Hybrid-Based Wound Dressings: Combination of Synthetic and Biopolymers
Abstract
1. Introduction
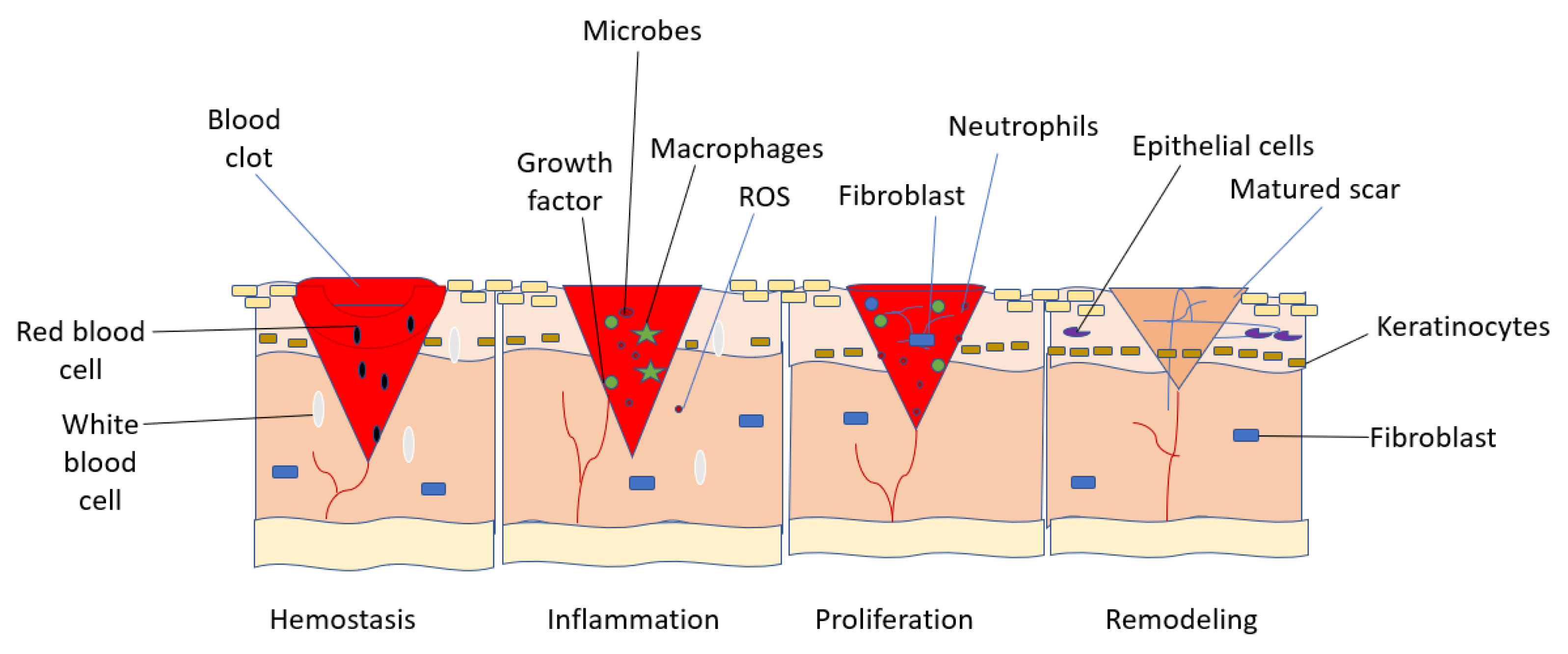
2. Wound Healing Mechanisms
- Hemostasis
- Inflammation
- Proliferation
- Remodeling
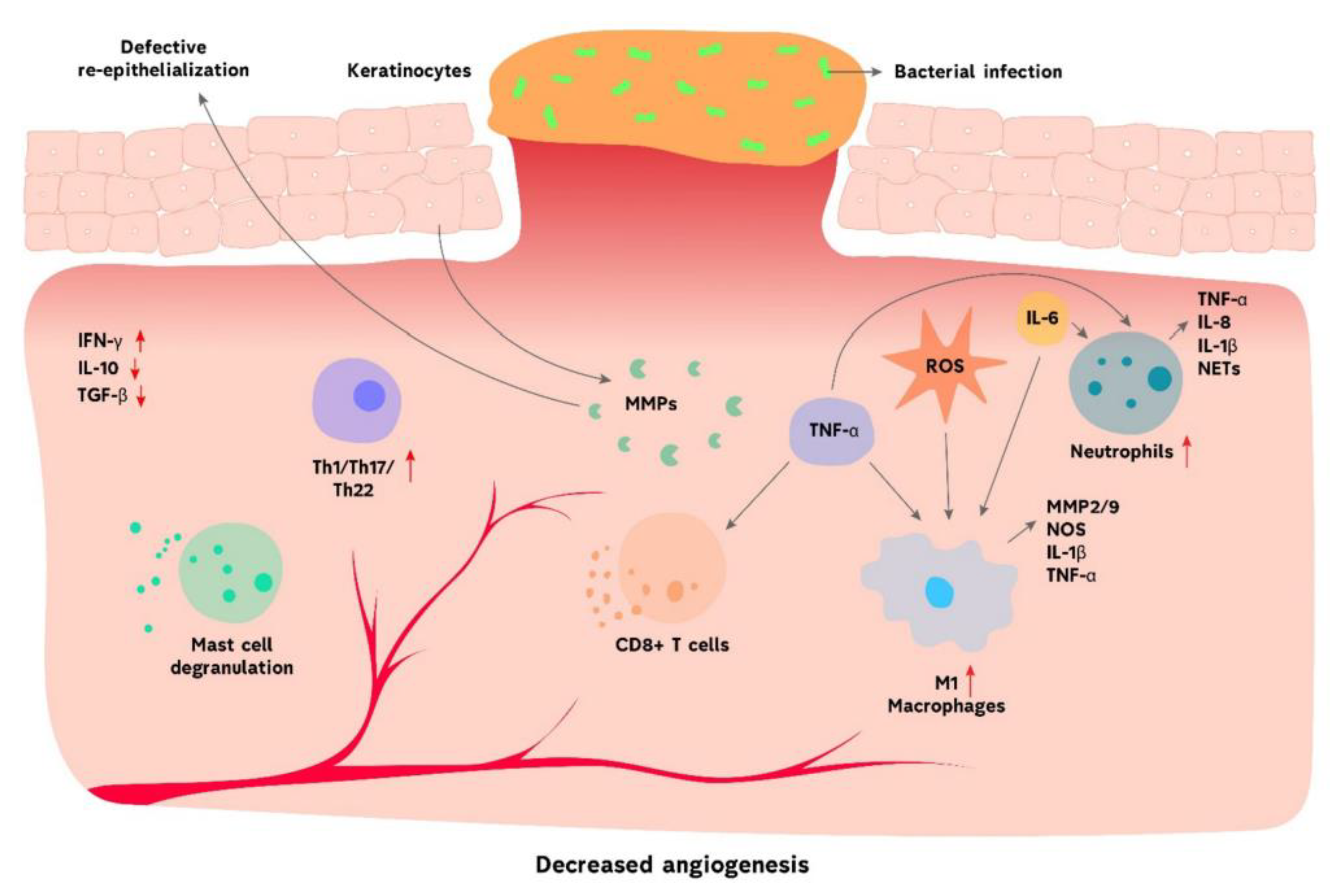
Chronic Wounds
Pathophysiology of Chronic Wounds
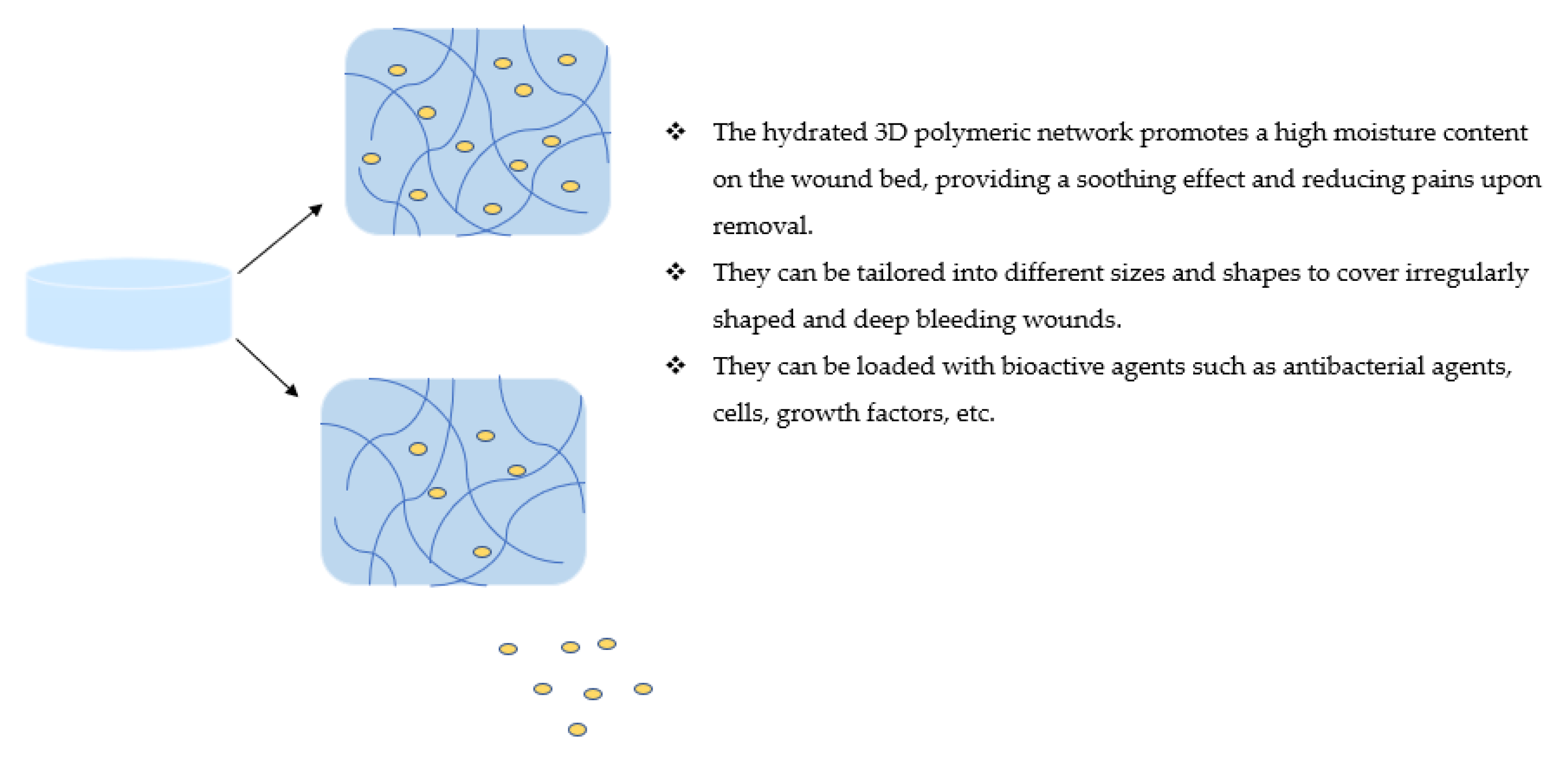
3. Hybrid Wound Dressings in Wound Healing
3.1. Foams
3.2. Hydrocolloids
3.3. Hydrogels
3.4. Nanofibers
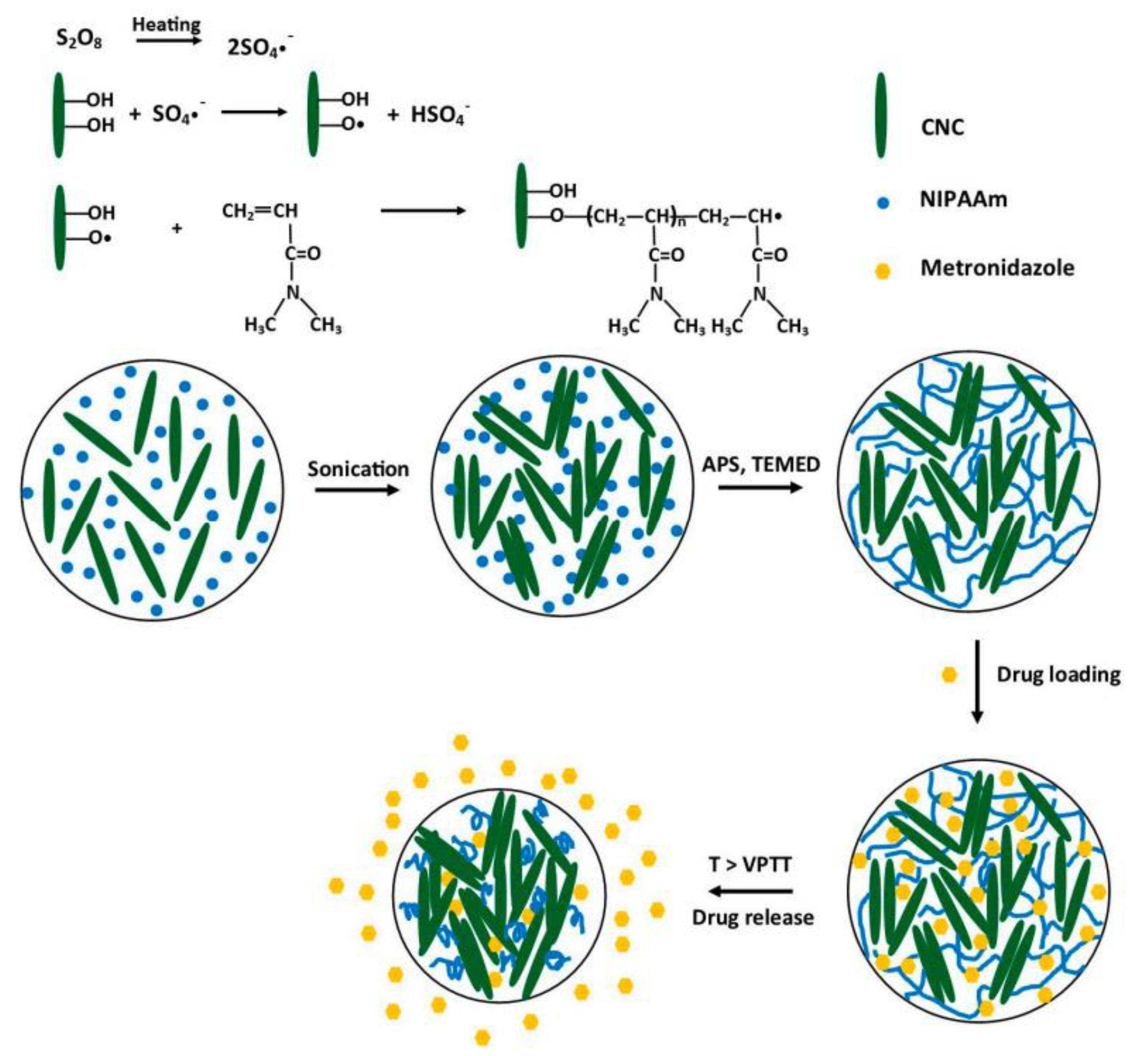
3.5. Nanogels
3.6. Films/Membranes
4. Future Perspective and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed]
- Pfalzgraff, A.; Brandenburg, K.; Weindl, G. Antimicrobial peptides and their therapeutic potential for bacterial skin infections and wounds. Front. Pharmacol. 2018, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Hacker, M.C.; Krieghoff, J.; Mikos, A.G. Synthetic Polymers. In Principles of Regenerative Medicine; Academic Press: Cambridge, MA, USA, 2019; pp. 559–590. [Google Scholar]
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef]
- Thomas, S.; Visakh, P.; Mathew, A.P. Advances in Natural Polymers. In Advanced Structured Materials; Sabu, T., Visakh, P.M., Mathew, A.P., Eds.; Springer: Cham, Switzerland, 2013. [Google Scholar]
- Rajeswari, S.; Prasanthi, T.; Sudha, N.; Swain, R.P.; Panda, S.; Goka, V. Natural polymers: A recent review. World J. Pharm. Pharm. Sci. 2017, 6, 472–494. [Google Scholar] [CrossRef][Green Version]
- Sionkowska, A. Current research on the blends of natural and synthetic polymers as new biomaterials. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Khan, M.F.; Akram, N.; Akhter, N.; Noreen, A.; Zuber, M. Recent trends on gellan gum blends with natural and synthetic polymers: A review. Int. J. Biol. Macromol. 2018, 109, 1068–1087. [Google Scholar] [CrossRef]
- Zilberman, M.; Egozi, D.; Shemesh, M.; Keren, A.; Mazor, E.; Baranes-Zeevi, M.; Goldstein, N.; Berdicevsky, I.; Gilhar, A.; Ullmann, Y. Hybrid wound dressings with controlled release of antibiotics: Structure-release profile effects and in vivo study in a guinea pig burn model. Acta Biomater. 2015, 22, 155–163. [Google Scholar] [CrossRef]
- Follmann, H.D.; Messias, I.; Queiroz, M.N.; Araujo, R.A.; Rubira, A.F.; Silva, R. Designing hybrid materials with multifunctional interfaces for wound dressing, electrocatalysis, and chemical separation. J. Colloid Interf. Sci. 2019, 533, 106–125. [Google Scholar] [CrossRef]
- Romić, M.D.; Špoljarić, D.; Klarić, M.Š.; Cetina-Čižmek, B.; Filipović-Grčić, J.; Hafner, A. Melatonin loaded lipid enriched chitosan microspheres–Hybrid dressing for moderate exuding wounds. J. Drug Deliv. Sci.Technol. 2019, 52, 431–439. [Google Scholar] [CrossRef]
- Mahsood, R.; Miraftab, M. Novel materials for moist wound management: Alginate-psyllium hybrid fibres. J. Wound Care. 2014, 23, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xi, Y.; Xue, Y.; Wang, M.; Liu, Y.; Guo, Y.; Lei, B. Injectable self-healing antibacterial bioactive polypeptide-based hybrid nanosystems for efficiently treating multidrug resistant infection, skin-tumor therapy, and enhancing wound healing. Adv. Funct. Mater. 2019, 29, 1806883. [Google Scholar] [CrossRef]
- Cheng, W.; Wang, M.; Chen, M.; Niu, W.; Li, Y.; Wang, Y.; Luo, M.; Xie, C.; Leng, T.; Lei, B. Injectable antibacterial antiinflammatory molecular hybrid hydrogel dressing for rapid MDRB-infected wound repair and therapy. Chem. Eng. J. 2021, 409, 128140. [Google Scholar] [CrossRef]
- Mercandetti, M.; Cohen, A.J. Wound healing and repair. EMedicine 2017, 14, 12–20. [Google Scholar]
- Dryden, S.V.; Shoemaker, W.G.; Kim, J.H. Wound management and nutrition for optimal wound healing. Atlas Oral Maxillofac. Surg. Clin. N. Am. 2013, 21, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Orgill, D.P.; Murphy, G.F. The Pathophysiologic Basis for Wound Healing and Cutaneous Regeneration. In Biomaterials for Treating Skin Loss; Woodhead Publishing: Sawston, UK, 2009; pp. 25–57. [Google Scholar]
- Guo, B.; Dong, R.; Liang, Y.; Li, M. Haemostatic materials for wound healing applications. Nat. Rev. Chem. 2021, 5, 773–791. [Google Scholar] [CrossRef]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef]
- Gardiner, E.E.; Andrews, R.K.; Corey, S.J. (Eds.) Structure and Function of Platelet Receptors Initiating Blood Clotting. A Systems Biology Approach to Blood. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 263–275. [Google Scholar]
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Kingsley, K.; Huff, J.L.; Rust, W.L.; Carroll, K.; Martinez, A.M.; Fitchmun, M.; Plopper, G.E. ERK1/2 mediates PDGF-BB stimulated vascular smooth muscle cell proliferation and migration on laminin-5. Biochem. Biophys. Res. Commun. 2002, 293, 1000–1006. [Google Scholar] [CrossRef]
- Schultz, G.S.; Chin, G.A.; Moldawer, L.; Diegelmann, R.F. Principles of Wound Healing. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; Fitridge, R., Thompson, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011; p. 23. Available online: https://www.ncbi.nlm.nih.gov/books/NBK534261/ (accessed on 30 June 2022).
- Wallace, H.A.; Basehore, B.M.; Zito, P.M. Wound Healing Phases. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of wound healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.; Young, A.; McNaught, C.E. The physiology of wound healing. Surgery 2014, 32, 445–450. [Google Scholar] [CrossRef]
- Young, A.; McNaught, C.E. The physiology of wound healing. Surgery 2011, 29, 475–479. [Google Scholar] [CrossRef]
- Dissemond, J.; Goos, M.; Wagner, S.N. The role of oxidative stress in the pathogenesis and therapy of chronic wounds. Hautarzt Z. Dermatol. Venerol. Verwandte Geb. 2002, 53, 718–723. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Das, A.; Sinha, M.; Datta, S.; Abas, M.; Chaffee, S.; Sen, C.K.; Roy, S. Monocyte and macrophage plasticity in tissue repair and regeneration. Am. J. Pathol. 2015, 185, 2596–2606. [Google Scholar] [CrossRef]
- Crane, M.J.; Daley, J.M.; van Houtte, O.; Brancato, S.K.; Henry, W.L., Jr.; Albina, J.E. The monocyte to macrophage transition in the murine sterile wound. PLoS ONE 2014, 9, e86660. [Google Scholar]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef]
- Gauglitz, G.G.; Korting, H.C.; Pavicic, T.; Ruzicka, T.; Jeschke, M.G. Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol. Med. 2011, 17, 113–125. [Google Scholar] [CrossRef]
- Shah, A.; Amini-Nik, S. The role of phytochemicals in the inflammatory phase of wound healing. Int. J. Mol. Sci. 2017, 18, 1068. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Skin Wound Care. 2012, 25, 304. [Google Scholar] [CrossRef]
- Yang, J.H.; Yoon, J.Y.; Moon, J.; Min, S.; Kwon, H.H.; Suh, D.H. Expression of inflammatory and fibrogenetic markers in acne hypertrophic scar formation: Focusing on role of TGF-β and IGF-1R. Arch. Dermatol. Res. 2018, 310, 665–673. [Google Scholar] [CrossRef]
- Lin, Z.Q.; Kondo, T.; Ishida, Y.; Takayasu, T.; Mukaida, N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J. Leukoc. Biol. 2003, 73, 713–721. [Google Scholar] [CrossRef]
- Ben Amar, M.; Wu, M. Re-epithelialization: Advancing epithelium frontier during wound healing. J. R. Soc. Interface 2014, 11, 20131038. [Google Scholar] [CrossRef]
- Yannas, I.V.; Tzeranis, D.S.; So, P.T. Regeneration of injured skin and peripheral nerves requires control of wound contraction, not scar formation. Wound Repair Regen. 2017, 25, 177–191. [Google Scholar] [CrossRef]
- Plikus, M.V.; Guerrero-Juarez, C.F.; Ito, M.; Li, Y.R.; Dedhia, P.H.; Zheng, Y.; Shao, M.; Gay, D.L.; Ramos, R.; His, T.C.; et al. Regeneration of fat cells from myofibroblasts during wound healing. Science 2017, 355, 748–752. [Google Scholar] [CrossRef]
- Agale, S.V. Chronic leg ulcers: Epidemiology, aetiopathogenesis, and management. Ulcers 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Arif, M.M.; Khan, S.M.; Gull, N.; Tabish, T.A.; Zia, S.; Khan, R.U.; Awais, S.M.; Butt, M.A. Polymer-based biomaterials for chronic wound management: Promises and challenges. Int. J. Pharm. 2021, 598, 120270. [Google Scholar] [CrossRef]
- van Koppen, C.J.; Hartmann, R.W. Advances in the treatment of chronic wounds: A patent review. Expert Opin. Ther. Pat. 2015, 25, 931–937. [Google Scholar] [CrossRef]
- Nirantharakumar, K.; Saeed, M.; Wilson, I.; Marshall, T.; Coleman, J.J. In-hospital mortality and length of stay in patients with diabetes having foot disease. J. Diabetes Complicat. 2013, 27, 454–458. [Google Scholar] [CrossRef]
- Nunan, R.; Harding, K.G.; Martin, P. Clinical challenges of chronic wounds: Searching for an optimal animal model to recapitulate their complexity. Dis. Models Mech. 2014, 7, 1205–1213. [Google Scholar] [CrossRef]
- FrykbergRobert, G. Challenges in the treatment of chronic wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of acute and chronic wound healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef]
- Zhong, W. Efficacy and toxicity of antibacterial agents used in wound dressings. Cutan. Ocul. Toxicol. 2015, 34, 61–67. [Google Scholar] [CrossRef]
- Khaliq, T.; Sohail, M.; Minhas, M.U.; Shah, S.A.; Jabeen, N.; Khan, S.; Hussain, Z.; Mahmood, A.; Kousar, M.; Rashid, H. Self-crosslinked chitosan/κ-carrageenan-based biomimetic membranes to combat diabetic burn wound infections. Int. J. Biol. Macromol. 2022, 197, 157–168. [Google Scholar] [CrossRef]
- Thomas, D.C.; Tsu, C.L.; Nain, R.A.; Arsat, N.; Fun, S.S.; Lah, N.A.S.N. The role of debridement in wound bed preparation in chronic wound: A narrative review. Ann. Med. Surg. 2021, 71, 102876. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The role of oxidative stress and antioxidants in diabetic wound healing. Oxid. Med. Cell. Longev. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, S.; Cheng, F.; He, X.; Liu, Z.; Wang, W.; Zhou, L.; Zhang, Q. Bioactive anti-inflammatory, antibacterial, conductive multifunctional scaffold based on MXene@ CeO2 nanocomposites for infection-impaired skin multimodal therapy. Chem. Eng. J. 2021, 424, 130148. [Google Scholar] [CrossRef]
- Skórkowska-Telichowska, K.; Czemplik, M.; Kulma, A.; Szopa, J. The local treatment and available dressings designed for chronic wounds. J. Am. Acad. Dermatol. 2013, 68, e117–e126. [Google Scholar] [CrossRef]
- Xu, Z.; Liang, B.; Tian, J.; Wu, J. Anti-inflammation biomaterial platforms for chronic wound healing. Biomater. Sci. 2021, 9, 4388–4409. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Gao, M.; Boakye-Yiadom, K.O.; Ho, W.; Yu, W.; Xu, X.; Zhang, X.Q. An intrinsically bioactive hydrogel with on-demand drug release behaviors for diabetic wound healing. Bioact. Mater. 2021, 6, 4592–4606. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhao, H.; Tu, C.; Xu, Z.; Ye, L.; Zhao, L.; Gu, Z.; Zhao, D.; Zhang, J.; Feng, Z. In situ hydrogel dressing loaded with heparin and basic fibroblast growth factor for accelerating wound healing in rat. Mater. Sci. Eng. C 2020, 116, 111169. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Hong, Y.L.; Wu, T.L. Novel silver and nanoparticle-encapsulated growth factor co-loaded chitosan composite hydrogel with sustained antimicrobility and promoted biological properties for diabetic wound healing. Mater. Sci. Eng. C 2021, 118, 111385. [Google Scholar] [CrossRef]
- Jones, V.; Grey, J.E.; Harding, K.G. Wound dressings. BMJ 2006, 332, 777–780. [Google Scholar] [CrossRef]
- Nielsen, J.; Fogh, K. Clinical utility of foam dressings in wound management: A review. Chronic. Wound Care Manag. Res. 2015, 2, 31. [Google Scholar]
- Bullough, L.; Johnson, S.; Forder, R. Evaluation of a foam dressing for acute and chronic wound exudate management. Br. J. Commun. Nurs. 2015, 20, S17–S24. [Google Scholar] [CrossRef]
- Charles, H.; Corser, R.; Varrow, S.; Hart, J. A non-adhesive foam dressing for exuding venous leg ulcers and pressure ulcers: Six case studies. J. Wound Care 2004, 13, 58–62. [Google Scholar] [CrossRef]
- Morena, A.G.; Stefanov, I.; Ivanova, K.; Pérez-Rafael, S.; Sánchez-Soto, M.; Tzanov, T. Antibacterial polyurethane foams with incorporated lignin-capped silver nanoparticles for chronic wound treatment. Ind. Eng. Chem. Res. 2020, 59, 4504–4514. [Google Scholar] [CrossRef]
- Oh, S.T.; Kim, W.R.; Kim, S.H.; Chung, Y.C.; Park, J.S. The preparation of polyurethane foam combined with pH-sensitive alginate/bentonite hydrogel for wound dressings. Fibers Polym. 2011, 12, 159–165. [Google Scholar] [CrossRef]
- Wojcik, M.; Kazimierczak, P.; Benko, A.; Palka, K.; Vivcharenko, V.; Przekora, A. Superabsorbent curdlan-based foam dressings with typical hydrocolloids properties for highly exuding wound management. Mater. Sci. Eng. C 2021, 124, 112068. [Google Scholar] [CrossRef] [PubMed]
- Vivcharenko, V.; Wojcik, M.; Palka, K.; Przekora, A. Highly porous and superabsorbent biomaterial made of marine-derived polysaccharides and ascorbic acid as an optimal dressing for exuding wound management. Materials 2021, 14, 1211. [Google Scholar] [CrossRef] [PubMed]
- Namviriyachote, N.; Lipipun, V.; Akkhawattanangkul, Y.; Charoonrut, P.; Ritthidej, G.C. Development of polyurethane foam dressing containing silver and asiaticoside for healing of dermal wound. Asian J. Pharm. Sci. 2019, 14, 63–77. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Ou, F.; Wu, Y.; Sun, X.; Chen, X.; Li, H.; Sun, D.; Zhang, L. Smart multi-layer PVA foam/CMC mesh dressing with integrated multi-functions for wound management and infection monitoring. Mater. Des. 2020, 194, 108913. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings–a review. BioMedicine 2015, 5, 1–5. [Google Scholar] [CrossRef]
- Fletcher, J.; Moore, Z.; Anderson, I.; Matsuzaki, K. Pressure ulcers and hydrocolloids made easy. Wound Int. 2011, 2, 1–6. [Google Scholar]
- Lohi, J.; Sipponen, A.; Jokinen, J.J. Local dressings for pressure ulcers: What is the best tool to apply in primary and second care? J. Wound Care 2010, 19, 123–127. [Google Scholar] [CrossRef]
- Sweeney, I.R.; Miraftab, M.; Collyer, G. A critical review of modern and emerging absorbent dressings used to treat exuding wounds. Int. Wound J. 2012, 9, 601–612. [Google Scholar] [CrossRef]
- Meaume, S.; Gemmen, E. Cost effectiveness and wound management in France: Pressure ulcers and venous leg ulcers. J. Wound Care 2002, 11, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J. The benefits of using hydrocolloids. Nurs. Times 2003, 9, 57. [Google Scholar]
- Lee, O.J.; Kim, J.H.; Moon, B.M.; Chao, J.R.; Yoon, J.; Ju, H.W.; Lee, J.M.; Park, H.J.; Kim, D.W.; Kim, S.J.; et al. Fabrication and characterization of hydrocolloid dressing with silk fibroin nanoparticles for wound healing. Tissue Eng. Regen. Med. 2016, 13, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.G.; Kim, K.S.; Yousaf, A.M.; Kim, D.W.; Jang, S.W.; Son, M.W.; Kim, Y.H.; Yong, C.S.; Kim, J.O.; Choi, H.G. Mechanical properties and in vivo healing evaluation of a novel Centella asiatica-loaded hydrocolloid wound dressing. Int. J. Pharm. 2015, 490, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels-a review. Am. J. Polym. Sci 2014, 4, 25–31. [Google Scholar]
- Wang, W.; Bai, H.; Zhao, Y.; Kang, S.; Yi, H.; Zhang, T.; Song, S. Synthesis of chitosan cross-linked 3D network-structured hydrogel for methylene blue removal. Int. J. Biol. Macromol. 2019, 141, 98–107. [Google Scholar] [CrossRef]
- Kumar, A.; Jaiswal, M. Design and in vitro investigation of nanocomposite hydrogel based in situ spray dressing for chronic wounds and synthesis of silver nanoparticles using green chemistry. J. Appl. Polym. Sci. 2016, 133, 1–14. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced hydrogels as wound dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Gonzalez, J.S.; Ludueña, L.N.; Ponce, A.; Alvarez, V.A. Poly (vinyl alcohol)/cellulose nanowhiskers nanocomposite hydrogels for potential wound dressings. Mater. Sci. Eng. C 2014, 34, 54–61. [Google Scholar] [CrossRef]
- Gupta, A.; Kowalczuk, M.; Heaselgrave, W.; Britland, S.T.; Martin, C.; Radecka, I. The production and application of hydrogels for wound management: A review. Eur. Polym. J. 2019, 111, 134–151. [Google Scholar] [CrossRef]
- Wu, H.; Qin, Z.; Yu, X.; Li, J.; Lv, H.; Yang, X. On-demand removable hydrogels based on photolabile cross-linkings as wound dressing materials. J. Mater. Chem. B 2019, 7, 5669–5676. [Google Scholar] [CrossRef]
- Yin, M.; Wang, X.; Yu, Z.; Wang, Y.; Wang, X.; Deng, M.; Zhao, D.; Ji, S.; Jia, N.; Zhang, W. γ-PGA hydrogel loaded with cell-free fat extract promotes the healing of diabetic wounds. J. Mater. Chem. B 2020, 8, 8395–8404. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Zhu, C.; Lei, H.; Fan, D.; Duan, Z.; Li, X.; Li, Y.; Cao, J.; Wang, S.; Yu, Y. Novel enzymatic crosslinked hydrogels that mimic extracellular matrix for skin wound healing. J. Mater. Sci. 2018, 53, 5909–5928. [Google Scholar] [CrossRef]
- Sood, N.; Bhardwaj, A.; Mehta, S.; Mehta, A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2016, 23, 748–770. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; An, X.; Fan, Z. Aramid nanofibers reinforced polyvinyl alcohol/tannic acid hydrogel with improved mechanical and antibacterial properties for potential application as wound dressing. J. Mech. Behav. Biomed. Mater. 2021, 118, 104452. [Google Scholar] [CrossRef]
- Kong, F.; Fan, C.; Yang, Y.; Lee, B.H.; Wei, K. 5-hydroxymethylfurfural-embedded poly (vinyl alcohol)/sodium alginate hybrid hydrogels accelerate wound healing. Int. J. Biol. Macromol. 2019, 138, 933–949. [Google Scholar] [CrossRef]
- Zubik, K.; Singhsa, P.; Wang, Y.; Manuspiya, H.; Narain, R. Thermo-responsive poly (N-isopropylacrylamide)-cellulose nanocrystals hybrid hydrogels for wound dressing. Polymers 2017, 9, 119. [Google Scholar] [CrossRef]
- Rasool, A.; Ata, S.; Islam, A. Stimuli responsive biopolymer (chitosan) based blend hydrogels for wound healing application. Carbohydr. Polym. 2019, 203, 423–429. [Google Scholar] [CrossRef]
- Xue, H.; Hu, L.; Xiong, Y.; Zhu, X.; Wei, C.; Cao, F.; Zhou, W.; Sun, Y.; Endo, Y.; Liu, M.; et al. Quaternized chitosan-Matrigel-polyacrylamide hydrogels as wound dressing for wound repair and regeneration. Carbohydr. Polym. 2019, 226, 115302. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Adeli, H.; Mansoori-Moghadam, Z.; Moghaddam, A. Design and optimization of process parameters of polyvinyl (alcohol)/chitosan/nano zinc oxide hydrogels as wound healing materials. Carbohydr. Polym. 2019, 207, 542–554. [Google Scholar] [CrossRef]
- Chen, G.; Yu, Y.; Wu, X.; Wang, G.; Ren, J.; Zhao, Y. Bioinspired multifunctional hybrid hydrogel promotes wound healing. Adv. Funct. Mater. 2018, 28, 1801386. [Google Scholar] [CrossRef]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Jangde, R.; Srivastava, S.; Singh, M.R.; Singh, D. In vitro and in vivo characterization of quercetin loaded multiphase hydrogel for wound healing application. Int. J. Biol. Macromol. 2018, 115, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Xuan, H.; Wu, S.; Fei, S.; Li, B.; Yang, Y.; Yuan, H. Injectable nanofiber-polysaccharide self-healing hydrogels for wound healing. Mater. Sci. Eng. C 2021, 128, 112264. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Fu, Y.N.; Wei, Y.; Zhao, L.; Tao, L. Self-adapting hydrogel to improve the therapeutic effect in wound-healing. ACS Appl. Mater. Interfaces 2018, 10, 26046–26055. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Gondil, V.S.; Chhibber, S. A novel wound dressing consisting of PVA-SA hybrid hydrogel membrane for topical delivery of bacteriophages and antibiotics. Int. J. Pharm. 2019, 572, 118779. [Google Scholar] [CrossRef]
- Yin, M.; Wan, S.; Ren, X.; Chu, C.C. Development of inherently antibacterial, biodegradable, and biologically active chitosan/pseudo-protein hybrid hydrogels as biofunctional wound dressings. ACS Appl. Mater. Interfaces. 2021, 13, 14688–14699. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, H.; Liang, Y.; Xuan, M.; Liu, G.; Xie, H. Hyaluronic acid-methacrylic anhydride/polyhexamethylene biguanide hybrid hydrogel with antibacterial and proangiogenic functions for diabetic wound repair. Chin. Chem. Lett. 2022, 33, 5030–5034. [Google Scholar] [CrossRef]
- Liu, T.; Liu, G.; Zhang, J.; Ding, Z.; Li, Y.; Sigdel, K.; Wang, X.; Xie, H. L-Arginine based polyester amide/hyaluronic acid hybrid hydrogel with dual anti-inflammation and antioxidant functions for accelerated wound healing. Chin. Chem. Lett. 2022, 33, 1880–1884. [Google Scholar] [CrossRef]
- Bercea, M.; Gradinaru, L.M.; Morariu, S.; Plugariu, I.A.; Gradinaru, R.V. Tailoring the properties of PVA/HPC/BSA hydrogels for wound dressing applications. React. Funct. Polym. 2022, 170, 105094. [Google Scholar] [CrossRef]
- Bahadoran, M.; Shamloo, A.; Nokoorani, Y.D. Development of a polyvinyl alcohol/sodium alginate hydrogel-based scaffold incorporating bFGF-encapsulated microspheres for accelerated wound healing. Sci. Rep. 2020, 10, 1–18. [Google Scholar]
- Tajik, F.; Eslahi, N.; Rashidi, A.; Rad, M.M. Hybrid antibacterial hydrogels based on PVP and keratin incorporated with lavender extract. J. Polym. Res. 2021, 28, 316. [Google Scholar] [CrossRef]
- Mori, H.M.; Kawanami, H.; Kawahata, H.; Aoki, M. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement. Alternat. Med. 2016, 16, 144. [Google Scholar] [CrossRef]
- Kazemi, M.; Mohammadifar, M.; Aghadavoud, E.; Vakili, Z.; Aarabi, M.H.; Talaei, S.A. Deep skin wound healing potential of lavender essential oil and licorice extract in a nanoemulsion form: Biochemical, histopathological and gene expression evidence. J. Tissue Viability 2020, 29, 116–124. [Google Scholar] [CrossRef]
- Lei, Q.; Zhang, Y.; Zhang, W.; Li, R.; Ao, N.; Zhang, H. A synergy between dopamine and electrostatically bound bactericide in a poly (vinyl alcohol) hybrid hydrogel for treating infected wounds. Carbohydr. Polym. 2021, 272, 118513. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef]
- Andreu, V.; Mendoza, G.; Arruebo, M.; Irusta, S. Smart dressings based on nanostructured fibers containing natural origin antimicrobial, anti-inflammatory, and regenerative compounds. Materials 2015, 8, 5154–5193. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Windbergs, M. Functional electrospun fibers for the treatment of human skin wounds. Eur. J. Pharm. Biopharm. 2017, 119, 283–299. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in nanofibers for wound dressing: A review. Eur. Polym. J. 2019, 117, 304–336. [Google Scholar] [CrossRef]
- de Oliveira, A.E.; Bonfim, D.P.F.; Salussoglia, A.I.P.; Medeiros, G.B.; Guerra, V.G.; Aguiar, M.L. Nanofiber Production Techniques Applied to Filtration Processes. In Environmental, Ethical, and Economical Issues of Nanotechnology; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2022; pp. 31–60. [Google Scholar]
- Liu, X.; Xu, H.; Zhang, M.; Yu, D.G. Electrospun medicated nanofibers for wound healing. Membranes 2021, 11, 770. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, Y.; Yang, Z.; Lin, Q.; Qiao, K.; Chen, X.; Peng, Y. Influence of dialdehyde bacterial cellulose with the nonlinear elasticity and topology structure of ECM on cell adhesion and proliferation. RSC Adv. 2014, 4, 3998–4009. [Google Scholar] [CrossRef]
- Bagheri, M.; Validi, M.; Gholipour, A.; Makvandi, P.; Sharifi, E. Chitosan nanofiber biocomposites for potential wound healing applications: Antioxidant activity with synergic antibacterial effect. Bioeng. Transl. Med. 2022, 7, e10254. [Google Scholar] [CrossRef]
- Teaima, M.H.; Elasaly, M.K.; Omar, S.A.; El-Nabarawi, M.A.; Shoueir, K.R. Wound healing activities of polyurethane modified chitosan nanofibers loaded with different concentrations of linezolid in an experimental model of diabetes. J. Drug Deliv. Sci. Technol. 2022, 67, 102982. [Google Scholar] [CrossRef]
- Amiri, N.; Ajami, S.; Shahroodi, A.; Jannatabadi, N.; Darban, S.A.; Bazzaz, B.S.F.; Pishavar, E.; Kalalinia, F.; Movaffagh, J. Teicoplanin-loaded chitosan-PEO nanofibers for local antibiotic delivery and wound healing. Int. J. Biol. Macromol. 2022, 162, 645–656. [Google Scholar] [CrossRef]
- Hashemikia, S.; Farhangpazhouh, F.; Parsa, M.; Hasan, M.; Hassanzadeh, A.; Hamidi, M. Fabrication of ciprofloxacin-loaded chitosan/polyethylene oxide/silica nanofibers for wound dressing application: In vitro and in vivo evaluations. Int. J. Pharm. 2021, 597, 120313. [Google Scholar] [CrossRef]
- Ionescu, O.M.; Iacob, A.T.; Mignon, A.; Van Vlierberghe, S.; Baican, M.; Danu, M.; Ibănescu, C.; Simionescu, N.; Profire, L. Design, preparation and in vitro characterization of biomimetic and bioactive chitosan/polyethylene oxide based nanofibers as wound dressings. Int. J. Biol. Macromol. 2021, 193, 996–1008. [Google Scholar] [CrossRef]
- Kharat, Z.; Goushki, M.A.; Sarvian, N.; Asad, S.; Dehghan, M.M.; Kabiri, M. Chitosan/PEO nanofibers containing Calendula officinalis extract: Preparation, characterization, in vitro and in vivo evaluation for wound healing applications. Int. J. Pharm. 2021, 609, 121132. [Google Scholar] [CrossRef]
- Kalalinia, F.; Taherzadeh, Z.; Jirofti, N.; Amiri, N.; Foroghinia, N.; Beheshti, M.; Bazzaz, B.S.F.; Hashemi, M.; Shahroodi, A.; Pishavar, E.; et al. Evaluation of wound healing efficiency of vancomycin-loaded electrospun chitosan/poly ethylene oxide nanofibers in full thickness wound model of rat. Int. J. Biol. Macromol. 2021, 177, 100–110. [Google Scholar] [CrossRef]
- Mirhosseini, M.M.; Haddadi-Asl, V.; Zargarian, S.S. Fabrication and characterization of hydrophilic poly (ε-caprolactone)/pluronic P123 electrospun fibers. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Shahrousvand, M.; Haddadi-Asl, V.; Shahrousvand, M. Step-by-step design of poly (ε-caprolactone)/chitosan/Melilotus officinalis extract electrospun nanofibers for wound dressing applications. Int. J. Biol. Macromol. 2021, 180, 36–50. [Google Scholar] [CrossRef]
- Fahimirad, S.; Abtahi, H.; Satei, P.; Ghaznavi-Rad, E.; Moslehi, M.; Ganji, A. Wound healing performance of PCL/chitosan based electrospun nanofiber electrosprayed with curcumin loaded chitosan nanoparticles. Carbohydr. Polym. 2021, 259, 117640. [Google Scholar] [CrossRef]
- Zhou, L.; Cai, L.; Ruan, H.; Zhang, L.; Wang, J.; Jiang, H.; Wu, Y.; Feng, S.; Chen, J. Electrospun chitosan oligosaccharide/polycaprolactone nanofibers loaded with wound-healing compounds of Rutin and Quercetin as antibacterial dressings. Int. J. Biol. Macromol. 2021, 183, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Hussein, Y.; El-Fakharany, E.M.; Kamoun, E.A.; Loutfy, S.A.; Amin, R.; Taha, T.H.; Salim, S.A.; Amer, M. Electrospun PVA/hyaluronic acid/L-arginine nanofibers for wound healing applications: Nanofibers optimization and in vitro bioevaluation. Int. J. Biol. Macromol. 2020, 164, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.; El-Beheri, N.G.; Agwa, M.M.; Eltaher, H.M.; Alseqely, M.; Sadik, W.S.; El-Khordagui, L. Antibiotic-free combinational hyaluronic acid blend nanofibers for wound healing enhancement. Int. J. Biol. Macromol. 2021, 167, 1552–1563. [Google Scholar]
- Ionescu, O.M.; Mignon, A.; Iacob, A.T.; Simionescu, N.; Confederat, L.G.; Tuchilus, C.; Profire, L. New hyaluronic acid/polyethylene oxide-based electrospun nanofibers: Design, characterization and in vitro biological evaluation. Polymers 2021, 13, 1291. [Google Scholar] [CrossRef] [PubMed]
- El-Aassar, M.R.; Ibrahim, O.M.; Fouda, M.M.; El-Beheri, N.G.; Agwa, M.M. Wound healing of nanofiber comprising Polygalacturonic/Hyaluronic acid embedded silver nanoparticles: In-vitro and in-vivo studies. Carbohydr. Polym. 2020, 238, 116175. [Google Scholar] [CrossRef]
- Movahedi, M.; Asefnejad, A.; Rafienia, M.; Khorasani, M.T. Potential of novel electrospun core-shell structured polyurethane/starch (hyaluronic acid) nanofibers for skin tissue engineering: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2020, 146, 627–637. [Google Scholar] [CrossRef]
- Eskandarinia, A.; Kefayat, A.; Gharakhloo, M.; Agheb, M.; Khodabakhshi, D.; Khorshidi, M.; Sheikhmoradi, V.; Rafienia, M.; Salehi, H. A propolis enriched polyurethane-hyaluronic acid nanofibrous wound dressing with remarkable antibacterial and wound healing activities. Int. J. Biol. Macromol. 2020, 149, 467–476. [Google Scholar] [CrossRef]
- Lin, M.; Liu, Y.; Gao, J.; Wang, D.; Xia, D.; Liang, C.; Li, N.; Xu, R. Synergistic effect of co-delivering ciprofloxacin and tetracycline hydrochloride for promoted wound healing by utilizing coaxial PCL/gelatin nanofiber membrane. Int. J. Mol. Sci. 2022, 23, 1895. [Google Scholar] [CrossRef]
- Li, A.; Li, L.; Li, X.; Liang, W.; Lang, M.; Cheng, B.; Li, J. Antibacterial, antioxidant and anti-inflammatory PLCL/gelatin nanofiber membranes to promote wound healing. Int. J. Biol. Macromol. 2022, 194, 914–923. [Google Scholar] [CrossRef]
- Yang, J.; Liu, C.L.; Ding, Y.N.; Sun, T.C.; Bai, X.H.; Cao, Z.K.; Ramakrishna, S.; Zhang, J.; Long, Y.Z. Synergistic antibacterial polyacrylonitrile/gelatin nanofibers coated with metal-organic frameworks for accelerating wound repair. Int. J. Biol. Macromol. 2021, 189, 698–704. [Google Scholar] [CrossRef]
- Sen, S.; Bal, T.; Rajora, A.D. Green nanofiber mat from HLM–PVA–Pectin (Hibiscus leaves mucilage–polyvinyl alcohol–pectin) polymeric blend using electrospinning technique as a novel material in wound-healing process. Appl. Nanosci. 2022, 12, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Bedir, T.; Kalkandelen, C.; Başar, A.O.; Şaşmazel, H.T.; Ustundag, C.B.; Sengor, M.; Gunduz, O. Coaxial and emulsion electrospinning of extracted hyaluronic acid and keratin based nanofibers for wound healing applications. Eur. Polym. J. 2021, 142, 110158. [Google Scholar] [CrossRef]
- Sanchez Ramirez, D.O.; Cruz-Maya, I.; Vineis, C.; Tonetti, C.; Varesano, A.; Guarino, V. Design of asymmetric nanofibers-membranes based on polyvinyl alcohol and wool-keratin for wound healing applications. J. Funct. Biomater. 2021, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Mohammadi, M.; Arab-Bafrani, Z.; Karimi, F.; Javid, N. Designing hybrid nanofibers based on keratin-poly (vinyl alcohol) and poly (Ɛ-caprolactone) for application as wound dressing. J. Ind. Text. 2021. [Google Scholar] [CrossRef]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef]
- Chen, W.; Zou, Y.; Zhong, Z.; Haag, R. Cyclo (RGD)-decorated reduction-responsive nanogels mediate targeted chemotherapy of integrin overexpressing human glioblastoma in vivo. Small 2017, 13, 1601997. [Google Scholar] [CrossRef]
- Peng, S.; Ouyang, B.; Xin, Y.; Zhao, W.; Shen, S.; Zhan, M.; Lu, L. Hypoxia-degradable and long-circulating zwitterionic phosphorylcholine-based nanogel for enhanced tumor drug delivery. Acta Pharm. Sin. B 2021, 11, 560–571. [Google Scholar] [CrossRef]
- Pinelli, F.; Saadati, M.; Zare, E.N.; Makvandi, P.; Masi, M.; Sacchetti, A.; Rossi, F. A perspective on the applications of functionalized nanogels: Promises and challenges. Int. Mater. Rev. 2022, 1–25. [Google Scholar] [CrossRef]
- Fasiku, V.O.; Omolo, C.A.; Kiruri, L.W.; Devnarain, N.; Faya, M.; Mocktar, C.; Govender, T. A hyaluronic acid-based nanogel for the co-delivery of nitric oxide (NO) and a novel antimicrobial peptide (AMP) against bacterial biofilms. Int. J. Biol. Macromol. 2022, 206, 381–397. [Google Scholar] [CrossRef]
- Amato, G.; Grimaudo, M.A.; Alvarez-Lorenzo, C.; Concheiro, A.; Carbone, C.; Bonaccorso, A.; Puglisi, G.; Musumeci, T. Hyaluronan/Poly-L-lysine/Berberine Nanogels for Impaired Wound Healing. Pharmaceutics 2020, 13, 34. [Google Scholar] [CrossRef]
- Rusu, A.G.; Chiriac, A.P.; Nita, L.E.; Ghilan, A.; Rusu, D.; Simionescu, N.; Tartau, L.M. Nanostructured hyaluronic acid-based hydrogels encapsulating synthetic/natural hybrid nanogels as promising wound dressings. Biochem. Eng. J. 2022, 179, 108341. [Google Scholar] [CrossRef]
- Zhu, J.; Li, F.; Wang, X.; Yu, J.; Wu, D. Hyaluronic acid and polyethylene glycol hybrid hydrogel encapsulating nanogel with hemostasis and sustainable antibacterial property for wound healing. ACS Appl. Mater. Interfaces 2018, 10, 13304–13316. [Google Scholar] [CrossRef]
- Weller, C.D.; Team, V.; Sussman, G. First-line interactive wound dressing update: A comprehensive review of the evidence. Front. Pharmacol. 2020, 11, 155. [Google Scholar] [CrossRef]
- Savencu, I.; Iurian, S.; Porfire, A.; Bogdan, C.; Tomuță, I. Review of advances in polymeric wound dressing films. React. Funct. Polym. 2021, 168, 105059. [Google Scholar] [CrossRef]
- Alven, S.; Peter, S.; Mbese, Z.; Aderibigbe, B.A. Polymer-Based Wound Dressing Materials Loaded with Bioactive Agents: Potential Materials for the Treatment of Diabetic Wounds. Polymers 2022, 14, 724. [Google Scholar] [CrossRef]
- Benskin, L.L. Evidence for Polymeric Membrane Dressings as a Unique Dressing Subcategory, Using Pressure Ulcers as an Example. Adv. Wound Care 2018, 7, 419–426. [Google Scholar] [CrossRef]
- Colobatiu, L.; Gavan, A.; Potarniche, A.V.; Rus, V.; Diaconeasa, Z.; Mocan, A.; Tomuta, I.; Mirel, S.; Mihaiu, M. Evaluation of bioactive compounds-loaded chitosan films as a novel and potential diabetic wound dressing material. React. Funct. Polym. 2019, 145, 104369. [Google Scholar] [CrossRef]
- Dharmalingam, K.; Anandalakshmi, R. Functionalization of cellulose-based nanocomposite hydrogel films with zinc oxide complex and grapefruit seed extract for potential applications in treating chronic wounds. Polymer 2020, 202, 122620. [Google Scholar] [CrossRef]
- Paneysar, J.S.; Barton, S.; Ambre, P.; Coutinho, E. Novel temperature responsive films impregnated with silver nano particles (Ag-NPs) as potential dressings for wounds. J. Pharm. Sci. 2022, 111, 810–817. [Google Scholar] [CrossRef]
- Alruwaili, N.K.; Ahmad, N.; Alzarea, A.I.; Alomar, F.A.; Alquraini, A.; Akhtar, S.; Shahari, M.S.B.; Zafar, A.; Elmowafy, M.; Elkomy, M.H.; et al. Arabinoxylan-Carboxymethylcellulose Composite Films for Antibiotic Delivery to Infected Wounds. Polymers 2022, 14, 1769. [Google Scholar] [CrossRef]
- Hosseini Salekdeh, S.S.; Daemi, H.; Zare-Gachi, M.; Rajabi, S.; Bazgir, F.; Aghdami, N.; Nourbakhsh, M.S.; Baharvand, H. Assessment of the efficacy of tributylammonium alginate surface-modified polyurethane as an antibacterial elastomeric wound dressing for both noninfected and infected full-thickness wounds. ACS Appl. Mater. Interfaces 2019, 12, 3393–3406. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, F.; Scoutaris, N.; Douroumis, D.; Boateng, J. 3D printed chitosan dressing crosslinked with genipin for potential healing of chronic wounds. Int. J. Pharm. 2019, 560, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.M.; Sudhakar, K.; Suneetha, M.; Won, S.Y.; Han, S.S. Fungal-derived carboxymethyl chitosan blended with polyvinyl alcohol as membranes for wound dressings. Int. J. Biol. Macromol. 2021, 190, 792–800. [Google Scholar] [CrossRef]
- Ahmed, A.; Niazi, M.B.K.; Jahan, Z.; Samin, G.; Pervaiz, E.; Hussain, A.; Mehran, M.T. Enhancing the thermal, mechanical and swelling properties of PVA/starch nanocomposite membranes incorporating g-C3N4. J. Polym. Environ. 2020, 28, 100–115. [Google Scholar] [CrossRef]
- Ahmed, A.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A.; Pervaiz, E.; Janjua, H.A.; Hussain, Z. In vitro and in vivo study of superabsorbent PVA/Starch/g-C3N4/Ag@ TiO2 NPs hydrogel membranes for wound dressing. Eur. Polym. J. 2020, 130, 109650. [Google Scholar] [CrossRef]
- Brianezi, S.F.S.; Castro, K.C.; Piazza, R.D.; Melo, M.D.S.F.; Pereira, R.M.; Marques, R.F.C.; Campos, M.G.N. Preparation and characterization of chitosan/mPEG-PCL blended membranes for wound dressing and controlled gentamicin release. Mater. Res. 2018, 21. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Elbarbary, A.M.; Hegazy, D.E.; Maziad, N.A. Radiation synthesis of pH-sensitive 2-(dimethylamino)ethyl methacrylate/polyethylene oxide/ZnS nanocomposite hydrogel membrane for wound dressing application. J. Drug Deliv. Sci. Technol. 2022, 73, 103399. [Google Scholar] [CrossRef]
- Yang, C.; Huang, Y.; Dan, N.; Chen, Y. Polyglutamic Acid-Grafted Halloysite-Modified Collagen-Sodium Alginate Oxide Composite Membrane for Wound Dressing Application. ACS Appl. Polym. Mater. 2022, 4, 3994–4002. [Google Scholar] [CrossRef]
- Ceylan, S. Propolis loaded and genipin-crosslinked PVA/chitosan membranes; characterization properties and cytocompatibility/genotoxicity response for wound dressing applications. Int. J. Biol. Macromol. 2021, 181, 1196–1206. [Google Scholar] [CrossRef]
- Hassan, M.A.; Abd El-Aziz, S.; Elbadry, H.M.; Samy, A.; Tamer, T.M. Prevalence, antimicrobial resistance profile, and characterization of multi-drug resistant bacteria from various infected wounds in North Egypt. Saudi J. Biol. Sci. 2022, 29, 2978–2988. [Google Scholar] [CrossRef]
- Dodda, J.M.; Azar, M.G.; Sadiku, R. Crosslinking Trends in Multicomponent Hydrogels for Biomedical Applications. Macromol. Biosci. 2021, 21, 2100232. [Google Scholar] [CrossRef]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New developments in medical applications of hybrid hydrogels containing natural polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef]



| Synthetic Polymer | Biopolymer/Semisynthetic Polymers | Bioactive Agents | Therapeutic Outcomes | References |
|---|---|---|---|---|
| Foam Wound Dressings | ||||
| Polyurethane | Chitosan, sodium alginate, and hydroxypropyl methylcellulose | Silver nanoparticles and asiaticoside | Non-cytotoxic, compatible and accelerated wound closure, and the formation of granulation tissue. | [69] |
| Polyvinyl alcohol | Sodium carboxymethylcellulose | Stearyl trimethyl ammonium chloride | An excellent absorption capacity of excess exudate, good inhibition effect against E. coli and S. aureus with good hemostatic capability. | [70] |
| Hydrocolloids | ||||
| Styrene–isoprene–styrene | Sodium carboxymethyl cellulose | Silk fibroin nanoparticles | Non-toxic, supports the regeneration of the dermis layer, decreased the burn wound size and increased the density of collagen fibers in vivo. | [77] |
| Styrene–isoprene–styrene copolymer and petroleum hydrocarbon resin | Sodium alginate | Centella Asiatica | Biocompatible, induced accelerated collagen deposition, regeneration of dermis, mature epidermis, and hair follicle in vivo | [78] |
| Hydrogel | ||||
| Poly (vinyl alcohol) | Sodium alginate | 5-hydroxymethylfurfural and silver nanoparticles | Induced proliferation and migration of human skin fibroblasts and collagen production. Good compatibility and accelerated wound healing in vivo by increasing collagen production, enhancing angiogenesis/vascularization, inducing re-epithelialization, and reducing inflammation. | [91] |
| Poly(N-isopropyl acrylamide) | Cellulose nanocrystals | Metronidazole | Slow and sustained drug release. | [92] |
| Poly (N-vinyl-2-pyrrolidone) and poly acrylic acid | Chitosan | Silver sulfadiazine | Controlled drug release | [93] |
| Polyacrylamide | Chitosan | - | Promoted collagen deposition, induced skin adnexal regeneration, and accelerated wound healing. | [94] |
| Polyvinyl (alcohol) | Chitosan | Nano zinc oxide | A significant antibacterial activity against E. coli and S. aureus, non-toxic, biocompatible, and effective for treating infected and exuding wounds. | [95] |
| Benzaldehyde-terminated polyethylene glycol | Chitosan | Vascular endothelial growth factor | Excellent tissue adhesion, hemostasis, and blood cell coagulation effects. | [96] |
| Polyethylene glycol | Chitosan | Silver nanoparticles | The high absorption capacity of the exudates and good antimicrobial and antioxidant properties in vitro. Improved wound healing in vivo in diabetic rabbit models. | [97] |
| Carbopol | Gelatin | Quercetin | Accelerated wound healing with a significant wound closure time in vivo. | [98] |
| - | Sodium alginate, carboxymethyl chitosan | - | Promoted wound healing in vivo in a full-thickness wound model. Facilitated re-epithelialization together with vascularization and skin regeneration. | [99] |
| Poly(ethylene glycol)- | Chitosan | - | Covered irregularly shaped wounds with good hemostatic effect in vivo. | [100] |
| Poly(vinyl alcohol) | Sodium alginate | Minocycline | Hemocompatible, non-hemolytic, and antibacterial effects in vivo against methicillin-resistant S. aureus (MRSA)-induced murine burn wound model. | [101] |
| Arginine-based poly(ester urea urethane) | Glycidyl methacrylate-modified chitosan | - | Non-cytotoxic effect and excellent antibacterial activity (eliminated 91.81% of E. coli and 85.59% of S. aureus). | [102] |
| Polyhexamethylene biguanide | Hyaluronic acid | Salidroside | Effective against E. coli (97.85%) and S. aureus (98.56%). In vivo studies using diabetic rat models revealed that the hydrogel promoted the high formation of granulation tissue together with the formation of subcutaneous capillaries and high collagen deposition. | [103] |
| Arginine–poly(ester amide) | Hyaluronic acid | - | Induced keratinocytes and accelerated re-epithelialization with a uniform density of collagen deposition in vivo. | [104] |
| Poly(vinyl alcohol) | Hydroxypropyl cellulose | Bovine serum albumin, glutathione, and neomycin trisulfate | Controlled drug release | [105] |
| Polyvinyl alcohol | Sodium alginate | Polycaprolactone microspheres loaded with fibroblast growth factor (bFGF) | A sustained release of bFGF. Accelerated wound closure, cell-induced tissue regeneration and wound healing in vivo in a burn-wound rat model. Effective against S. aureus and E. coli. | [106] |
| Polyvinyl pyrrolidone | Keratin | Lavender oil | Controlled and tailored drug release profiles. | [107] |
| Polyvinyl alcohol | Sodium alginate | Bis-quaternary triphenyl-phosphonium salt | Prolonged antibacterial activity and good hemostasis effect with accelerated wound healing. | [110] |
| Nanofibers | ||||
| Polyethylene oxide | Chitosan | Silver and zinc oxide nanoparticles | Exhibited enhanced antibacterial activity against E. coli, S. aureus, and P. aeruginosa with a high antioxidant effect. Good blood compatibility with good fibroblast migration and proliferation on the wound margin in vitro. | [118] |
| Polyurethane | Chitosan | Linezolid | Promoted healing in streptozotocin-induced diabetic rats in vivo. | [119] |
| Polyethylene oxide | Chitosan | Teicoplanin | Sustained drug release with antibacterial effect against S. aureus. Non-cytotoxic effect and accelerated healing on a rat full-thickness wound model. | [120] |
| Polyethylene oxide | Chitosan | Ciprofloxacin | Effective against S. aureus and E. coli with no cytotoxic effect on HFFF2 human foreskin and L929 mouse fibroblasts. Reduced infection and inflammation in vivo in dorsal cutaneous wounds of the Balb/C mice. | [121] |
| Polyethylene oxide | Chitosan | Manuka honey, propolis, Calendula officinalis infusion, insulin, and L-arginine | Biodegradable with improved hemocompatibility and reduced cytotoxic effect. Significant radical scavenging effects and increased antimicrobial effects against S. aureus. | [122] |
| Polyethylene oxide | Chitosan | Calendula officinalis | High antibacterial effects against Gram-positive and Gram-negative bacteria with 96% and 94% reduction, respectively. Accelerated wound healing with 87.5% wound closure in 14 days. Improved collagen synthesis, re-epithelization and remodeling. | [123] |
| Poly(ethylene oxide) | Chitosan | Vancomycin | Accelerated wound healing in full-thickness wound models in vivo. | [124] |
| Polycaprolactone | Chitosan | - | Biodegradable and non-toxic. | [125] |
| Polycaprolactone | Chitosan | Melilotus officinalis | Effective against Bacillus and Shigella with no toxicity. | [126] |
| Polycaprolactone | Chitosan | Curcumin | Improved antibacterial activity against MRSA and increased antioxidant activity. Induced 96.4% wound healing in MRSA-infected wounds. | [127] |
| Polycaprolactone | Chitosan | Quercetin and rutin | Improved the hydrophilicity, water absorption capacity and the specific surface area. Good biocompatibility and antibacterial activity. | [128] |
| Polyvinyl alcohol | Hyaluronic acid | Cellulose nanocrystals, arginine | Accelerated wound healing. Excellent hemocompatibility and antibacterial activity against K. pneumonia. | [129] |
| Polyethylene oxide | Hyaluronic acid | Zinc oxide nanoparticles and cinnamon oil | High antibacterial activity in full-thickness wounds inoculated with S. aureus with accelerated healing. | [130] |
| Poly ethylene oxide | Hyaluronic acid | L-arginine, propolis, Calendula officinalis infusion, and Manuka honey | Outstanding cytocompatibility, antioxidant, and antimicrobial activities (against pathogen E. coli, S. aureus, and P. aeruginosa). | [131] |
| Polygalacturonic acid | Hyaluronic acid | Silver nanoparticles | Excellent antibacterial activity and accelerated wound healing on the albino rat model with high wound epithelization and collagen deposition | [132] |
| Polyurethane | Starch and hyaluronic acid | - | Non-toxic and biocompatible. | [133] |
| Polyurethane- | Hyaluronic acid | Ethanolic extract of propolis | Enhanced antibacterial activity against S. aureus and E. coli. Excellent biocompatibility on L929 fibroblast cells with accelerated wound healing and closure with improved development of dermis, hair follicles, and deposition of densely packed collagen on the healed wound area. | [134] |
| Polycaprolactone | Gelatin | Ciprofloxacin and tetracycline hydrochloride | Sustained drug release with excellent antibacterial activity against E. coli and S. aureus with biocompatibility on human skin fibroblast cells. | [135] |
| Poly (L-Lactic-co-caprolactone) | Gelatin | Epigallocatechin-3-O-gallate | Facilitated accelerated wound closure with good tissue organization and excellent hemostatic ability. | [136] |
| Polyacrylonitrile | Gelatin | ZIF-8@gentamicin | Synergistic antibacterial effects with accelerated wound healing time. | [137] |
| Polyvinyl alcohol | Pectin | Hibiscus rosa–Sinensis leaves | Accelerated wound healing on Swiss albino mice model with rapid epithelization in 8 days. | [138] |
| Poly (vinyl alcohol), poly (Ɛ-caprolactone) | Keratin | - | Improved cell-scaffold adhesion and proliferation of fibroblast cells of the nanofibers. Good antibacterial activity against Gram-negative and Gram-positive strains of bacteria. | [139,140,141] |
| Nanogels | ||||
| Divinyl sulfone | Hyaluronic acid | S-Nitroso-N-acetyl-DL-penicillamine | Significant antibacterial activity against P. aeruginosa, methicillin-resistant S. aureus, and E. coli, respectively. | [146] |
| Poly-L-lysine | Hyaluronan | Berberine | Accelerated wound closure in vivo. | [147] |
| Poly(aspartic acid) | Maleoyl–chitosan | Amoxicillin | Good stability in physiological conditions and biocompatible in vivo | [148] |
| Methacrylated methoxy polyethylene glycol | Aminoethyl methacrylate hyaluronic acid | Chlorhexidine diacetate | Prolonged drug release and extended antibacterial activity. Promoted rapid hemostasis and accelerated wound healing in vivo. | [149] |
| Membrane/Films | ||||
| Poly(vinyl alcohol) | Chitosan | An alcoholic extract containing a mixture of Arnica montana, Geum urbanum, Plantago lanceolata, Symphytum officinale, Tagetes patula and Calendula officinalis | Good proliferative effect and antioxidant activity, biocompatible and induced wound contraction with a complete re-epithelialization and a deposition of dense collagen in vivo on a streptozotocin-induced diabetic rat model. | [154] |
| Sodium carboxymethylcellulose and hydroxypropylmethylcellulose | Zinc oxide complex and grapefruit seed extract | The release of grapefruit seed extract and zinc from the films was sustained. Good antibacterial activity against S. aureus and E. coli was significant. | [155] | |
| N-isopropyl acrylamide | Pullulan | Silver nanoparticles | The antibacterial activity of the films was effective against S. aureus and E. coli. Good biocompatibility on HeK293 cells and temperature-responsive nature. | [156] |
| - | Carboxymethylcellulose and arabinoxylan | Amikacin | Initial rapid drug release followed by a sustained release. | [157] |
| Polyurethane | tributyl ammonium alginate | - | Good antibacterial activity against E. coli and S. aureus. Promoted rapid healing with enhanced deposition of collagen and the formation of matured blood vessels. | [158] |
| Polyethylene glycol | Chitosan | - | Non-cytotoxic on human skin fibroblast cell lines with over 90% of the cells being viable. High flexibility and adherence to a mucosal surface in vitro. | [159] |
| Poly (vinyl alcohol) | Carboxymethyl chitosan | - | Effective against E. coli and Staphylococcus bacteria. They were also biocompatible with fibroblasts and keratinocytes in vitro. | [160] |
| Poly (vinyl alcohol) | Starch | Carbon nitride | Excellent mechanical and thermal stability, swelling capability, hydrophilicity, moisture retention capacity, and water vapor transmission. | [161] |
| Poly (vinyl alcohol) | Starch | Titania and silver nanoparticles | Excellent antibacterial activity against S. aureus and E. coli with a maximum zone of inhibition of 33.25 and 37.33 mm, respectively. Complete healing in seven days in vivo in partial and full thickness excision wounds. | [162] |
| Methoxy polyethylene glycol and polycaprolactone | Chitosan | Gentamicin | Inhibited S. aureus and E. coli growth. Thermally stable with high moisture content and swelling capability. | [163] |
| 2-(dimethylamino)ethyl methacrylate-polyethylene oxide | Colistin, gentamicin, and neomycin | The drug release of colistin and neomycin was high at pH 4 and high for gentamicin at pH 7. | [164] | |
| Polyglutamic acid | Sodium Alginate | - | Increased elongation at the break, tensile strength, and biocompatibility. | [165] |
| Poly (vinyl alcohol) | Chitosan | Propolis | Improved cell proliferation rate, water uptake and hydrophilicity. The good genotoxic potential is suitable for wound healing applications. | [166] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aderibigbe, B.A. Hybrid-Based Wound Dressings: Combination of Synthetic and Biopolymers. Polymers 2022, 14, 3806. https://doi.org/10.3390/polym14183806
Aderibigbe BA. Hybrid-Based Wound Dressings: Combination of Synthetic and Biopolymers. Polymers. 2022; 14(18):3806. https://doi.org/10.3390/polym14183806
Chicago/Turabian StyleAderibigbe, Blessing Atim. 2022. "Hybrid-Based Wound Dressings: Combination of Synthetic and Biopolymers" Polymers 14, no. 18: 3806. https://doi.org/10.3390/polym14183806
APA StyleAderibigbe, B. A. (2022). Hybrid-Based Wound Dressings: Combination of Synthetic and Biopolymers. Polymers, 14(18), 3806. https://doi.org/10.3390/polym14183806





