Activation Mechanism of Coal Gangue and Its Impact on the Properties of Geopolymers: A Review
Abstract
1. Introduction
- (1)
- To summarize the physicochemical characteristics and heavy metal content of CG and analyze its performance trend.
- (2)
- To investigate all the activation methods and mechanisms of CG.
- (3)
- To discuss the effects of activated CG on coal gangue geopolymer (CGG).
2. Chemical Composition and Reaction Mechanism of Geopolymers
2.1. Chemical Composition of Geopolymers
2.2. Geopolymer Reaction Mechanism
3. CG Properties and Preparation Process
3.1. Physical Characteristics
3.2. Chemical and Mineral Composition
3.3. Heavy-Metal Element Content
3.4. Preparation Process of Coal Gangue Powder
4. Method and Mechanism of CG Activation
4.1. Physical Activation
4.1.1. Mechanical Activation
4.1.2. Calcined Activation
4.1.3. Microwave Activation
4.2. Chemical Activation
4.3. Composite Activation
5. The Performance of CGG
5.1. Fresh Properties
5.2. Mechanical Property
| Cementing Material (Particles; Calcined) | Alkali-Activator | Optimum Condition | Compressive Strength (MPa) | Refs | ||
|---|---|---|---|---|---|---|
| 3 d | 7 d | 28 d | ||||
| Coal gangue (<80 μm; 700 °C), Blast furnace slag | NaOH, Na2SiO3 | CG:BFS = 60:40, Na2SiO3 modulu: 0.8, alkali dosage: 18% | 24.5 | 29.5 | 46.4 | [104] |
| Coal gangue (<80 μm; 700 °C), Blast furnace slag | CaSO4·2H2O, NaOH, Na2SiO3 | CG:BFS = 60:40, CaSO4·2H2O dosage: 6%, Na2SiO3 modulus: 0.8, Na2SiO3: 16% | 17 | 29.8 | 40.1 | [64] |
| Coal gangue (<75 μm), Calcium carbide residue | NaOH, Na2SiO3 | CG:CCR = 70:30, Na2SiO3 modulus: 2.3, NaOH:Na2SiO3 = 30:70 | 18.7 | 24.7 | 44 | [89] |
| Coal gangue (<80 μm), Fly ash | NaOH, CaCO3 | CG:FA = 50:50, NaOH:CaCO3 = 36.4:65.6, curing temperature: 100 °C | - | 71 | 101 | [106] |
| Coal gangue (<100 μm), Blast furnace slag, Fly ash | NaOH, Na2SiO3, KOH | CG:BFS:FA = 50:25:25, Na2SiO3 modulus: 2.2, NaOH:Na2SiO3:KOH = 5.1:66.4:28.5 | 18.8 | 23.3 | 29.4 | [107] |
| Coal gangue (700 °C), Blast furnace slag | NaOH, Na2SiO3 | CG:SFS = 50:50, Na2SiO3 modulus: 1.3, liquid-to-solid ratio: 0.36 | 41 | 86 | 93 | [108] |
| Coal gangue, Blast furnace slag, Slaked lime | NaOH, Na2SiO3 | CG:GBFG:SL = 55:40:5, Na2SiO3 modulus: 2.7, NaOH:Na2SiO3 = 27.7:72.3 | 39 | - | 60.5 | [103] |
| Coal gangue (<250 μm), Sludge | NaOH, Na2SiO3 | CG:S = 60:40, Na2SiO3 modulus: 1.0 | - | 30 | 39.8 | [109] |
| Coal gangue (<75 μm), Red mud | NaOH, Na2SiO3 | CG:RM = 20:80, Na2SiO3 modulus: 2.21 | 17 | 22.5 | 26.3 | [110] |
| Coal gangue (<80 μm), Blast furnace slag, Fly ash | NaOH, Na2SiO3, NaO | CG:BFS:FA = 50:40:10, Na2SiO3 modulus: 1.2 | 5.41 | - | 11.5 | [98] |
5.3. The Micro Performance
5.4. Heavy Metal Solidification
6. Discussion and Gap
- (1)
- According to different weathering ages and weathering degrees, the content of heavy metal elements varies greatly, Pb, Cr, and Ni elements exceeding the standard to different degrees, but there are few studies focused on CGG leaching, which is not conducive to the application of coal gangue preparation geopolymers.
- (2)
- There are many studies on CGG in the existing literature, but few study durability, which has a great impact on the service life of buildings. The lack of research on durability affects the promotion of gangue geopolymers.
- (3)
- The research purpose of CGG is to replace cement. Most of the existing studies are focused on the properties of cement materials. However, there are few studies on the combination of CGG and aggregate to prepare concrete, which cannot be applied in practical engineering quickly.
- (4)
- The admixture of CGG is millimeter-level mineral waste, while the research on CGG of nanoscale materials is less common. The morphology effects, activity effects, and micro-aggregate packing effects of nanoscale materials (such as nano-silica, graphene oxide, and nano-calcium carbonate) are much higher than those of millimeter-scale materials.
7. Conclusions
- (1)
- The CG performance difference in different parts is very significant. CG has poor physical characteristics, and most of its chemical composition is SiO2, Al2O3, and Fe2O3. Kaolinite is the main mineral composition. The higher crystallinity causes difficulty in generating hydration products to promote strength. In addition, because of the different sources of CG, it has different levels of heavy metal element content in the paint.
- (2)
- The CG activation methods are physical activation (mechanical activation, calcination activation, and microwave activation), chemical activation, and composite activation. With three activation methods, the activity of CG can be effectively improved, and the gelling properties of CG can be stimulated. The composite activation, in combination with other activation methods, effectively plays respective advantages, and activation is more efficient.
- (3)
- CG is a silica–alumina material. Reducing the content of CG or increasing the liquid–solid ratio of CGG can effectively improve fluidity. Increasing the content of the CGG base activator and the modulus of Na2SiO3 can shorten the setting time and improve fluidity. Adding high-calcium material can effectively promote the formation of C–S–H gel and increase the alkali initiator content as well as the modulus of Na2SiO3 to effectively dissolve the silicon–oxygen tetrahedra, improve the bonding effect, increase the curing temperature, and accelerate the hydrolysis of the polymer using an alkali initiator. Changing these factors appropriately can effectively improve the mechanical strength of CGG. The activation reaction of CG affects the microstructure of CGG. The higher the activity of CG, the denser the structure of CGG, and the increase in Na+ and H2O in CGG decreases the energy of the mineral polymer, the average bond length of Si–O, Al–O, H–O, and O–O becomes shorter, and the molecular structure of CGG becomes more stable. With the increase in Na/Al and H2O/Al, the distribution of bond angles becomes more concentrated, and the mechanical strength increases. CGG prepared after CG activation forms a three-dimensional network of silica–aluminate geopolymer with a tight pore structure, strong physical fixation, and chemical bonding, which can effectively solidify and adsorb heavy metal ions and radioactive wastes.
8. Outlook
- (1)
- The heavy metal elements of CG exceed the standard and the heavy metal curing of CGG needs much research.
- (2)
- Increasing the durability research of CGG can effectively popularize and apply CGG in the engineering field.
- (3)
- It is necessary to evaluate the concrete prepared by combining CGG with aggregate. The future sustainable development direction is to use CGG instead of cement in concrete.
- (4)
- Nanomaterials (such as nano-silica, graphene oxide, and nano-calcium carbonate) have been used in the research on concrete, and the use of nanoscale materials to prepare CGG should be considered in the future.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y. Energy globalization of China: Trade, investment, and embedded energy flows. J. Geogr. Sci. 2022, 32, 377–400. [Google Scholar] [CrossRef]
- Li, J.Y.; Wang, J.M. Comprehensive utilization and environmental risks of coal gangue: A review. J. Clean. Prod. 2019, 239, 117946. [Google Scholar] [CrossRef]
- Wang, B.; Ma, Y.N.; Lee, X.Q.; Wu, P.; Liu, F.; Zhang, X.Y.; Li, L.; Chen, M. Environmental-friendly coal gangue-biochar composites reclaiming phosphate from water as a slow-release fertilizer. Sci. Total Environ. 2020, 758, 143664. [Google Scholar] [CrossRef]
- Han, J.; Liu, X.B.; Yang, W.W.; Liu, J.P. Research of Comprehensive Utilization of Coal Gangue. DEStech Trans. Eng. Technol. Res. 2018, 2018, 24927. [Google Scholar] [CrossRef]
- Wu, H.; Wen, Q.B.; Hu, L.M.; Gong, M.; Tang, Z.L. Feasibility study on the application of coal gangue as landfill liner material. Waste Manag. 2017, 63, 161–171. [Google Scholar] [CrossRef]
- Tan, W.F.; Wang, L.A.; Huang, C. Environmental effects of coal gangue and its utilization. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 3716–3721. [Google Scholar] [CrossRef]
- Qi, L.Q.; Yuan, Y.T. Characteristics and the behavior in electrostatic precipitators of high-alumina coal fly ash from the Jungar power plant, Inner Mongolia, China. J. Hazard. Mater. 2011, 192, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Baic, I.; Witkowska, K.B. Hard Coal Mining Waste Management Technologies-Diagnosis of Current Development, Innovativeness Evaluation and SWOT Analysis. Rocz. Ochr. Srodowiska 2011, 13, 1315–1325. [Google Scholar]
- Li, J.Y.; You, L.F.; Wei, Y. The Current Situation of Comprehensive Utilization of Coal Gangue in China. Adv. Mater. Res. 2012, 1792, 915. [Google Scholar]
- Inyangh, I.; Daniels, J.L.; Otto, F.; Struthers, S. Environmental issues from coal mining and their solutions. Min. Sci. Technol. 2010, 20, 215–223. [Google Scholar]
- Li, M.; Zhang, J.; Li, A.; Zhou, N. Reutilisation of coal gangue and fly ash as underground backfill materials for surface subsidence control. J. Clean. Prod. 2020, 254, 120113. [Google Scholar] [CrossRef]
- Luo, L.; Li, K.; Fu, W.; Liu, C.; Yang, S.Y. Preparation, characteristics and mechanisms of the composite sintered bricks produced from shale, sewage sludge, coal gangue powder and iron ore tailings. Constr. Build. Mater. 2019, 232, 117250. [Google Scholar] [CrossRef]
- Zhan, X.; Fang, W.; Song, Z.; Chen, L.J.; Cui, Z.D. Development Model of Circular Eco-Industrial Park for Comprehensive Utilization of Coal Gangue in Coal Enterprise. Mater. Ence Forum 2014, 787, 71–75. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Q.; Yan, K. Novel Process for Alumina Extraction via the Coupling Treatment of Coal Gangue and Bauxite Red Mud. Ind. Eng. Chem. Res. 2014, 53, 4518–4521. [Google Scholar] [CrossRef]
- Wang, A.G.; Liu, P.; Mo, L.W.; Liu, K.W.; Ma, R.; Guan, Y.M.; Sun, D.S. Mechanism of thermal activation on granular coal gangue and its impact on the performance of cement mortars. J. Build. Eng. 2022, 45, 103616. [Google Scholar] [CrossRef]
- Benhelal, E.; Zahedi, G.; Shamsaei, E.; Bahadori, A. Global strategies and potentials to curb CO2 emissions in cement industry. J. Clean. Prod. 2013, 51, 142–161. [Google Scholar] [CrossRef]
- Lu, C.Y.; Wang, Q.P.; Liu, Y.X.; Xue, T.T.; Yu, Q.B.; Chen, S. Influence of new organic alkali activators on microstructure and strength of fly ash geopolymer. Ceram. Int. 2022, 48, 12442–12449. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Mohammed, A.A.; Mohammed, A.S. The role of nanomaterials in geopolymer concrete composites: A state-of-the-art review. J. Build. Eng. 2022, 49, 104062. [Google Scholar] [CrossRef]
- Ivana, V.K.; Serdar, M. Contribution to Understanding of Synergy between Red Mud and Common Supplementary Cementitious Materials. Materials 2022, 15, 1968. [Google Scholar]
- Mehdizadeh, H.; Cheng, X.F.; Mo, K.H.; Ling, T.C. Upcycling of waste hydrated cement paste containing high-volume supplementary cementitious materials via CO2 pre-treatment. J. Build. Eng. 2022, 52, 104396. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Ling, T.C. Reactivity activation of waste coal gangue and its impact on the properties of cement-based materials–A review. Constr. Build. Mater. 2020, 234, 117424. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers and geopolymeric materials. J. Therm. Anal. 1989, 35, 429. [Google Scholar] [CrossRef]
- Sun, D.S.; Wang, A.G.; Hu, P.H. Research of Geopolymer and Its Applications and Development Prospect. Mater. Rev. 2009, 23, 61–65. (In Chinese) [Google Scholar]
- Prachasaree, W.; Hawa, A. Prediction of Torsional Strength for Very High Early Strength Geopolymer. Medžiagotyra 2017, 23, 378–383. [Google Scholar] [CrossRef]
- Hani, A. Early strength and durability of metakaolin-based geopolymer concrete. Mag. Concr. Res. 2017, 69, 46–54. [Google Scholar]
- Ma, C.; Zhao, B.; Wang, L.M.; Long, G.C.; Xie, Y.J. Clean and low-alkalinity one-part geopolymeric cement: Effects of sodium sulfate on microstructure and properties. J. Clean. Prod. 2020, 252, 119279. [Google Scholar] [CrossRef]
- Jin, M.T.; Liao, M.Y.; Chen, L.; Jin, Z.F. Thermal Performance and Temperature Resistance of Straw-Geopolymer Composites. Appl. Mech. Mater. 2015, 3745, 15–18. [Google Scholar] [CrossRef]
- Liu, J.; Lv, C. Durability of Cellulosic-Fiber-Reinforced Geopolymers: A Review. Molecules 2022, 27, 796. [Google Scholar] [CrossRef]
- Luo, Y.; Klima, K.M.; Brouwers, H.J.H.; Yu, Q.L. Effects of ladle slag on Class F fly ash geopolymer: Reaction mechanism and high temperature behavior. Cem. Concr. Compos. 2022, 129, 104468. [Google Scholar] [CrossRef]
- Srividya, T.; Rajkumar, P.R.K.; Sivasakthi, M.; Sujitha, A.; Jeyalakshmi, R. A state-of-the-art on development of geopolymer concrete and its field applications. Case Stud. Constr. Mater. 2022, 16, e00812. [Google Scholar]
- Marvila, M.T.; Azevedo, A.R.G.D.; Vieira, C.M.F. Reaction mechanisms of alkali-activated materials. Rev. IBRACON Estrut. Mater. 2021, 14. [Google Scholar] [CrossRef]
- Provis, J.L. Geopolymers and other alkali activated materials: Why, how, and what? Mater. Struct. Constr. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Teixeira, M.M.; Garcez, D.A.A.R.; Barbosa, D.O.L.; Gustavo, D.C.X.; Fontes, V.C.M. Mechanical, physical and durability properties of activated alkali cement based on blast furnace slag as a function of %Na2O. Case Stud. Constr. Mater. 2021, 15, e00723. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.H.; Li, L.G. A review: The comparison between alkali-activated slag (Si+Ca) and metakaolin (Si+Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Reaction kinetics, gel character and strength of ambient temperature cured alkali activated slag–fly ash blends. Constr. Build. Mater. 2015, 80, 105–115. [Google Scholar] [CrossRef]
- Park, S.M.; Jang, J.G.; Lee, N.K.; Lee, H.K. Physicochemical properties of binder gel in alkali-activated fly ash/slag exposed to high temperatures. Cem. Concr. Res. 2016, 89, 72–79. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, S.M.; Guo, L.H. Application of Coal Gangue as a Coarse Aggregate in Green Concrete Production: A Review. Materials 2021, 14, 6803. [Google Scholar] [CrossRef]
- Guo, Y.X.; Yan, K.Z.; Cui, L.; Cheng, F.Q. Improved extraction of alumina from coal gangue by surface mechanically grinding modification. Powder Technol. 2016, 302, 33–41. [Google Scholar] [CrossRef]
- Zhu, X.L.; Guo, Z.H.; Yang, W.; Song, W.J. Durability of Concrete with Coal Gasification Slag and Coal Gangue Powder. Front. Mater. 2022, 8, 791178. [Google Scholar] [CrossRef]
- Wang, A.G.; Zhu, Y.Y.; Xu, H.Y.; Liu, K.W.; Jing, Y.; Sun, D.S. Research Progress on Coal Gangue Aggregate for Concrete. Bull. Chin. Ceram. Soc. 2019, 38, 2076–2086. (In Chinese) [Google Scholar]
- Rodríguez, J.; Tobón, J.; Frías, M.; Sánchez de Rojas, M.I. Aprovechamiento de un residuo del carbón para reducción del impacto ambiental de la minería del carbón en Colombia: Estudio del potencial de uso en la industria del cemento. Rev. CINTEX 2018, 23, 95–102. [Google Scholar] [CrossRef]
- Gu, B.W.; Wang, P.M. Analysis of Factors Affecting Pozzolanic Activity in Thermal Activated Coal Gangue. J. Build. Mater. 2009, 12, 6–11. (In Chinese) [Google Scholar]
- Wu, C.L.; Jiang, W.; Zhang, C.; Li, J.W.; Wu, S.; Wang, X.J.; Xu, Y.M.; Wang, W.L.; Feng, M.J. Preparation of solid-waste-based pervious concrete for pavement: A two-stage utilization approach of coal gangue. Constr. Build. Mater. 2022, 319, 125962. [Google Scholar] [CrossRef]
- Jin, J.X.; Qin, Z.F.; Lü, X.L.; Liu, T.; Zhang, G.S.; Shi, J.Y.; Zuo, S.H.; Li, D.Q. Rheology control of self-consolidating cement-tailings grout for the feasible use in coal gangue-filled backfill. Constr. Build. Mater. 2022, 316, 125836. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X. Properties of coal gangue-Portland cement mixture with carbonation. Fuel 2019, 245, 1–2. [Google Scholar] [CrossRef]
- Yi, C.; Ma, H.Q.; Zhu, H.G.; Li, W.J.; Xin, M.L.; Liu, Y.L.; Guo, Y.D. Study on chloride binding capability of coal gangue based cementitious materials. Constr. Build. Mater. 2018, 167, 649–656. [Google Scholar] [CrossRef]
- Guan, X.; Chen, J.X.; Zhu, M.Y.; Gao, J. Performance of microwave-activated coal gangue powder as auxiliary cementitious material. J. Mater. Res. Technol. 2021, 14, 2799–2811. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Tan, Y.; Wang, Y.C.; Liu, C.X. Mechanical properties and chloride permeability of green concrete mixed with fly ash and coal gangue. Constr. Build. Mater. 2020, 233, 117166. [Google Scholar] [CrossRef]
- Li, D.X.; Song, X.Y.; Gong, C.C.; Pan, Z.H. Research on cementitious behavior and mechanism of pozzolanic cement with coal gangue. Cem. Concr. Res. 2006, 36, 1752–1759. [Google Scholar] [CrossRef]
- Chen, H.B.; Chen, J.X.; Zhang, P.C.; Wu, J.C. Study on calcined fine ground coal gangue as admixture of high performance concrete. New Build. Mater. 2002, 05, 10–11. (In Chinese) [Google Scholar]
- Liu, Y.; Lei, S.; Lin, M.; Li, Y.; Ye, Z.; Fan, Y.M. Assessment of pozzolanic activity of calcined coal-series kaolin. Appl. Clay Sci. 2017, 143, 159–167. [Google Scholar] [CrossRef]
- Chen, Y.M.; Zhou, S.X.; Zhang, W.S. Effect of coal gangue with different kaolin contents on compressive strength and pore size of blended cement paste. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2008, 23, 12–15. [Google Scholar] [CrossRef]
- Shi, X.Y.; Cai, Q.X.; Qi, C.C.; Zhang, L.L.; Lu, X.; Zhou, W.; Zhao, B.Y. Co-utilization of reactivated cement pastes with coal gangue. Constr. Build. Mater. 2020, 270, 121423. [Google Scholar] [CrossRef]
- Dabbaghi, F.; Rashidi, M.; Nehdi, M.L.; Sadeghi, H.; Karimaei, M.; Rasekh, H.; Qaderi, F. Experimental and Informational Modeling Study on Flexural Strength of Eco-Friendly Concrete Incorporating Coal Waste. Sustainability 2021, 13, 7506. [Google Scholar] [CrossRef]
- Frías, M.; Rojas, M.I.S.D.; García, R.; Valdés, A.J.; Medina, C. Effect of activated coal mining wastes on the properties of blended cement. Cem. Concr. Compos. 2012, 34, 678–683. [Google Scholar] [CrossRef]
- Afrakoti, M.T.P.; Choobbasti, A.J.; Ghadakpour, M.; Kutanaei, S.S. Investigation of the effect of the coal wastes on the mechanical properties of the cement-treated sandy soil. Constr. Build. Mater. 2020, 239, 117848. [Google Scholar] [CrossRef]
- Chhaiba, S.; Blanco, V.M.T.; Diouri, A.; Bougarrani, S. Characterization and hydration of cements and pastes obtained from raw mix containing Moroccan oil shale and coal waste as a raw material. Constr. Build. Mater. 2018, 189, 539–549. [Google Scholar] [CrossRef]
- Gao, H.D.; Huang, Y.L.; Li, W.; Li, J.M.; Ouyang, S.Y.; Song, T.Q.; Lv, F.Y.; Zhai, W.; Ma, K. Explanation of heavy metal pollution in coal mines of china from the perspective of coal gangue geochemical characteristics. Environ. Sci. Pollut. Res. Int. 2021, 28, 65363–65373. [Google Scholar] [CrossRef]
- Hua, C.Y.; Zhou, G.Z.; Yin, X.; Wang, C.Z.; Chi, B.R.; Cao, Y.Y.; Wang, Y.; Zheng, Y.; Cheng, Z.R.; Li, R.Y. Assessment of heavy metal in coal gangue: Distribution, leaching characteristic and potential ecological risk. Environ. Sci. Pollut. Res. Int. 2018, 25, 32321–32331. [Google Scholar] [CrossRef]
- Liu, X.Y.; Jing, M.; Bai, Z.K. Heavy Metal Concentrations of Soil, Rock, and Coal Gangue in the Geological Profile of a Large Open-Pit Coal Mine in China. Sustainability 2022, 14, 1020. [Google Scholar] [CrossRef]
- Peng, W.H.; Liu, Y.Y.; Lin, M.L.; Liu, Y.; Zhu, C.; Sun, L.H.; Gui, H.R. Toxicity of coal fly ash and coal gangue leachate to Daphnia magna: Focusing on typical heavy metals. J. Clean. Prod. 2022, 330, 129946. [Google Scholar] [CrossRef]
- Zhang, H.J.; Ouyang, S.L. Release Characteristics of Heavy Metals from Coal Gangue under Simulation Leaching Conditions. Energy Explor. Exploit. 2014, 32, 413–422. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, L.J.; Wu, Y.G.; Fu, T.L.; Ran, Y. Enrichment of heavy metals in coal gangue by puff balls and mechanism research. Chin. J. Geochem. 2014, 33, 419–424. [Google Scholar] [CrossRef]
- Yang, X.Y.; Zhang, Y.; Lin, C. Microstructure Analysis and Effects of Single and Mixed Activators on Setting Time and Strength of Coal Gangue-Based Geopolymers. Gels 2022, 8, 195. [Google Scholar] [CrossRef]
- Gabor, M.; Halyag, P.N.; Carina, U.; Oliveira, F.P.; Ferenc, K. Mechanical Activation of Construction and Demolition Waste in Order to Improve Its Pozzolanic Reactivity. ACS Sustain. Chem. Eng. 2021, 9, 3416–3427. [Google Scholar]
- Ravaszová, S.; Dvořák, K. Effect of mechanical activation on the properties of Portland cement. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1205, 012003. [Google Scholar] [CrossRef]
- Mitrović, A.; Zdujić, M. Preparation of pozzolanic addition by mechanical treatment of kaolin clay. Int. J. Miner. Process. 2014, 132, 59–66. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Qiu, J.P.; Ma, Z.Y.; Sun, X.G. Eco-friendly treatment of coal gangue for its utilization as supplementary cementitious materials. J. Clean. Prod. 2021, 285, 124834. [Google Scholar] [CrossRef]
- Chen, J.X.; Guan, X.; Zhu, M.Y.; Gao, J.; Zhao, D. Mechanism on Activation of Coal Gangue Admixture. Adv. Civ. Eng. 2021, 2021, 5436482. [Google Scholar] [CrossRef]
- Li, Z.F.; Gao, Y.F.; Zhang, J.; Zhang, C.; Chen, J.P.; Liu, C. Effect of particle size and thermal activation on the coal gangue based geopolymer. Mater. Chem. Phys. 2021, 267, 124657. [Google Scholar] [CrossRef]
- Li, C.; Wan, J.H.; Sun, H.H.; Li, L.T. Investigation on the activation of coal gangue by a new compound method. J. Hazard. Mater. 2010, 179, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Guo, W. Research on Coal Gangue Activation and Its Activity Evaluation Method. Ph.D. Thesis, Nanjing Tech University, Nanjing, China, 2005. (In Chinese). [Google Scholar]
- Yuan, S.; Li, Y.J.; Han, Y.X.; Gao, P. Effects of carbonaceous matter additives on kinetics, phase and structure evolution of coal-series kaolin during calcination. Appl. Clay Sci. 2018, 165, 124–134. [Google Scholar] [CrossRef]
- Gong, C.C.; Li, D.X.; Wang, X.J.; Li, Z.J. Activity and structure of calcined coal gangue. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2007, 22, 749–753. [Google Scholar] [CrossRef]
- Guo, W.; Li, D.X.; Chen, J.H.; Yang, N.R. Structure and pozzolanic activity of calcined coal gangue during the process of mechanical activation. J. Wuhan Univ. Technol. Mater. Sci. Ed 2009, 24, 4. [Google Scholar] [CrossRef]
- Guo, W.; Zhu, J.P.; Li, D.X.; Chen, J.H.; Yang, N.R. Early hydration of composite cement with thermal activated coal gangue. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2010, 25, 162–166. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Zhu, Y.C.; Wang, A.G.; Sun, D.S.; Liu, K.W.; Liu, P.; Chu, Y.J. Valorization of calcined coal gangue as coarse aggregate in concrete. Cem. Concr. Compos. 2021, 121, 104057. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Xu, L.; Seetharaman, S.; Liu, L.L.; Wang, X.D.; Zhang, Z.T. Effects of chemistry and mineral on structural evolution and chemical reactivity of coal gangue during calcination: Towards efficient utilization. Mater. Struct. 2015, 48, 2279–2793. [Google Scholar] [CrossRef]
- Li, L.L.; Zhang, Y.M.; Zhang, Y.F.; Sun, J.M.; Hao, Z.F. The thermal activation process of coal gangue selected from Zhungeer in China. J. Therm. Anal. Calorim. 2016, 126, 1559–1566. [Google Scholar] [CrossRef]
- Li, Y.F.; Wang, W.X.; Yang, X.Y. Analysis of Thermal Activation and Phase Transformation of Coal Gangue. J. Chin. Ceram. Soc. 2007, 35, 1258. (In Chinese) [Google Scholar]
- Sun, Y.F.; Zhang, P.; Hu, J.P.; Liu, B.C.; Yang, J.K.; Liang, S.; Xiao, K.K.; Hou, H.J. A review on microwave irradiation to the properties of geopolymers: Mechanisms and challenges. Constr. Build. Mater. 2021, 294, 123491. [Google Scholar] [CrossRef]
- Zhao, Z.M.; Han, S.; Liu, Y.C.; Quan, S.C. On Experimental Study of Activation Coal Gangue-Portland Cement by Microwave Technology. Adv. Mater. Res. 2013, 838–841, 2658–2662. [Google Scholar] [CrossRef]
- Lan, X.J.; Liu, S.H.; Jing, Y. Microwave activation of coal gangue for Al compound. IOP Conf. Ser. Mater. Sci. Eng. 2019, 1205, 012003. [Google Scholar] [CrossRef]
- Zhang, X.M.; Zhao, Z.M.; Wang, C.J. Simulation of Microwave Activation Effect of Coal Gangue by ANSYS. Adv. Mater. Res. 2013, 838–841, 1898–1902. [Google Scholar] [CrossRef]
- Ubolluk, R.; Kanokwan, P.; Prinya, C. Effect of chemical admixtures on properties of high-calcium fly ash geopolymer. Int. J. Miner. Metall. Mater. 2011, 18, 364–369. [Google Scholar]
- Karunanithi, S. Chemical activation and curing regime of geopolymer concretes. Proc. Inst. Civ. Eng.-Constr. Mater. 2015, 168, 24–34. [Google Scholar]
- Geetha, S.; Ramamurthy, K. Properties of geopolymerised low-calcium bottom ash aggregate cured at ambient temperature. Cem. Concr. Compos. 2013, 43, 20–30. [Google Scholar] [CrossRef]
- Qin, B.K.; Ji, Y.H.; Bai, Z.L.; Zhao, Q.K. Research on mechanical behavior of calcined coal gangue cement mortar with different chemical activators. In Proceedings of the 2017 3rd International Forum on Energy, Environment Science and Materials (IFEESM 2017), Shenzhen, China, 25–26 November 2017. [Google Scholar]
- Li, Y.D.; Li, J.F.; Cui, J.; Shan, Y.; Niu, Y.F. Experimental study on calcium carbide residue as a combined activator for coal gangue geopolymer and feasibility for soil stabilization. Constr. Build. Mater. 2021, 312, 125465. [Google Scholar] [CrossRef]
- Fernando, P.T.; João, C.G.; Said, J. Alkali-activated binders: A review. Part 2. About materials and binders manufacture. Constr. Build. Mater. 2007, 22, 1315–1322. [Google Scholar]
- Liew, Y.M.; Heah, C.Y.; Mustafa, A.B.M.; Kamarudin, H. Structure and properties of clay-based geopolymer cements: A review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- Han, J.Y.; Song, X.Y.; Gao, Z.H. Excitation Effect of Soluble Glass on Composite System with Calcined Coal Gangue and Slag. Appl. Mech. Mater. 2012, 1801, 30–34. [Google Scholar] [CrossRef]
- Xu, J.; Kang, A.H.; Wu, Z.G.; Gong, Y.F.; Xiao, P. The effect of mechanical-thermal synergistic activation on the mechanical properties and microstructure of recycled powder geopolymer. J. Clean. Prod. 2021, 327, 129477. [Google Scholar] [CrossRef]
- Liu, S.H.; Lan, X.J.; Gao, H. Complex Activation of Coal Gangue to Al Compounds. Adv. Mater. Res. 2011, 402, 734–737. [Google Scholar] [CrossRef]
- Li, Y.; Yao, Y.; Liu, X.M.; Sun, H.H.; Ni, W. Improvement on pozzolanic reactivity of coal gangue by integrated thermal and chemical activation. Fuel 2013, 109, 527–533. [Google Scholar] [CrossRef]
- Song, X.Y.; Han, J.Y.; Gao, Z.H. Thermally and Chemically Added-Calcium Composite Activation of Coal Gangue and its Application in Cement. Adv. Mater. Res. 2011, 1368, 1016–1019. [Google Scholar] [CrossRef]
- van Deventer, J.S.J.; Provis, J.L.; Duxson, P.; Lukey, G.C. Reaction mechanisms in the geopolymeric conversion of inorganic waste to useful products. J. Hazard. Mater. 2007, 139, 506–513. [Google Scholar] [CrossRef]
- Guo, L.Z.; Zhou, M.; Wang, X.Y.; Li, C.; Jia, H.Q. Preparation of coal gangue-slag-fly ash geopolymer grouting materials. Constr. Build. Mater. 2022, 328, 126997. [Google Scholar] [CrossRef]
- Kashani, A.; Provis, J.L.; Qiao, G.G.; Deventer, J.S.J.V. The interrelationship between surface chemistry and rheology in alkali activated slag paste. Constr. Build. Mater. 2014, 65, 583–591. [Google Scholar] [CrossRef]
- Dai, X.D.; Aydın, S.; Yardımcı, M.Y.; Lesage, K.; De, S.G. Effects of activator properties and GGBFS/FA ratio on the structural build-up and rheology of AAC. Cem. Concr. Res. 2020, 138, 106253. [Google Scholar] [CrossRef]
- Heah, C.Y.; Kamarudin, H.; Bakri, A.M.M.A.; Bnhussain, M.; Luqman, M.; Nizar, I.K.; Ruzaidi, C.M.; Liew, Y.M. Study on solids-to-liquid and alkaline activator ratios on kaolin-based geopolymers. Constr. Build. Mater. 2012, 35, 912–922. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, H.J.; Zhang, Z.H.; Gao, X.; Zhang, C.S.; Wu, Q.S. Effect of fly ash microsphere on the rheology and microstructure of alkali-activated fly ash/slag pastes. Cem. Concr. Res. 2018, 109, 198–207. [Google Scholar] [CrossRef]
- Huang, G.D.; Ji, Y.S.; Li, J.; Hou, Z.H.; Dong, Z.C. Improving strength of calcinated coal gangue geopolymer mortars via increasing calcium content. Constr. Build. Mater. 2018, 166, 760–768. [Google Scholar] [CrossRef]
- Yang, X.Y.; Zhang, Y.; Lin, C. Compressive and Flexural Properties of Ultra-Fine Coal Gangue-Based Geopolymer Gels and Microscopic Mechanism Analysis. Gels 2022, 8, 145. [Google Scholar] [CrossRef]
- Wang, C.B.; Liu, C.X.; Zhang, L.H.; Wang, C.; Xu, S.Z.; Yang, J.X. Exploring calcined coal gangue fines as the total substitute of fly ash in the production of alkali-activated slag/fly ash materials. Case Stud. Constr. Mater. 2022, 17, e01332. [Google Scholar] [CrossRef]
- Zhang, D.M.; Sun, F.J.; Liu, T.T.; Chindaprasirt, P. Study on Preparation of Coal Gangue-Based Geopolymer Concrete and Mechanical Properties. Adv. Civ. Eng. 2021, 2021, 5117584. [Google Scholar] [CrossRef]
- Liu, C.J.; Deng, X.W.; Liu, J.; Hui, D. Mechanical properties and microstructures of hypergolic and calcined coal gangue based geopolymer recycled concrete. Constr. Build. Mater. 2019, 221, 691–708. [Google Scholar] [CrossRef]
- Ma, H.Q.; Zhu, H.G.; Chen, H.Y.; Ni, Y.D.; Wang, T.; Yang, S. Effects and mechanisms of slag reinforced coal gangue geopolymers. IOP Conf. Ser. Mater. Sci. Eng. 2019, 474, 012040. [Google Scholar] [CrossRef]
- Xu, Z.F.; Zou, X.T.; Chen, J. Preparation of Thermal Activation Sludge and Coal Gangue Polymer. Integr. Ferroelectr. 2015, 160, 1–9. [Google Scholar]
- Geng, J.J.; Zhou, M.; Zhang, T.; Wang, W.X.; Wang, T.; Zhou, X.; Wang, X.S.; Hou, H.B. Preparation of blended geopolymer from red mud and coal gangue with mechanical co-grinding preactivation. Mater. Struct. 2017, 50, 109. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Li, L.F.; Ma, X.; Hao, W. Compositional, microstructural and mechanical properties of ambient condition cured alkali-activated cement. Constr. Build. Mater. 2016, 113, 237–245. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.S.; Song, Q.C. The effect of Na + and H 2 O on structural and mechanical properties of coal gangue-based geopolymer: Molecular dynamics simulation and experimental study. Constr. Build. Mater. 2020, 268, 121081. [Google Scholar] [CrossRef]
- Zhou, S.Q.; Lu, C.H.; Zhu, X.Y.; Li, F. Preparation and Characterization of High-Strength Geopolymer Based on BH-1 Lunar Soil Simulant with Low Alkali Content. Engineering 2021, 7, 1631–1645. [Google Scholar] [CrossRef]
- Cheng, S.K.; Shui, Z.H.; Sun, T.; Yu, R.; Zhang, G.Z.; Ding, S. Effects of fly ash, blast furnace slag and metakaolin on mechanical properties and durability of coral sand concrete. Appl. Clay Sci. 2017, 141, 111–117. [Google Scholar] [CrossRef]
- Xu, Z.F.; Ye, D.F.; Dai, T.; Dai, Y. Research on Preparation of Coal Waste-Based Geopolymer and Its Stabilization/Solidification of Heavy Metals. Integr. Ferroelectr. 2021, 217, 214–224. [Google Scholar]
- Liu, F.; Tang, R.; Wang, B.M.; Yuan, X.S. Experimental Study on Solidification of Pb2+ in Fly Ash-Based Geopolymers. Sustainability 2021, 13, 12621. [Google Scholar] [CrossRef]
- Ji, Z.H.; Su, L.Y.; Pei, Y.S. Synthesis and toxic metals (Cd, Pb, and Zn) immobilization properties of drinking water treatment residuals and metakaolin-based geopolymers. Mater. Chem. Phys. 2020, 242, 122535. [Google Scholar] [CrossRef]
- Sanguanpak, S.; Wannagon, A.; Saengam, C.; Chiemchaisri, W.; Chiemchaisri, C. Porous metakaolin-based geopolymer granules for removal of ammonium in aqueous solution and anaerobically pretreated piggery wastewater. J. Clean. Prod. 2021, 297, 126643. [Google Scholar] [CrossRef]
- Zhao, S.J.; Xia, M.; Yu, L.; Huang, X.; Jiao, B.Q.; Li, D.W. Optimization for the preparation of composite geopolymer using response surface methodology and its application in lead-zinc tailings solidification. Constr. Build. Mater. 2021, 266, 120969. [Google Scholar] [CrossRef]
- Wang, C.; Hu, H.C.; Yang, Z.Y.; Zhong, Y.K.; Sun, M.G.; Gan, T.; Bao, B.Q. Quantifying gel properties of industrial waste-based geopolymers and their application in Pb2+ and Cu2+ removal. J. Clean. Prod. 2021, 315, 128203. [Google Scholar] [CrossRef]
- Li, J.L.; Duan, Y.; Zhou, S.K.; Rong, L.S.; Liu, Y.J.; Chu, L.P.; Li, Q.; Yang, L. Immobilization of uranium soil by geopolymer coupled with nHAP. Ceram. Int. 2021, 47, 30815–30825. [Google Scholar] [CrossRef]
- Zhou, S.K.; Li, J.L.; Rong, L.S.; Xiao, J.; Liu, Y.J.; Duan, Y.; Chu, L.P.; Li, Q.; Yang, L. Immobilization of uranium soils with alkali-activated coal gangue–based geopolymer. J. Radioanal. Nucl. Chem. 2021, 329, 1155–1166. [Google Scholar] [CrossRef]

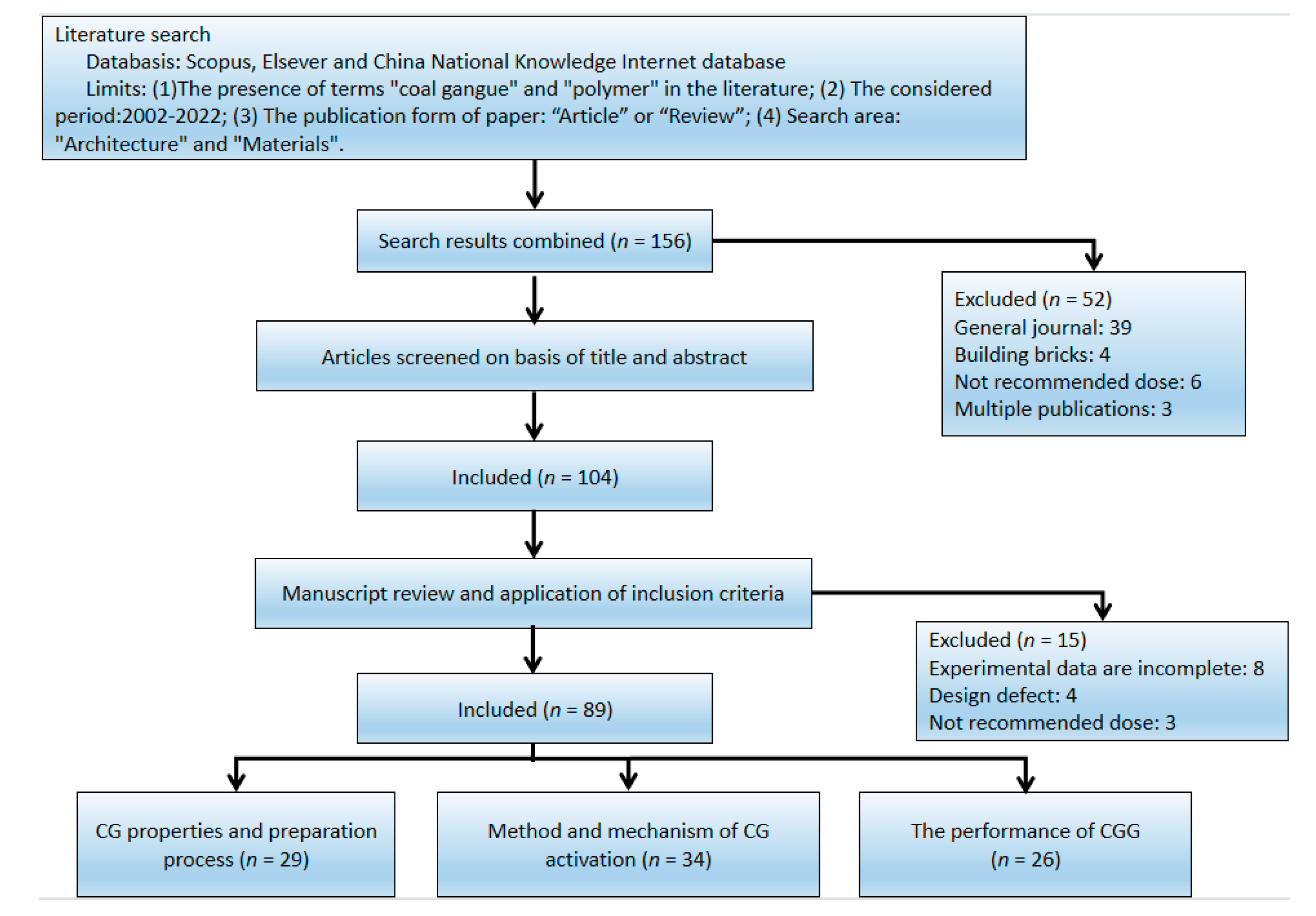
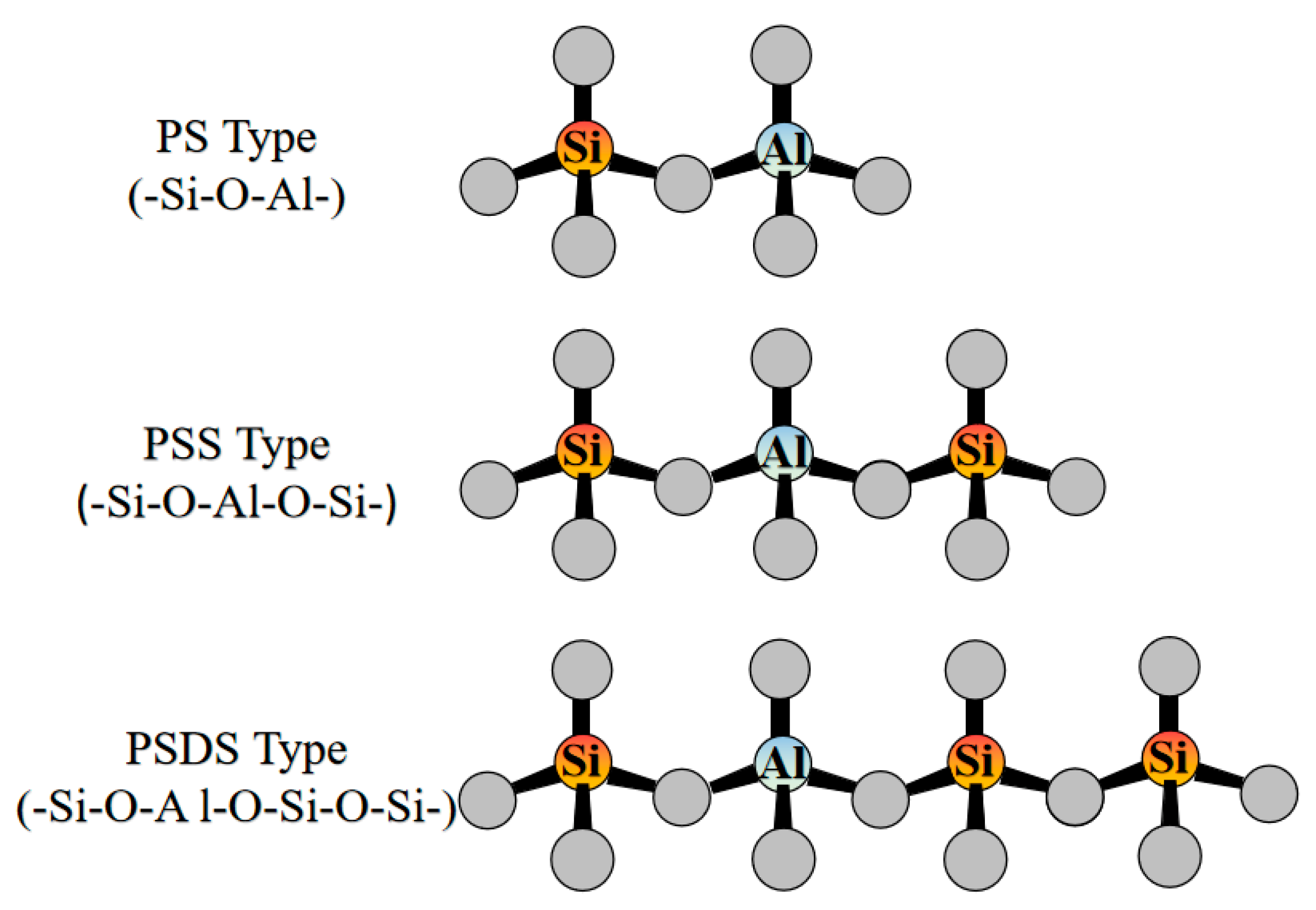
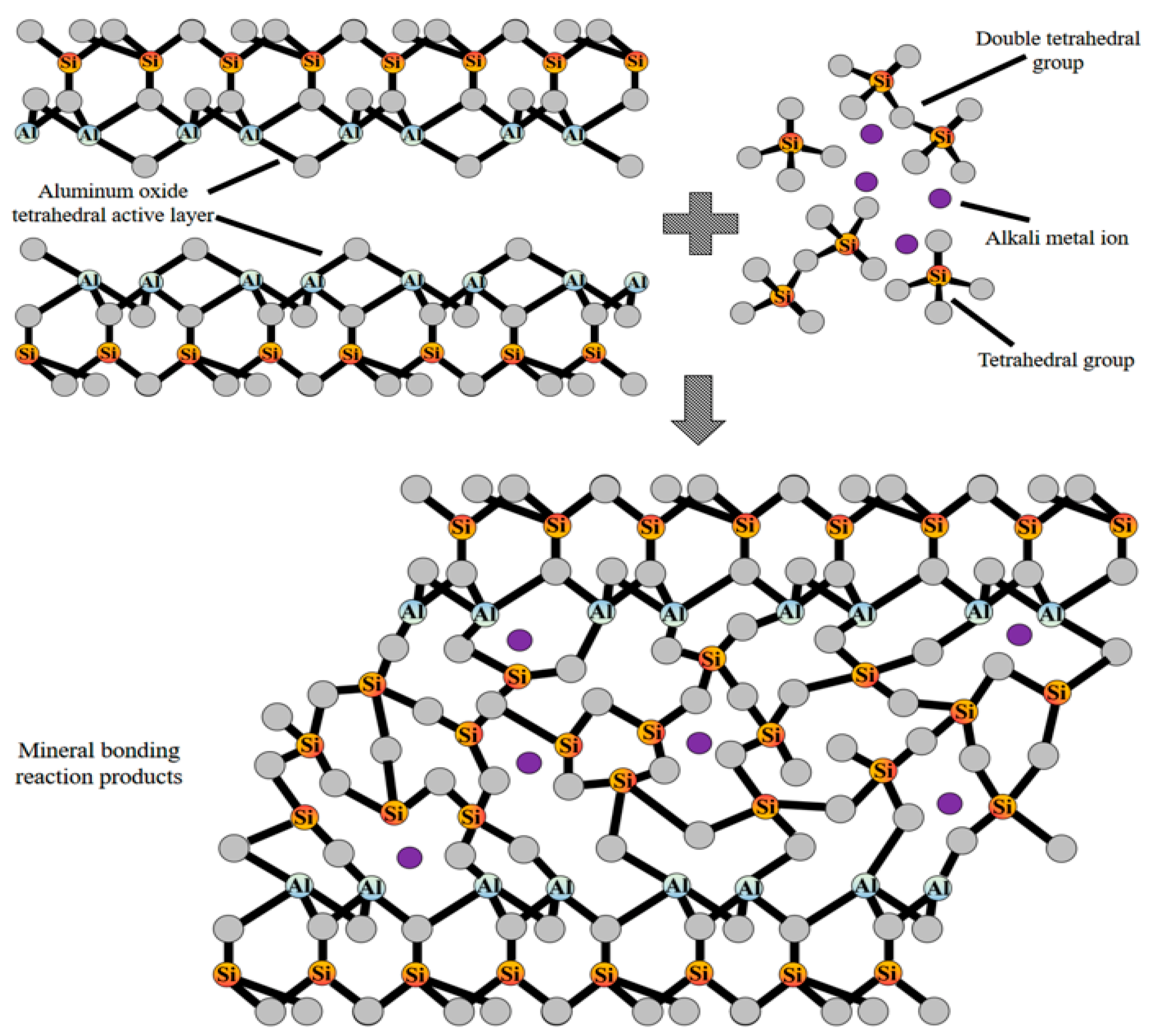
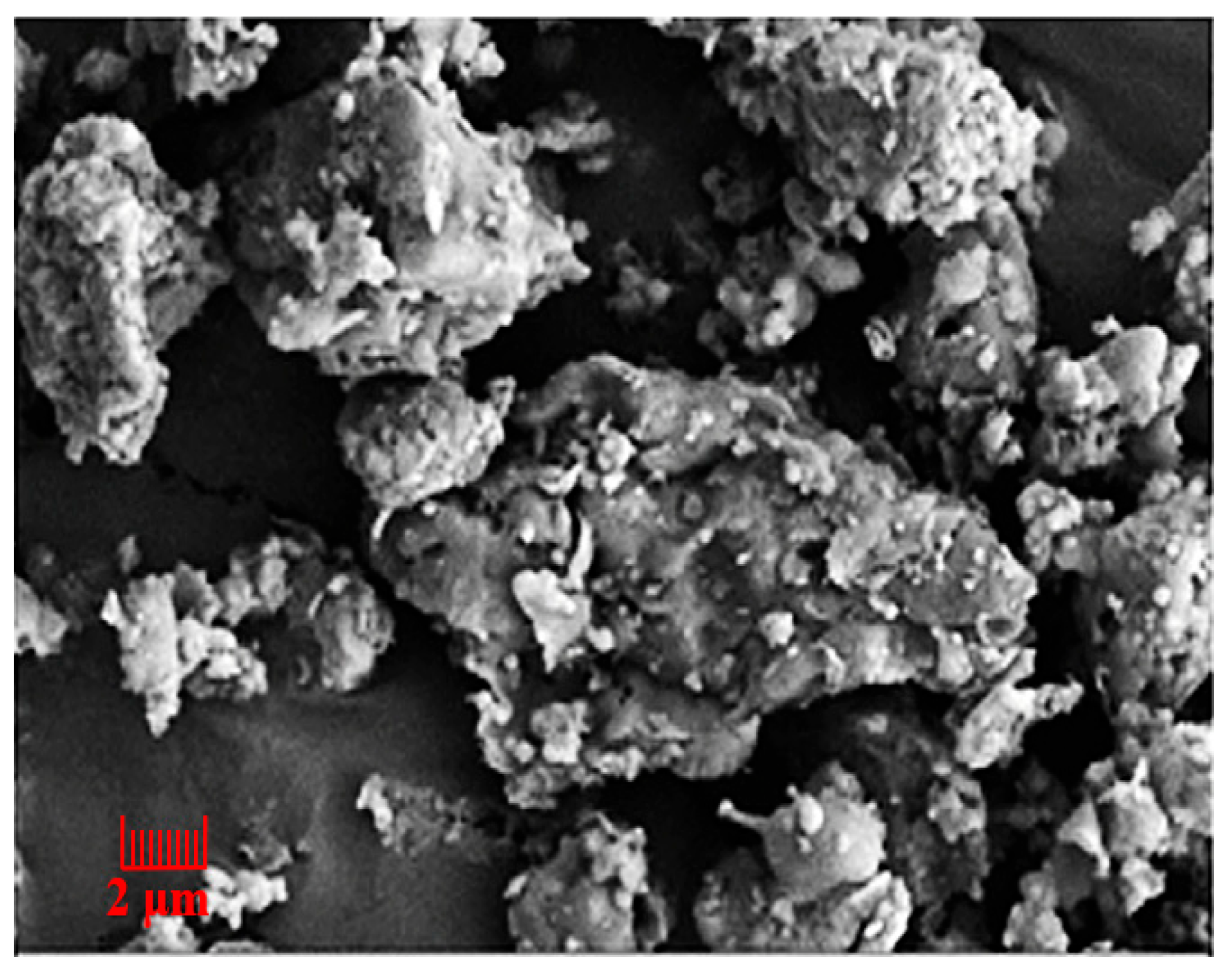
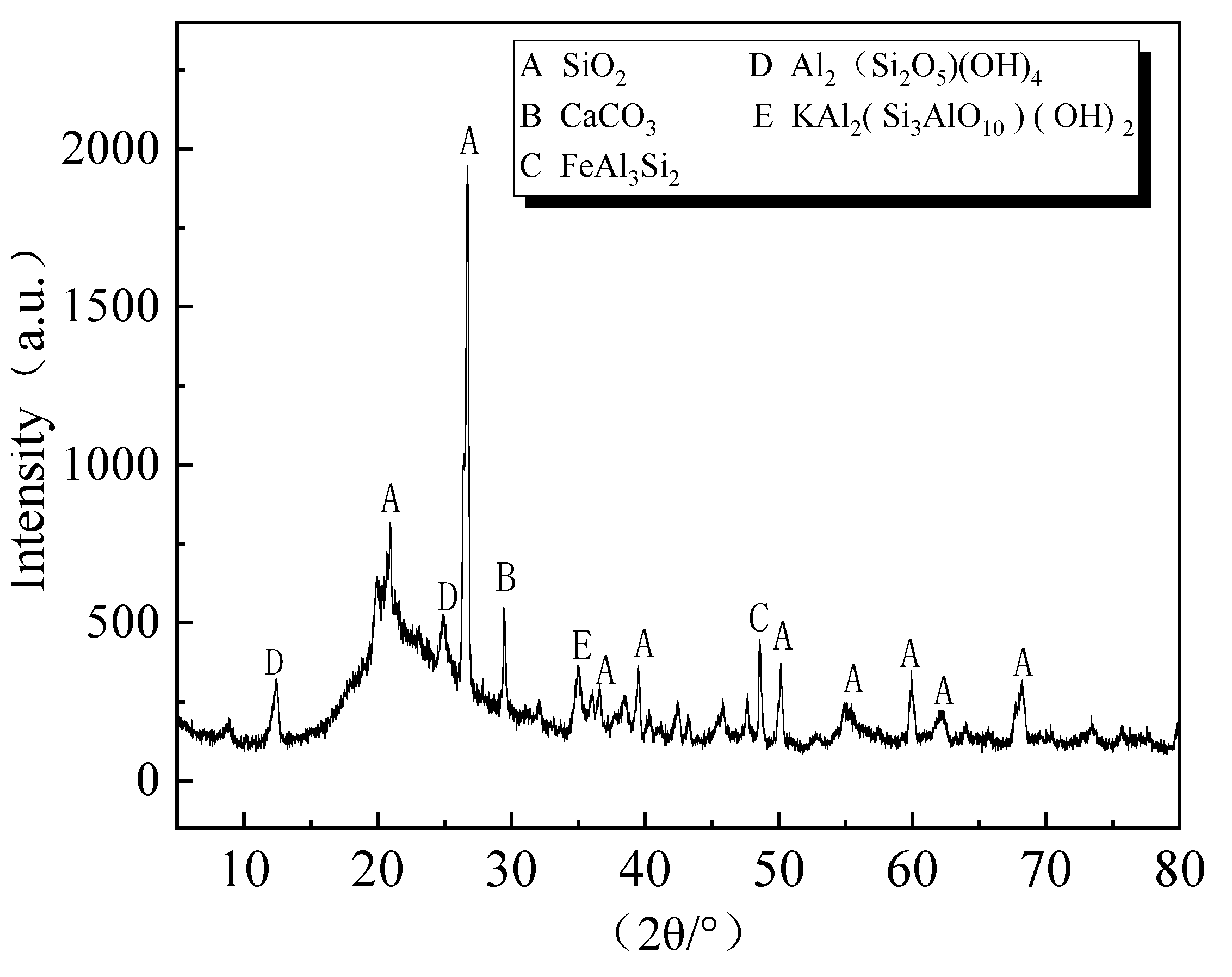
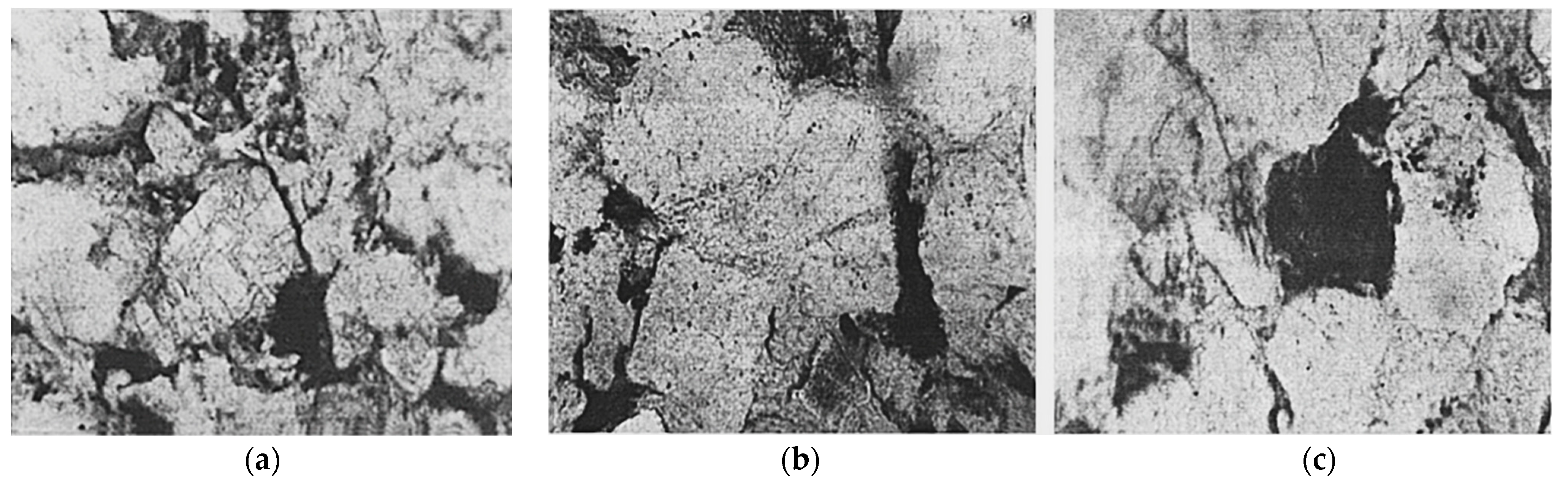
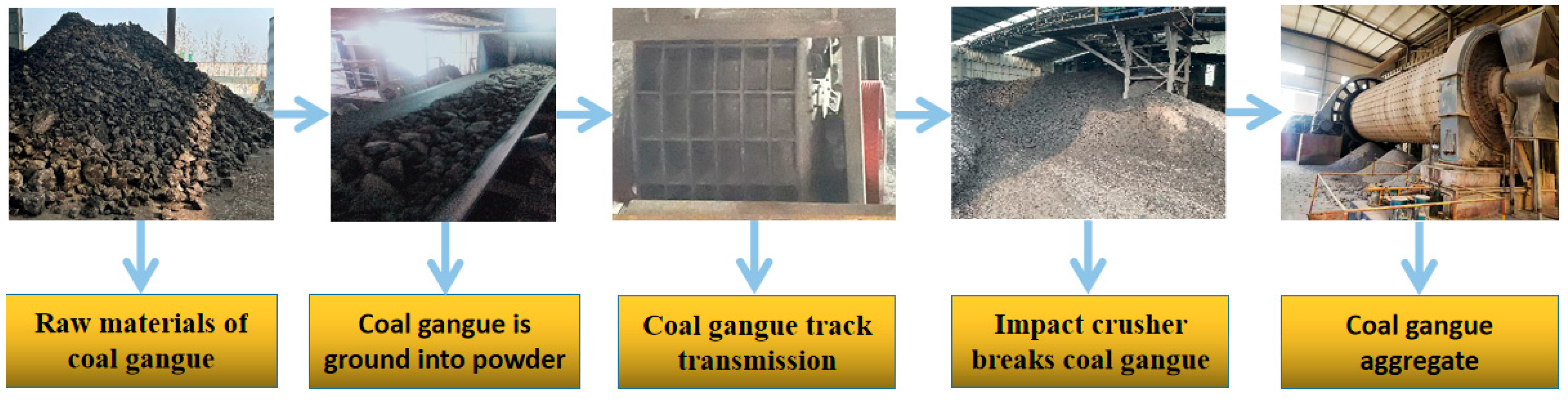
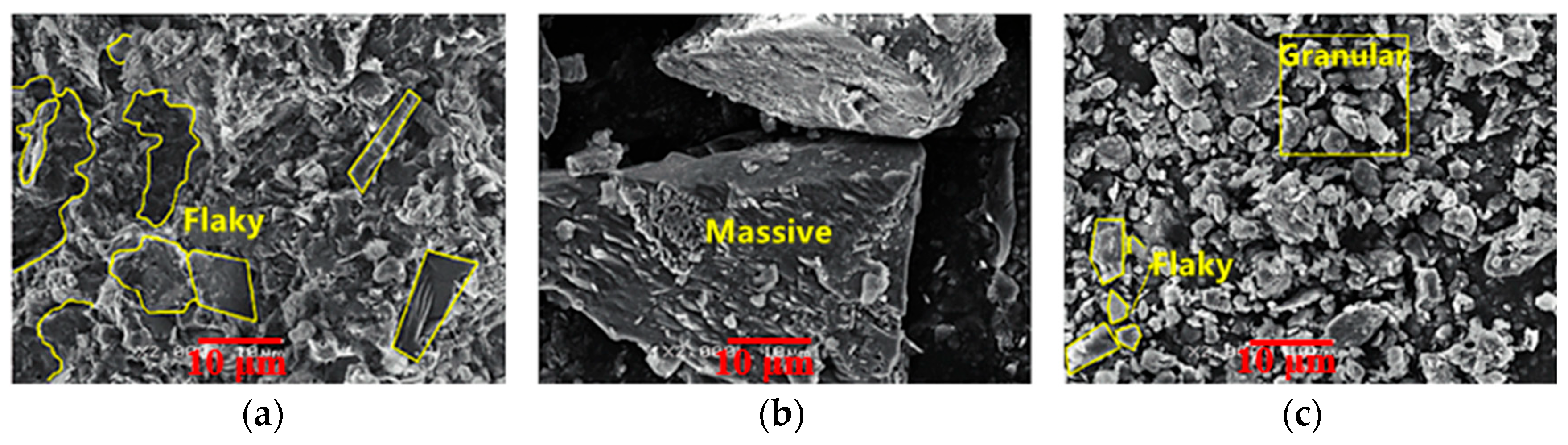
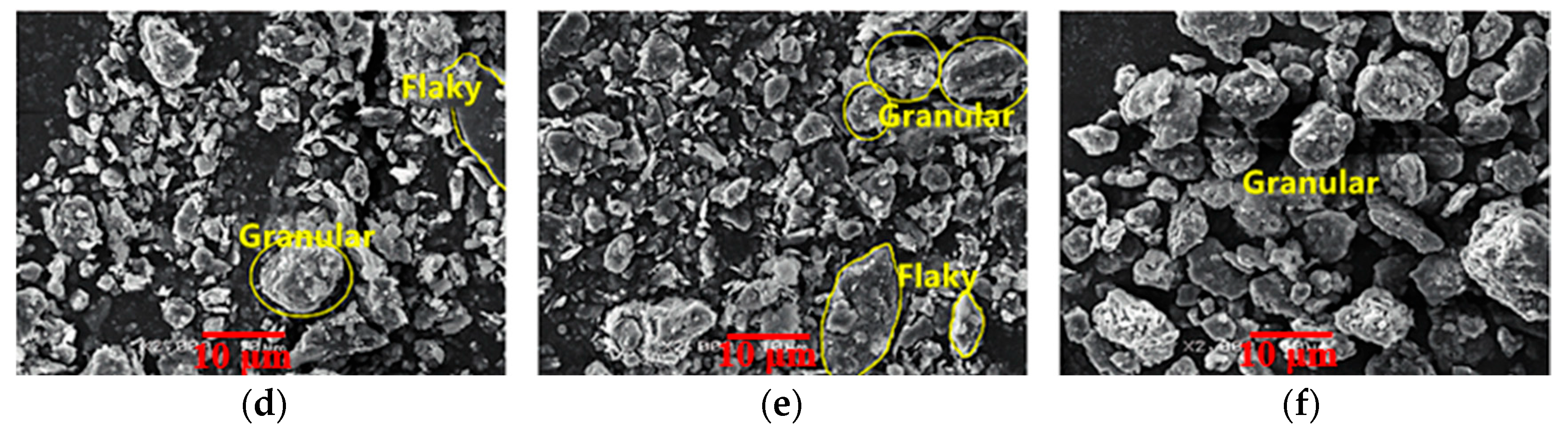
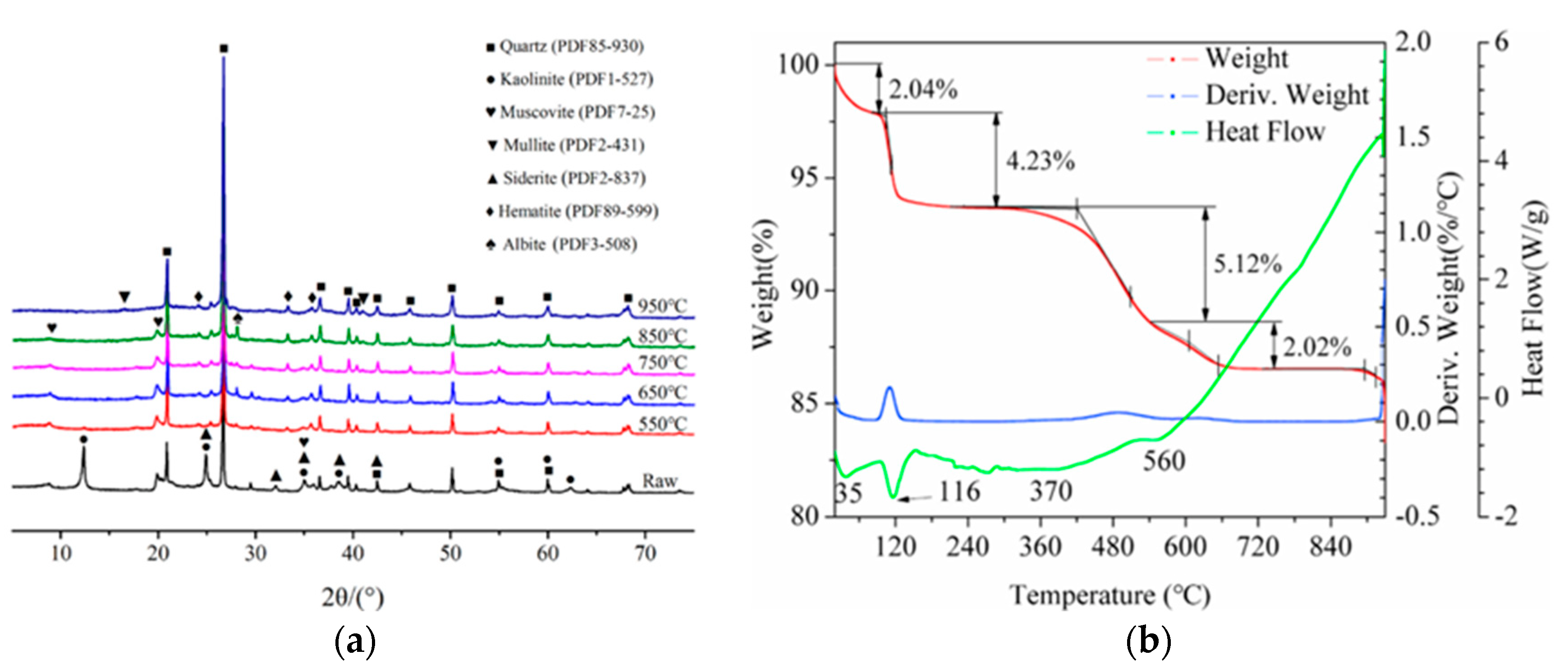
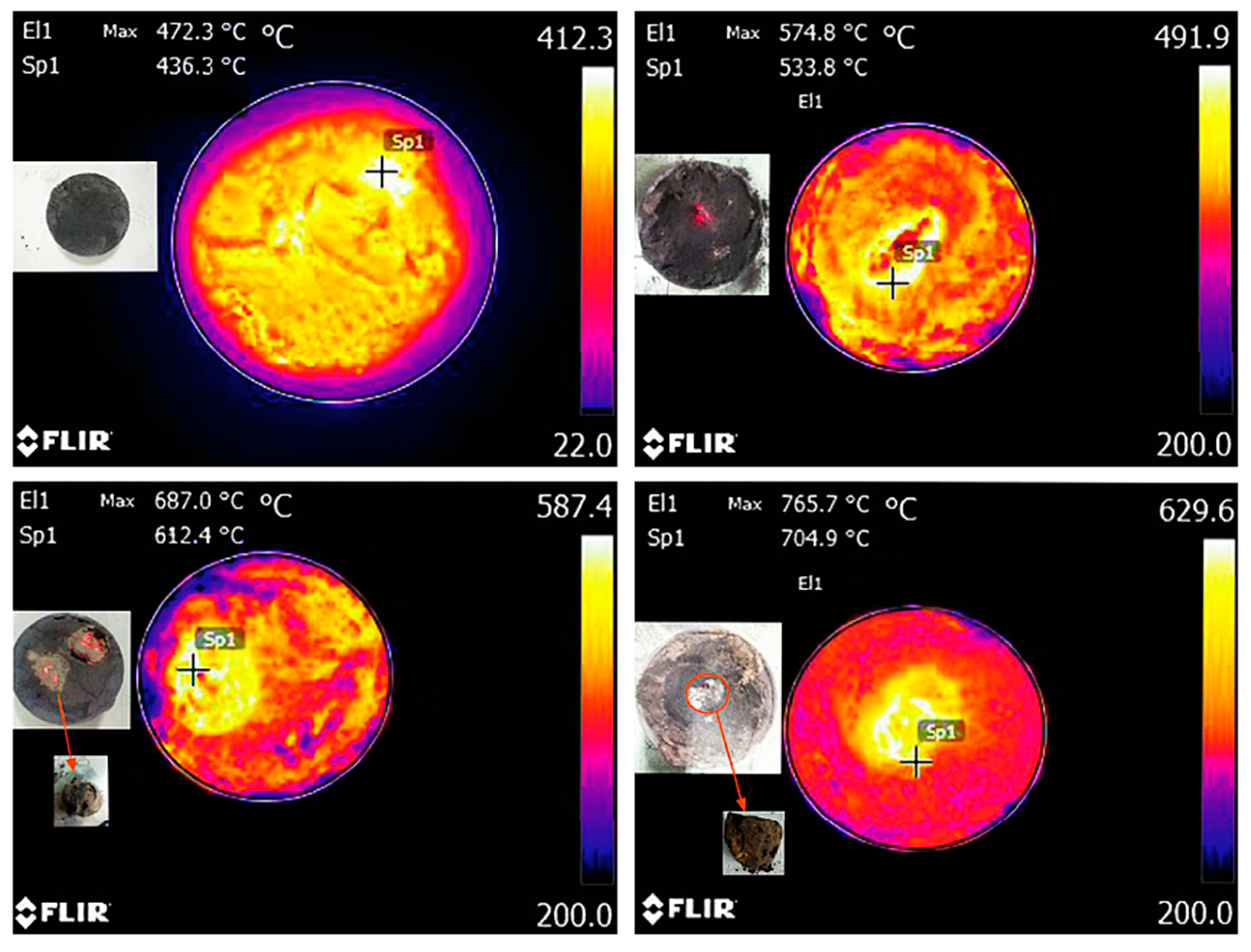

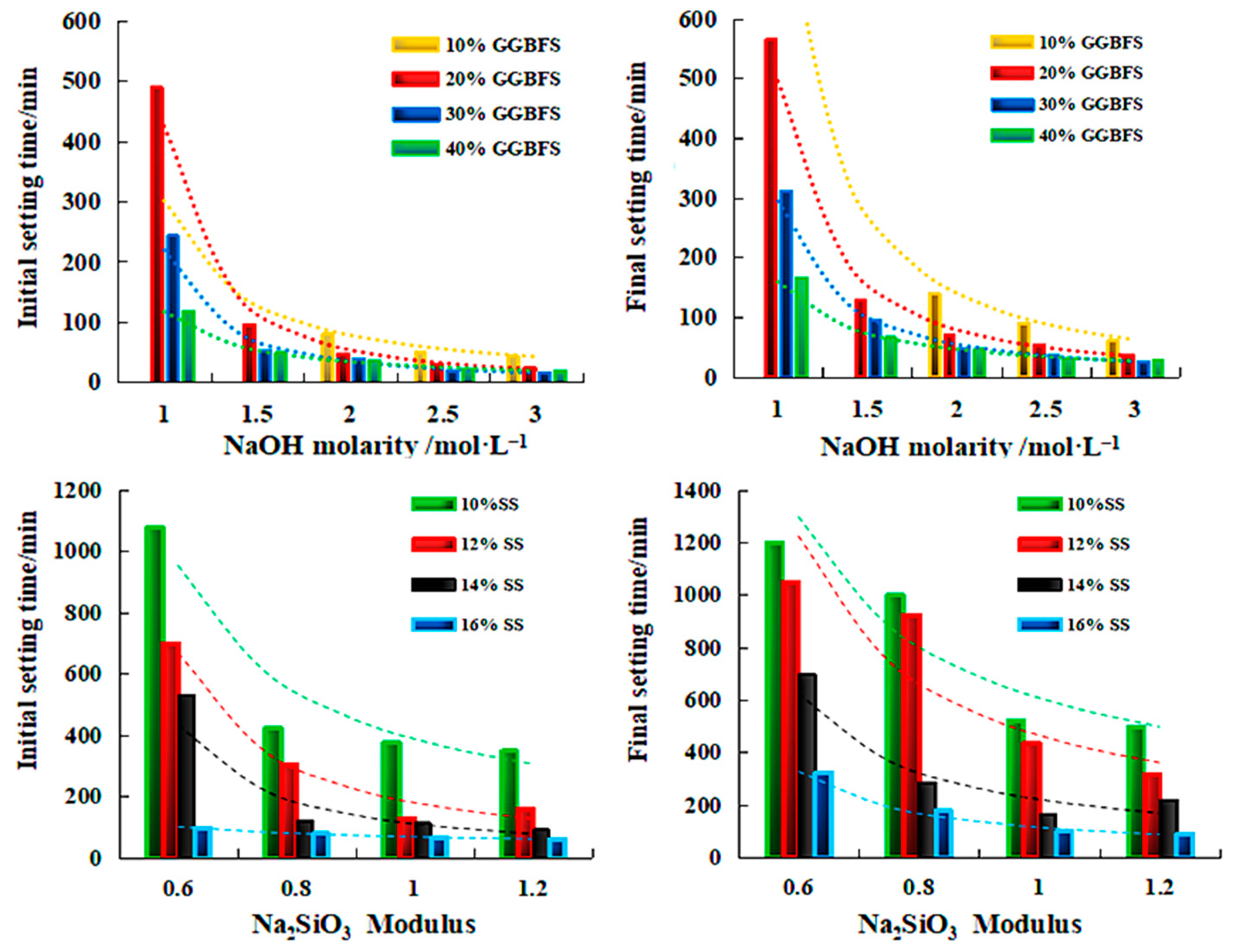
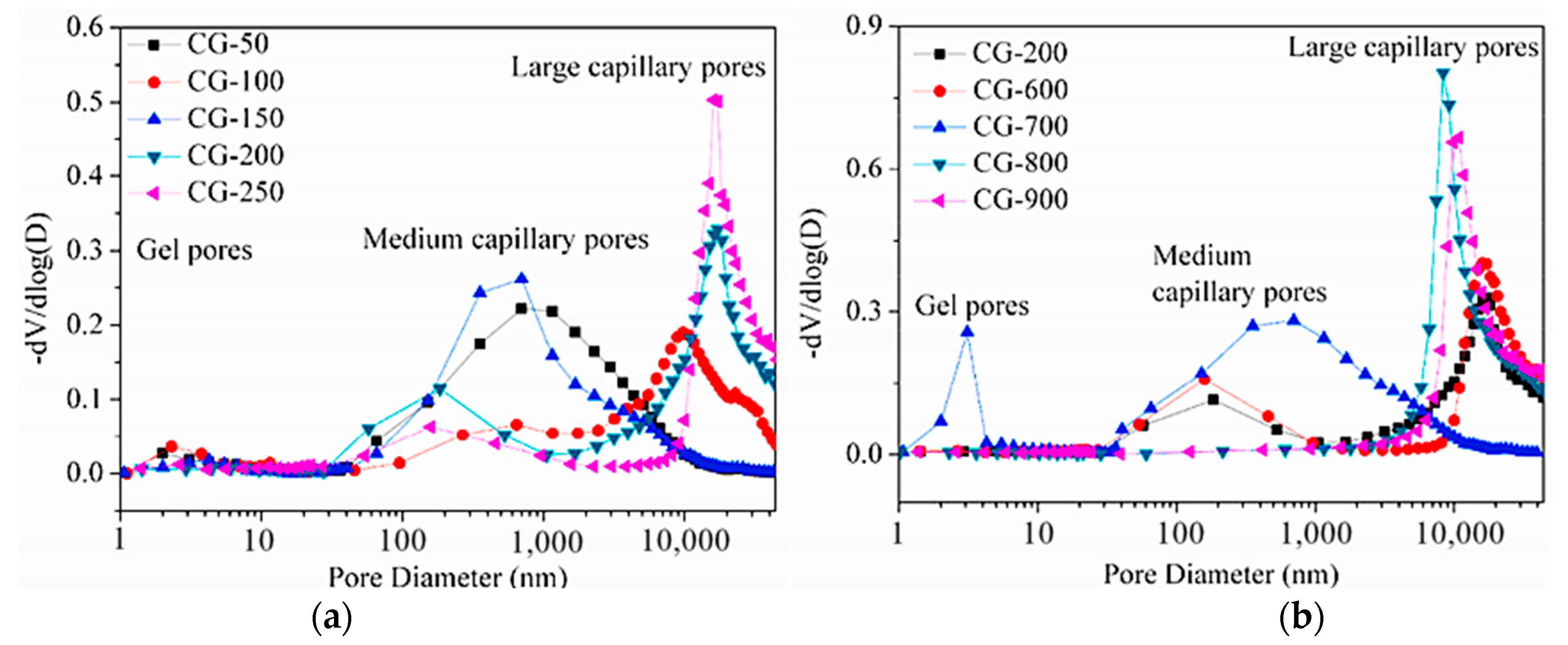
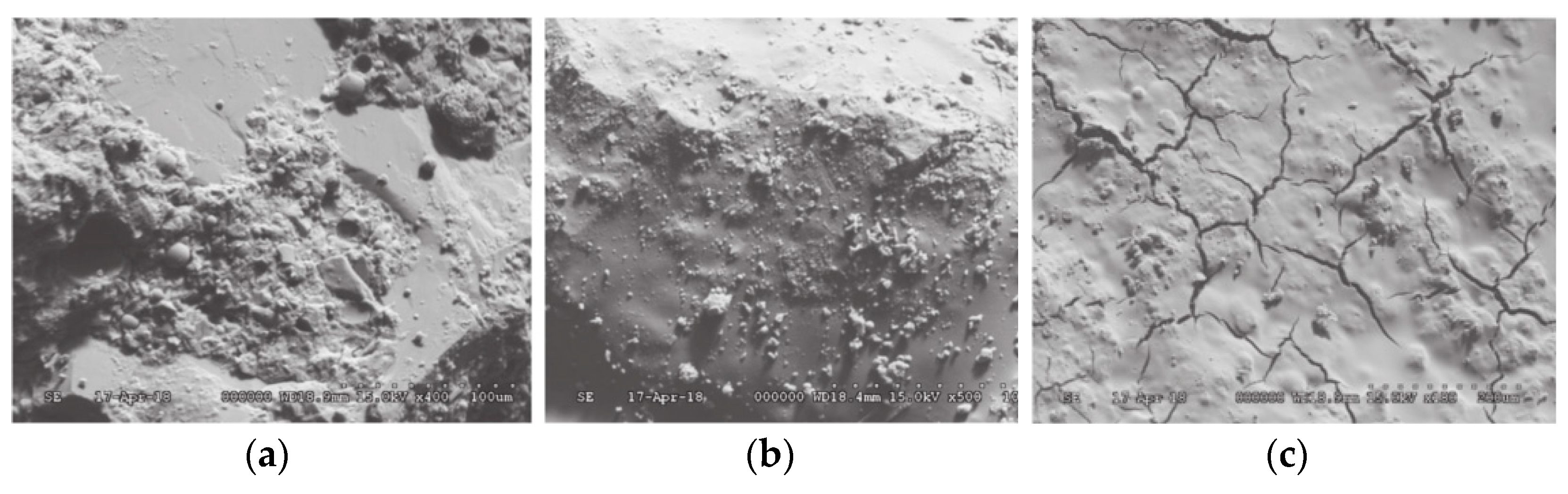
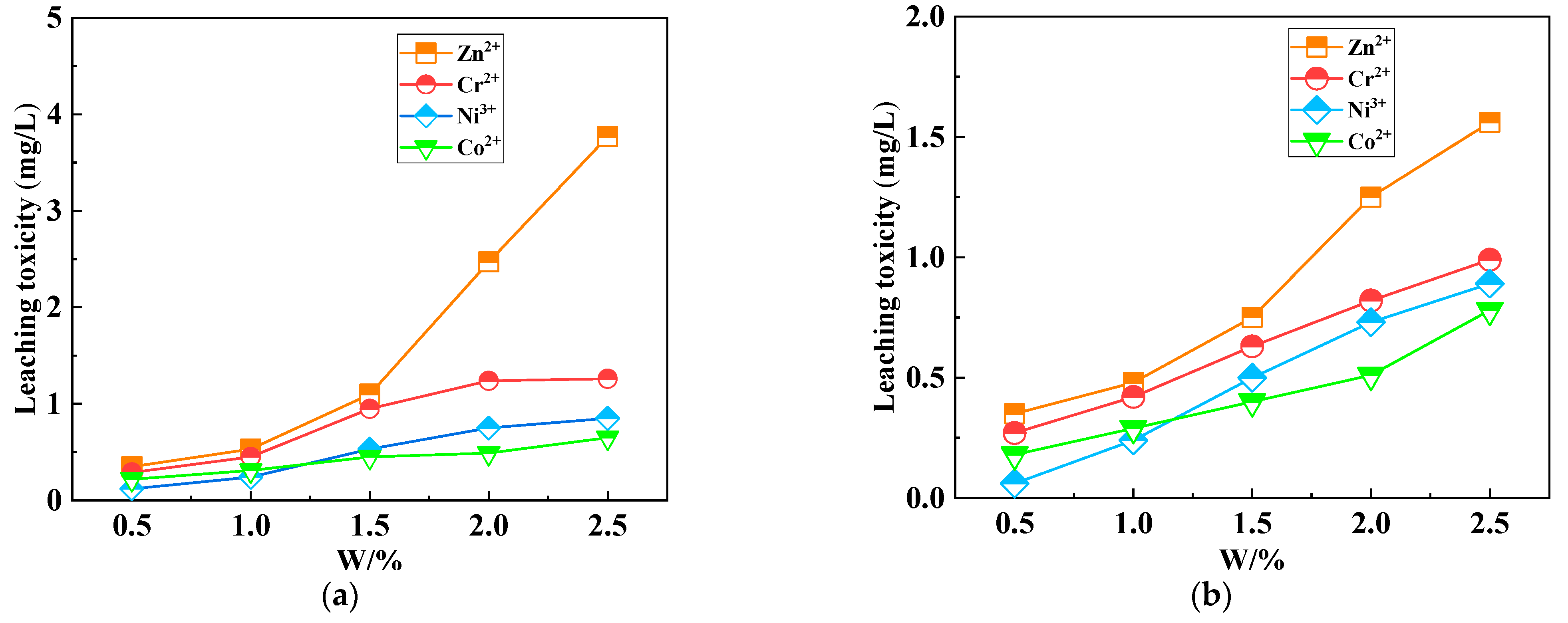
| Sources | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | TiO2 | L.O.I | Refs. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| North China | Shandong | 59.54 | 16.31 | 6.55 | 1.82 | 1.52 | - | - | - | 12.27 | [43] |
| Huaibei | 54.12 | 22.38 | 3.56 | 0.71 | 0.98 | 0.64 | 1.13 | - | - | [15] | |
| Fuxin | 48.78 | 21.86 | 5.38 | 0.82 | 3.87 | - | - | - | 12.14 | [44] | |
| Heilongjiang | 58.82 | 27.87 | 8.31 | - | 0.78 | - | 1.23 | 1.04 | 1.43 | [45] | |
| Shanxi | 56.56 | 36.78 | 1.95 | 0.22 | 0.62 | 0.42 | - | 2.10 | - | [46] | |
| Ordos | 45.9 | 16.0 | 4.71 | 1.37 | 0.74 | 0.99 | 3.36 | 0.78 | 8.03 | [47] | |
| Hebei | 52.4 | 42.26 | 0.14 | 0.08 | 0.76 | 0.03 | 0.02 | - | 2.52 | [48] | |
| Beijing | 49.90 | 24.41 | 6.42 | 1.59 | 0.82 | 1.46 | 2.06 | 0.88 | 11.76 | [49] | |
| South China | Chongqing | 58.10 | 24.50 | 5.31 | 1.05 | 5.73 | 1.14 | 1.54 | - | - | [50] |
| Liuzhi | 46.50 | 16.40 | 13.85 | 3.57 | 10.67 | 1.48 | 1.83 | - | [42] | ||
| Yichang | 49.03 | 34.18 | 0.73 | - | 0.20 | - | 0.12 | 1.72 | 13.50 | [51] | |
| Pingxiang | 52.56 | 16.57 | 3.35 | 2.01 | 1.24 | 0.21 | 2.39 | - | 20.71 | [42] | |
| Xuzhou | 57.95 | 19.02 | 5.32 | 0.82 | 3.16 | - | - | - | - | [52] | |
| Xuzhou | 60.24 | 18.50 | 2.58 | 0.52 | 1.48 | 0.14 | 1.53 | - | - | [53] | |
| Iran | 37.8 | 13.14 | 2.85 | 0.73 | 0.76 | 0.28 | 2.02 | 1.17 | 40.96 | [54] | |
| Spain | 56.4 | 26.34 | 6.42 | 1.07 | 1.06 | 0.17 | 4.02 | 1.21 | 2.38 | [55] | |
| America | 34.4 | 14.4 | 3.4 | 0.93 | 1.3 | 0.34 | 3.5 | 1.2 | 38.7 | [56] | |
| Jerada | 62.41 | 7.95 | 4.14 | 0.97 | 20.38 | 0.14 | 1.02 | - | - | [57] | |
| Sample | Cd | As | Pb | Cr | Cu | Ni | Zn | Refs. |
|---|---|---|---|---|---|---|---|---|
| CG | 0.1 | 1.56 | 20.19 | 28.72 | 17.17 | 10.59 | 44.54 | [60] |
| 0.2 | 33.45 | 59.1 | 26.82 | 32.29 | 123.21 | [61] | ||
| 0.51 | 20.26 | 46.76 | 60.16 | 16.49 | 78.23 | [62] | ||
| 0.58 | 2.156 | 62.218 | 59.972 | 36.635 | 138.199 | [58] | ||
| 1.097 | 7.71 | 5.087 | 102.84 | 5.131 | [63] | |||
| 0.66 | 3.2 | 52.98 | 76.53 | 35.90 | 120.32 | 24.30 | [59] | |
| GB5085.3-2007 | ≤15 | ≤5 | ≤5 | ≤1 | ≤100 | ≤5 | ≤100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, R.; Guo, X.; Guan, J.; Yao, X.; Hao, Y. Activation Mechanism of Coal Gangue and Its Impact on the Properties of Geopolymers: A Review. Polymers 2022, 14, 3861. https://doi.org/10.3390/polym14183861
Han R, Guo X, Guan J, Yao X, Hao Y. Activation Mechanism of Coal Gangue and Its Impact on the Properties of Geopolymers: A Review. Polymers. 2022; 14(18):3861. https://doi.org/10.3390/polym14183861
Chicago/Turabian StyleHan, Ruicong, Xiaoning Guo, Junfeng Guan, Xianhua Yao, and Ying Hao. 2022. "Activation Mechanism of Coal Gangue and Its Impact on the Properties of Geopolymers: A Review" Polymers 14, no. 18: 3861. https://doi.org/10.3390/polym14183861
APA StyleHan, R., Guo, X., Guan, J., Yao, X., & Hao, Y. (2022). Activation Mechanism of Coal Gangue and Its Impact on the Properties of Geopolymers: A Review. Polymers, 14(18), 3861. https://doi.org/10.3390/polym14183861









