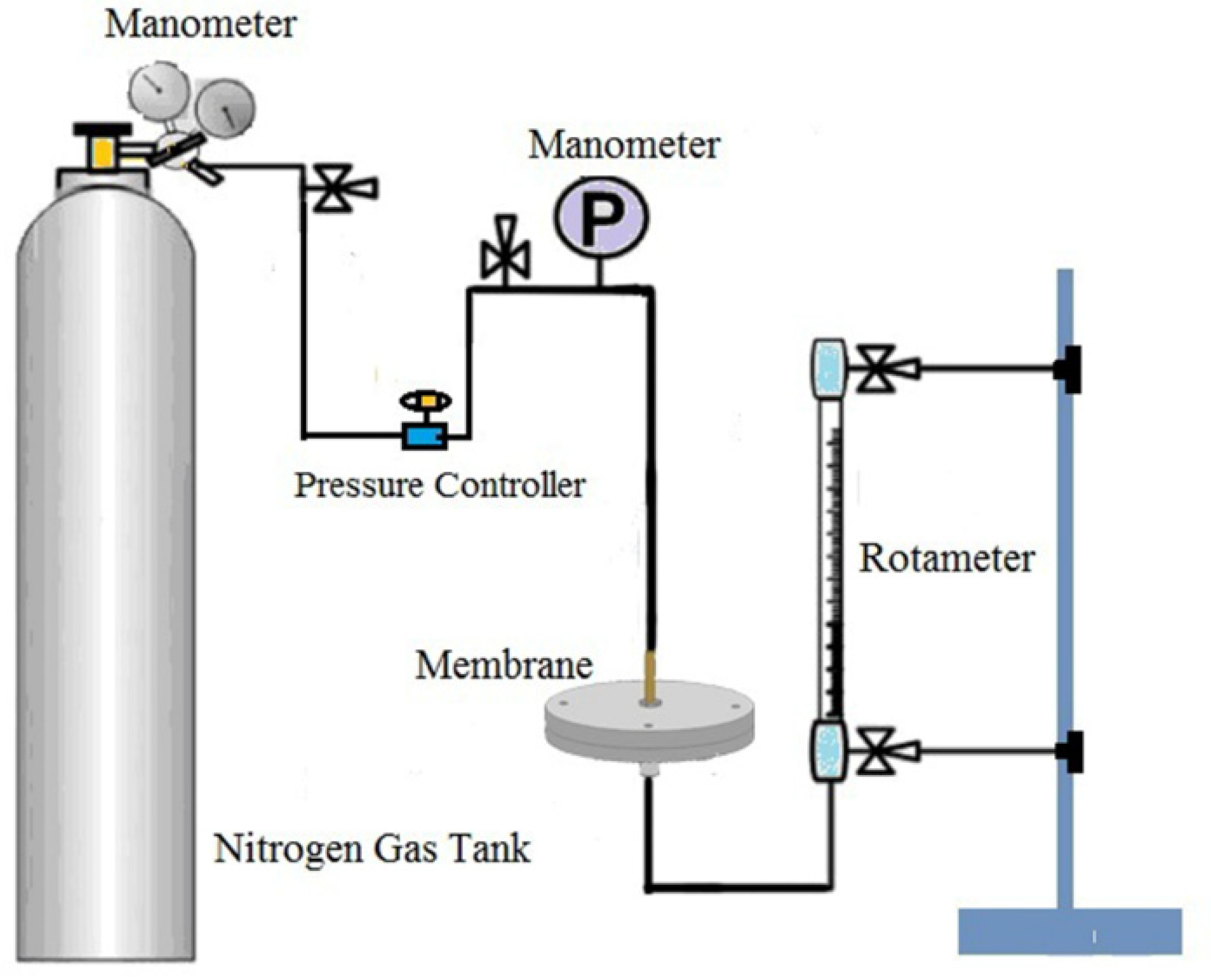
2.3.5. Nitrogen Flux through the Membranes
2.3.6. Porosity and Pore Size Distribution
2.3.7. Mechanical Strength
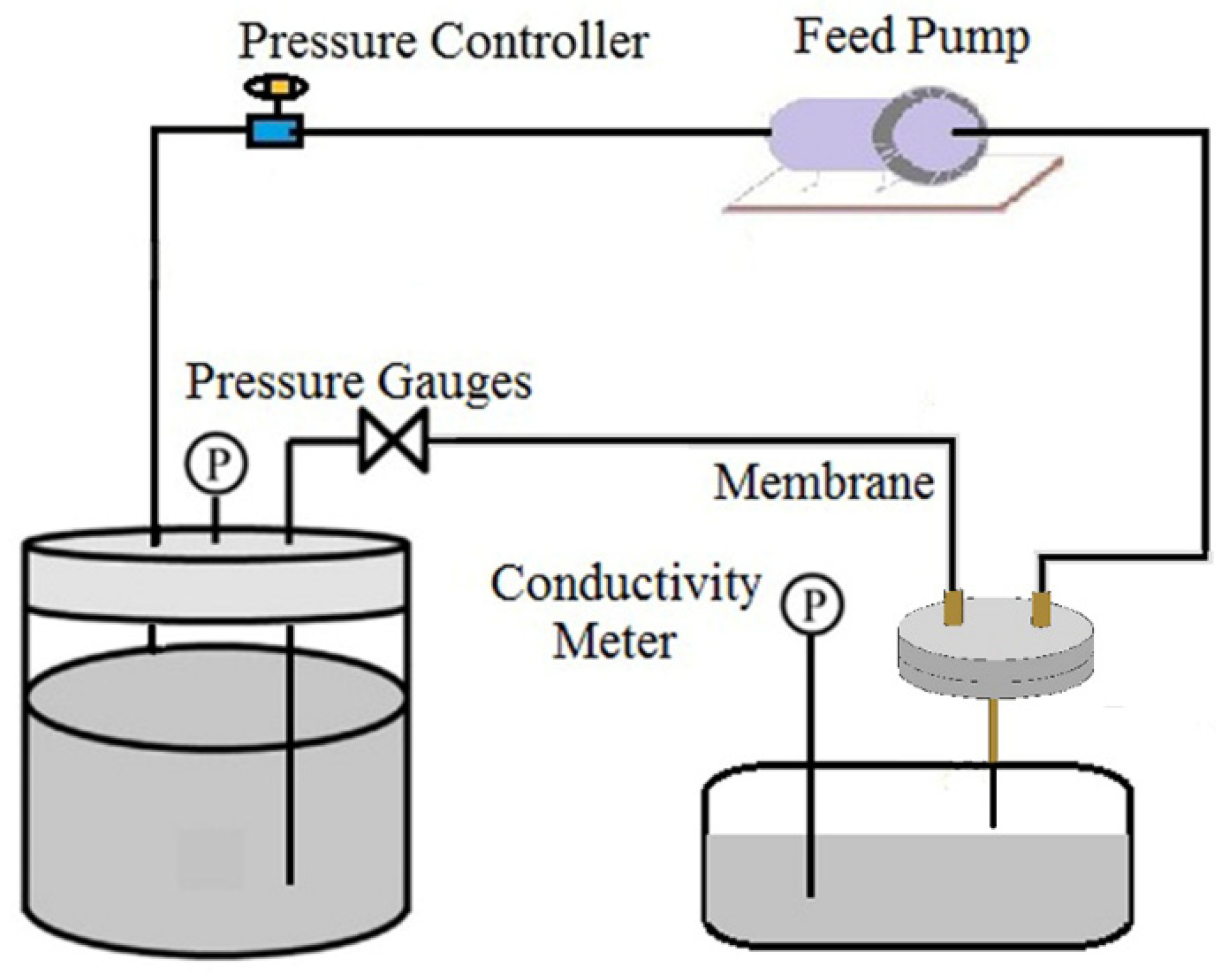
2.4. VMD Experiment
3. Results and Discussion
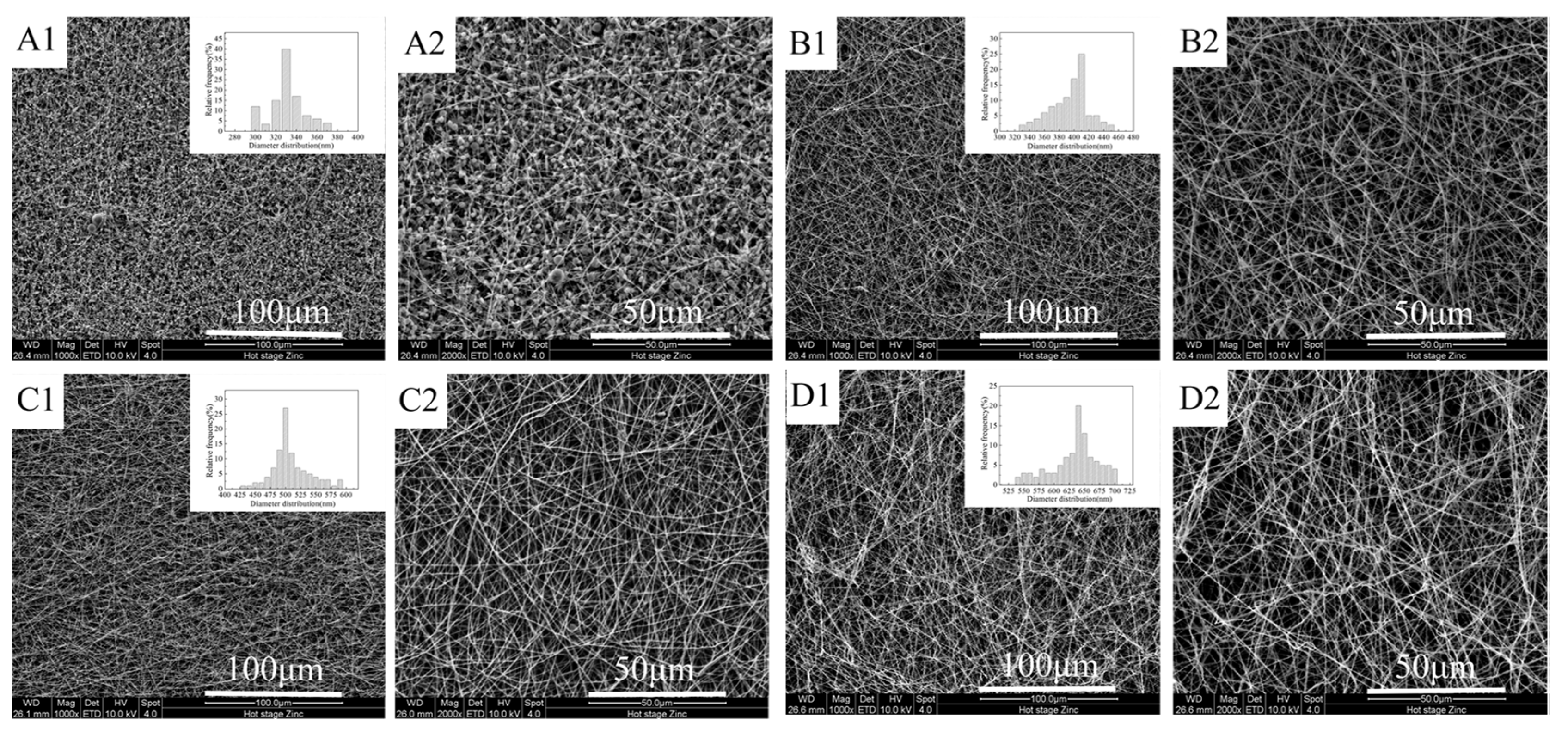
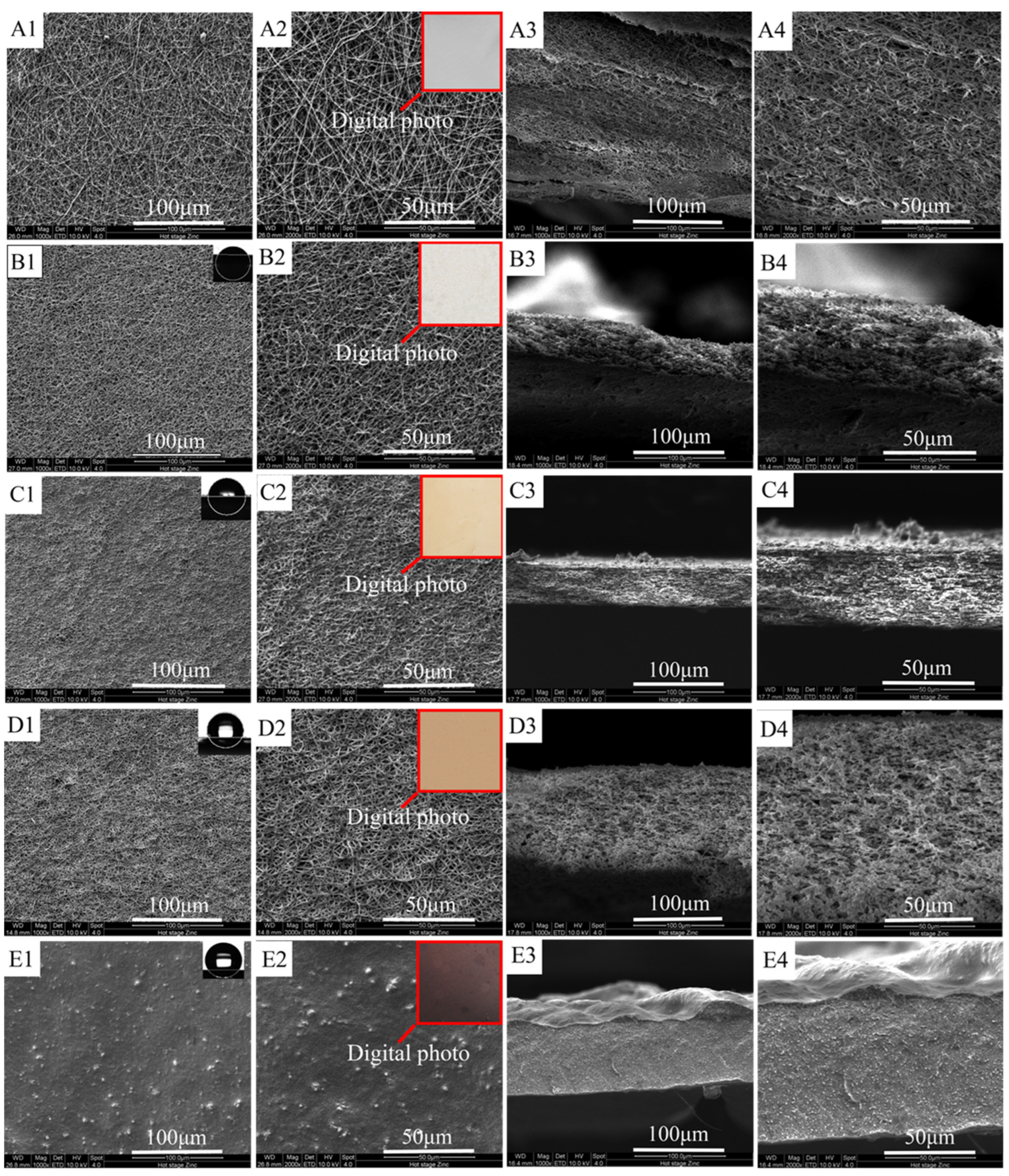
3.1. Membrane Morphology
3.1.1. Effects of FEP/PVA Mass Ratios
3.1.2. Effects of Sintering Temperature
3.2. DSC Analysis
3.3. WCA Analysis
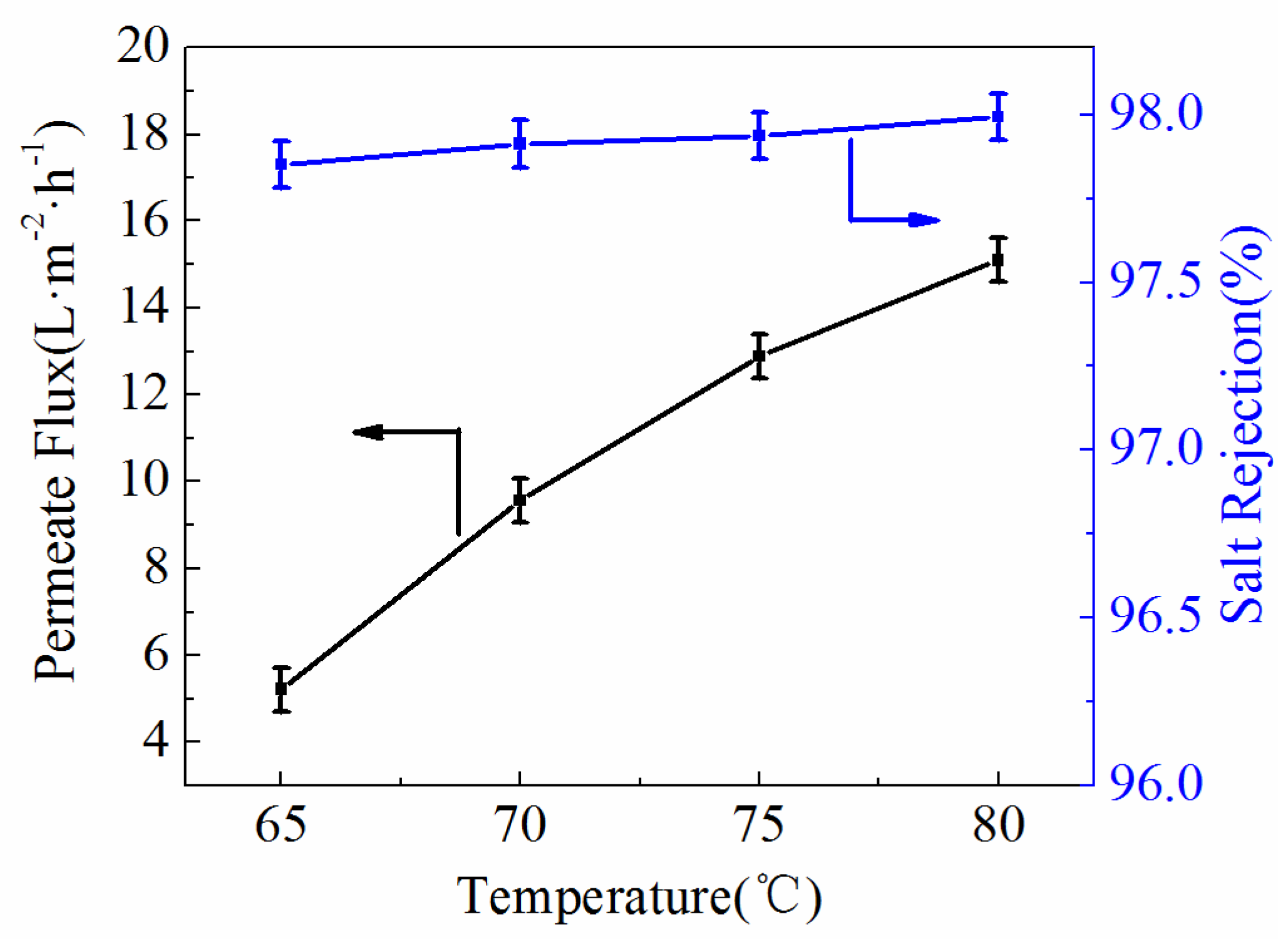
3.4. Permeability
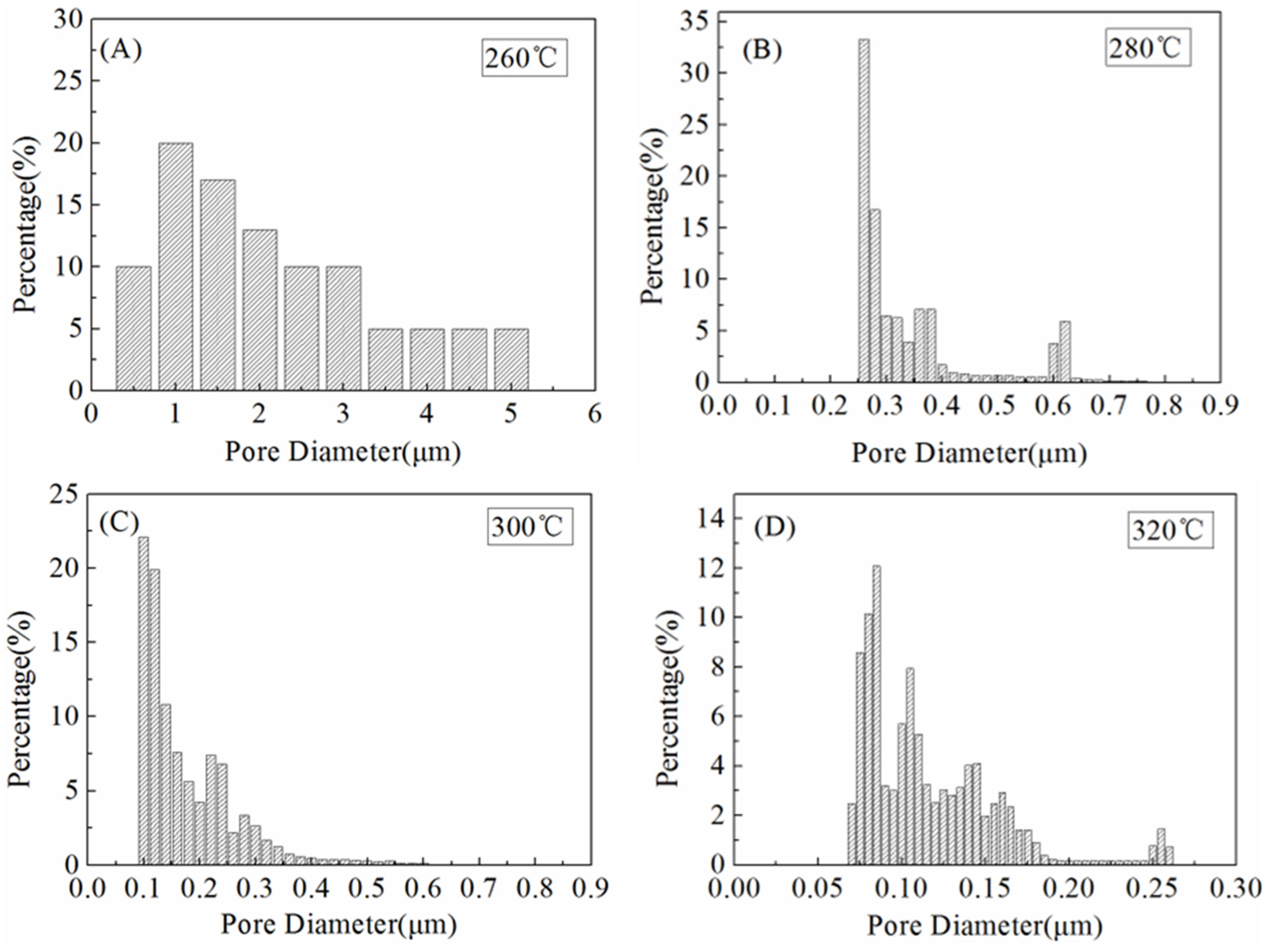
3.5. Pore Size Distribution
3.6. Mechanical Strength
3.7. VMD Experiment
3.8. Comparison with Other VMD Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbol | Definition |
| Xc | crystallinity value |
| ΔHm | melting enthalpy (J) |
| ΔHm100 | 100% melting enthalpy (J) |
| ε | porosity |
| W1 | weight of wet membrane (g) |
| W2 | the weight of dry membrane (g) |
| ρ | the density (g/mL) |
| d | the average thickness of the membrane (mm) |
| J | nitrogen flux (m3·m−2·h−1) |
| L | nitrogen flow (m3·h−1) |
| A | membrane area (m2) |
| R | rejection |
| Cf | concentration of the ink in the feed |
| Cp | concentration of the permeate solution |
References
- Formhals, A. Process and Apparatus for Preparing Artificial Threads. U.S. Patent 1,975,504, 2 October 1934. [Google Scholar]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Ghayour, H.; Ismail, A.F.; Nur, H.; Berto, F. Electrospun Nano-Fibers for Biomedical and Tissue Engineering Applications: A Comprehensive Review. Materials 2020, 13, 2153. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Wu, S.; Ma, Y.; Zhao, Y.; Chen, Z.; Frenkel, I.; Strzalka, J.; Zhou, H.; Zhu, X.; He, X. Strong tough hydrogels via the synergy of freeze-casting and salting out. Nature 2021, 590, 594–599. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Wang, G.; Liu, Y.; Kang, W.; Deng, N.; Zhuang, X.; Zhou, X. Research progress of ultrafine alumina fiber prepared by sol-gel method: A review. Chem. Eng. J. 2020, 421, 127744. [Google Scholar] [CrossRef]
- Kang, K.W.; Choi, C.W.; Jin, J.W. A Wet-Spinning Process for Producing Carbon Nanotube/Polyvinylidene Fluoride Fibers Having Highly Consistent Electrical and Mechanical Properties. Polymers 2021, 13, 4048. [Google Scholar] [CrossRef]
- Chang, L.; Wang, F.; Guo, Y.; Li, J.; Gong, Y.; Shi, Q. Green Preparation of Thermochromic Starch-Based Fibers through a Wet-Spinning Process. ACS Appl. Polym. Mater. 2020, 3, 436–444. [Google Scholar] [CrossRef]
- Lakra, R.; Balakrishnan, M.; Basu, S. Development of cellulose acetate-chitosan-metal organic framework forward osmosis membrane for recovery of water and nutrients from wastewater. J. Environ. Chem. Eng. 2021, 9, 105882. [Google Scholar] [CrossRef]
- Barambu, N.U.; Bilad, M.R.; Bustam, M.A.; Huda, N.; Jaafar, J.; Narkkun, T.; Faungnawakij, K. Development of Polysulfone Membrane via Vapor-Induced Phase Separation for Oil/Water Emulsion Filtration. Polymers 2020, 12, 2519. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chao, C.M.; Wang, D.K.; Liu, K.M.; Tseng, H.H. Enhancing the antifouling properties of a PVDF membrane for protein separation by grafting branch-like zwitterions via a novel amphiphilic SMA-HEA linker. J. Membr. Sci. 2021, 624, 119126. [Google Scholar] [CrossRef]
- Wang, R.; Hu, Q.H.; Wang, Q.Y.; Xiang, Y.L.; Huang, S.H.; Liu, Y.Z.; Li, S.Y.; Chen, Q.L.; Zhou, Q.H. Efficiently selective removal of Pb(II) by magnetic ion-imprinted membrane based on polyacrylonitrile electro-spun nanofibers. Sep. Purif. Technol. 2022, 284, 120280. [Google Scholar] [CrossRef]
- Ye, S.; Zhou, X.; Xu, Y.; Lai, W.; Yan, K.; Huang, L.; Ling, J.; Zheng, L. Photocatalytic performance of multi-walled carbon nanotube/BiVO4 synthesized by electro-spinning process and its degradation mechanisms on oxytetracycline. Chem. Eng. J. 2019, 373, 880–890. [Google Scholar] [CrossRef]
- Cheng, H.; Zhou, Z.; Liu, T. Electro-spinning fabrication of nitrogen, phosphorus co-doped porous carbon nanofiber as an electro-chemiluminescent sensor for the determination of cyproheptadine. RSC Adv. 2020, 10, 23091–23096. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Hararak, B.; Fernando, G.F. Improved procedure for electro-spinning and carbonisation of neat solvent-fractionated softwood Kraft lignin. Sci. Rep. 2021, 11, 16237. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, A.; Shafiee, A.; Aliahmad, N.; Agarwal, M. Overview of nano-fiber mats fabrication via electrospinning and morphology analysis. Textiles 2021, 1, 206–226. [Google Scholar] [CrossRef]
- Kotrotsos, A. An innovative synergy between solution electrospinning process technique and self-healing of materials. A critical review. Polym. Eng. Sci. 2021, 61, 5–21. [Google Scholar] [CrossRef]
- Rihova, M.; Ince, A.E.; Cicmancova, V.; Hromadko, L.; Castkova, K.; Pavlinak, D.; Vojtova, L.; Macak, J.M. Water-born 3D nanofiber mats using cost-effective centrifugal spinning: Comparison with electrospinning process: A complex study. J. Appl. Polym. Sci. 2021, 138, 49975. [Google Scholar] [CrossRef]
- Mailley, D.; Hebraud, A.; Schlatter, G. A review on the impact of humidity during electrospinning: From the nanofiber structure engineering to the applications. Macromol. Mater. Eng. 2021, 306, 2100115. [Google Scholar] [CrossRef]
- Kugarajah, V.; Ojha, A.K.; Ranjan, S.; Dasgupta, N.; Ganesapillai, M.; Dharmalingam, S.; Elmoll, A.; Hosseini, S.A.; Muthulakshmi, L.; Vijayakumar, S.; et al. Future applications of electrospun nanofibers in pressure driven water treatment: A brief review and research update. J. Environ. Chem. Eng. 2021, 9, 105107. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, J.; Lu, T.; Tang, G.; Wu, S.; Ma, W.; Huang, C. Robust, functionalized reduced graphene-based nanofibrous membrane for contaminated water purification. Chem. Eng. J. 2021, 404, 126347. [Google Scholar] [CrossRef]
- Cao, M.; Chen, Y.; Huang, X.; Sun, L.; Xu, J.; Yang, K.; Zhao, X.; Lin, L. Construction of PA6-rGO nanofiber membrane via electrospraying combining electrospinning processes for emulsified oily sewage purification. J. Taiwan Inst. Chem. Eng. 2021, 118, 232–244. [Google Scholar] [CrossRef]
- Subramanian, S.; Seeram, R. New directions in nanofiltration applications-Are nanofibers the right materials as membranes in desalination? Desalination 2013, 308, 198–208. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, F.; Zhang, H. Emulsion electrospinning: Fundamentals, food applications and prospects. Trends Food Sci. Technol. 2018, 80, 175–186. [Google Scholar] [CrossRef]
- Lett, J.A.; Sagadevan, S.; Fatimah, I.; Hoque, M.E.; Lokanathan, Y.; Léonard, E.; Alshahateet, S.F.; Schirhagl, R.; Oh, W.C. Recent advances in natural polymer-based hydroxyapatite scaffolds: Properties and applications. Eur. Polym. J. 2021, 148, 110360. [Google Scholar] [CrossRef]
- Zhou, T.; Yao, Y.; Xiang, R.; Wu, Y. Formation and characterization of polytetrafluoroethylene nanofiber membranes for vacuum membrane distillation. J. Membr. Sci. 2014, 453, 402–408. [Google Scholar] [CrossRef]
- Feng, M.; Wu, Y.; Feng, Y.; Dong, Y.; Liu, Y.; Peng, J.; Wang, N.; Xu, S.; Wang, D. Highly wearable, machine-washable, and self-cleaning fabric-based triboelectric nanogenerator for wireless drowning sensors. Nano Energy 2022, 93, 106835. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, C.; Huang, Q.; Liu, H.; Guo, Z.; Sun, K. Robust preparation of tubular PTFE/FEP ultrafine fibers-covered porous membrane by electrospinning for continuous highly effective oil/water separation. J. Membr. Sci. 2018, 568, 87–96. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, Q.; Liu, H.; Xiao, C.; Sun, K. A facile and environmental-friendly strategy for preparation of poly (tetrafluoroethylene-co-hexafluoropropylene) hollow fiber membrane and its membrane emulsification performance. Chem. Eng. J. 2020, 384, 123345. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, C.; Huang, Q.; Liu, H.; Zhao, J.C. Progress on polymeric hollow fiber membrane preparation technique from the perspective of green and sustainable development. Chem. Eng. J. 2021, 403, 126295. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Ghoneim, R.; Ezzat, H.A.; Yahia, I.S.; Elhaes, H.; Ibrahim, M.A. Electronic and physical studies for Teflon FEP as a thermal control in low earth orbit reinforced with ZnO and SiO2 nanoparticles. J. Mol. Model. 2021, 27, 295. [Google Scholar] [CrossRef]
- Chen, K.; Xiao, C.; Huang, Q.; Liu, H.; Liu, H.; Wu, Y.; Liu, Z. Study on vacuum membrane distillation (VMD) using FEP hollow fiber membrane. Desalination 2015, 375, 24–32. [Google Scholar] [CrossRef]
- Wu, T.; Moghadam, F.; Li, K. High-performance porous graphene oxide hollow fiber membranes with tailored pore sizes for water purification. J. Membr. Sci. 2022, 645, 120216. [Google Scholar] [CrossRef]
- Chen, H.; Li, C.; Liu, L.; Meng, B.; Yang, N.; Sunarso, J.; Liu, L.; Liu, S.; Wang, X. ZIF-67 membranes supported on porous ZnO hollow fibers for hydrogen separation from gas mixtures. J. Membr. Sci. 2022, 653, 120550. [Google Scholar] [CrossRef]
- Jahan, Z.; Niazi, M.B.K.; Hägg, M.B.; Gregersen, Ø.W. Decoupling the effect of membrane thickness and CNC concentration in PVA based nanocomposite membranes for CO2/CH4 separation. Sep. Purif. Technol. 2018, 204, 220–225. [Google Scholar] [CrossRef]
- Jahan, Z.; Niazi, M.B.K.; Hagg, M.B.; Gregersen, Ø.W.; Hussain, A. Phosphorylated nanocellulose fibrils/PVA nanocomposite membranes for biogas upgrading at higher pressure. Sep. Sci. Technol. 2020, 55, 1524–1534. [Google Scholar] [CrossRef]
- Jahan, Z.; Niazi, M.B.K.; Hägg, M.B.; Gregersen, Ø.W. Cellulose nanocrystal/PVA nanocomposite membranes for CO2/CH4 separation at high pressure. J. Membr. Sci. 2018, 554, 275–281. [Google Scholar] [CrossRef]
- Bonyadi, E.; Niknejad, A.S.; Ashtiani, F.Z.; Bazgir, S.; Kargari, A. A well-designed polystyrene/polycarbonate membrane for highly saline water desalination using DCMD process. Desalination 2022, 528, 115604. [Google Scholar] [CrossRef]
- Lalia, B.S.; Guillen-Burrieza, E.; Arafat, H.A.; Hashaikeh, R. Fabrication and characterization of polyvinylidenefluoride-co-hexafluoropropylene (PVDF-HFP) electrospun membranes for direct contact membrane distillation. J. Membr. Sci. 2013, 428, 104–115. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Gutierrez, L.; Derese, S.; Nxumalo, E.N.; Verliefde, A.R.; Mamba, B.B.; Mhlanga, S.D. A review of nanoparticle-enhanced membrane distillation membranes: Membrane synthesis and applications in water treatment. J. Chem. Technol. Biotechnol. 2019, 94, 2757–2771. [Google Scholar] [CrossRef]
- Gustafson, R.D.; McGaughey, A.L.; Ding, W.; McVety, S.C.; Childress, A.E. Morphological changes and creep recovery behavior of expanded polytetrafluoroethylene (ePTFE) membranes used for membrane distillation. J. Membr. Sci. 2019, 584, 236–245. [Google Scholar] [CrossRef]
- Ramlow, H.; Ferreira, R.K.M.; Marangoni, C.; Machado RA, F. Ceramic membranes applied to membrane distillation: A comprehensive review. Int. J. Appl. Ceram. Technol. 2019, 16, 2161–2172. [Google Scholar] [CrossRef]

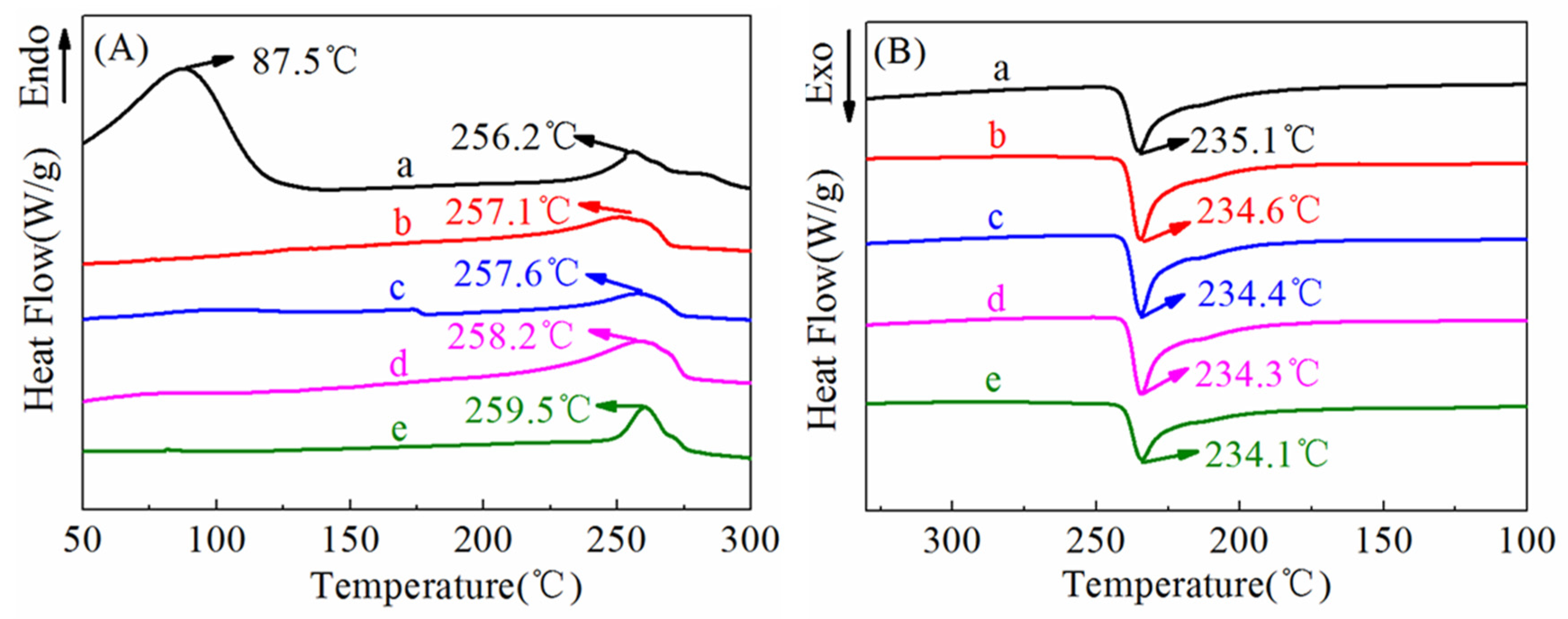


| Sintering Temperature (°C) | Tm (°C) | Tc (°C) | ΔHm (J/g) | Xc (%) |
|---|---|---|---|---|
| - | 256.2 | 235.1 | 27.4 | 31.2 |
| 260 | 257.1 | 234.6 | 35.9 | 40.9 |
| 280 | 257.6 | 234.4 | 39.1 | 44.5 |
| 300 | 258.2 | 234.3 | 40.9 | 46.6 |
| 320 | 259.5 | 234.1 | 45.2 | 51.8 |
| Sintering Temperature (°C) | - | 260 | 280 | 300 | 320 |
|---|---|---|---|---|---|
| WCA (°) | 0 | 58.9 ± 1.9 | 88.8 ± 1.8 | 124.2 ± 2.1 | 131.9 ± 1.7 |
| Sintering Temperature (°C) | 280 | 300 | 320 | |
|---|---|---|---|---|
| Thickness (μm) | 43 ± 5 | 48 ± 2 | 82 ± 3 | 86 ± 2 |
| Porosity (%) | 96.1 ± 1 | 84.0 ± 2 | 62.7 ± 1 | 31.5 ± 1 |
| N2 flux (m3·m−2·h−1) | - | - | 20.2 ± 3 | 1.37 ± 2 |
| LEP (MPa) | - | 0.04 | 0.18 | 0.32 |
| Membrane Code | Porosity (%) | Feed Solution | Feed Temperature (°C) | Vacuum (MPa) | Permeate Flux (L·m−2·h−1) | Reference |
|---|---|---|---|---|---|---|
| PVDF flat-sheet | - | 3.5 wt.% NaCl | 30 | 0.05 | 12.6 | [38] |
| PTFE flat-sheet | 60.0 | 5 wt.% Acetone | 80 | 0.07 | 12.5 | [30] |
| PTFE ultrafine fiber | 79.8 | 3.5 wt.% NaCl | 80 | 0.03 | 15.9 | [40] |
| FEP ultrafine fiber | 62.7 | 3.5 wt.% NaCl | 80 | 0.06 | 15.1 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Ling, H.; Liu, H.; Zhao, W.; Xiao, C. Design of Robust FEP Porous Ultrafiltration Membranes by Electrospinning-Sintered Technology. Polymers 2022, 14, 3802. https://doi.org/10.3390/polym14183802
Chen K, Ling H, Liu H, Zhao W, Xiao C. Design of Robust FEP Porous Ultrafiltration Membranes by Electrospinning-Sintered Technology. Polymers. 2022; 14(18):3802. https://doi.org/10.3390/polym14183802
Chicago/Turabian StyleChen, Kaikai, Haoyang Ling, Hailiang Liu, Wei Zhao, and Changfa Xiao. 2022. "Design of Robust FEP Porous Ultrafiltration Membranes by Electrospinning-Sintered Technology" Polymers 14, no. 18: 3802. https://doi.org/10.3390/polym14183802
APA StyleChen, K., Ling, H., Liu, H., Zhao, W., & Xiao, C. (2022). Design of Robust FEP Porous Ultrafiltration Membranes by Electrospinning-Sintered Technology. Polymers, 14(18), 3802. https://doi.org/10.3390/polym14183802







