Polyhydroxybutyrate Rice Hull and Torrefied Rice Hull Biocomposites
Abstract
:1. Introduction
2. Methods and Materials
2.1. Materials
2.2. Torrefaction
2.3. Milling and Particle Size Analysis
2.4. CHNSO Analysis
2.5. Differential Scanning Calorimeter (DSC)
2.6. Thermogravimetric Analysis (TGA)
2.7. Extrusion and Injection Molding
2.8. Mechanical Testing
2.9. Fourier Transform Infrared (FTIR) Spectroscopy
2.10. Thermomechanical Analysis (TMA)
2.11. Scanning Electron Microscope
3. Results and Discussion
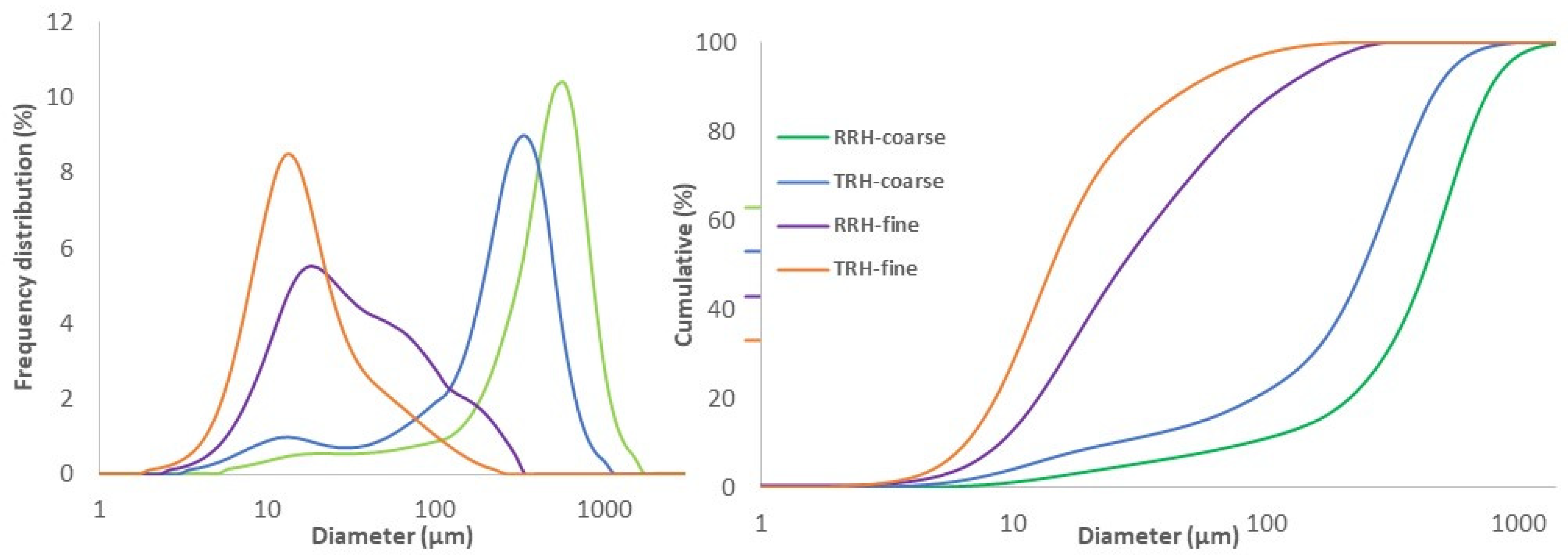
3.1. Milling and Particle Size Analysis
3.2. CHNSO Analysis
3.3. Differential Scanning Calorimeter (DSC)
3.4. Thermogravimetric Analysis
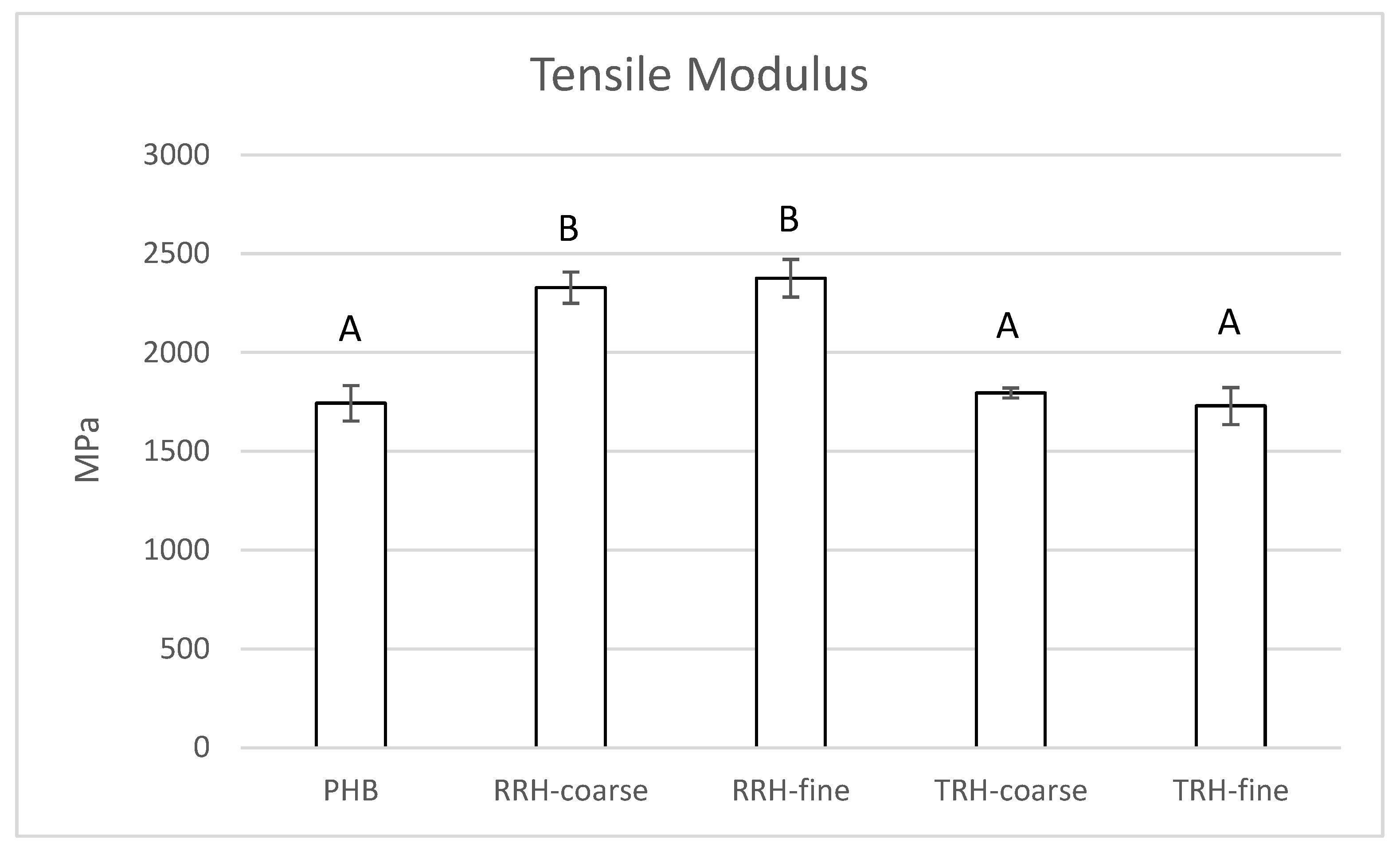
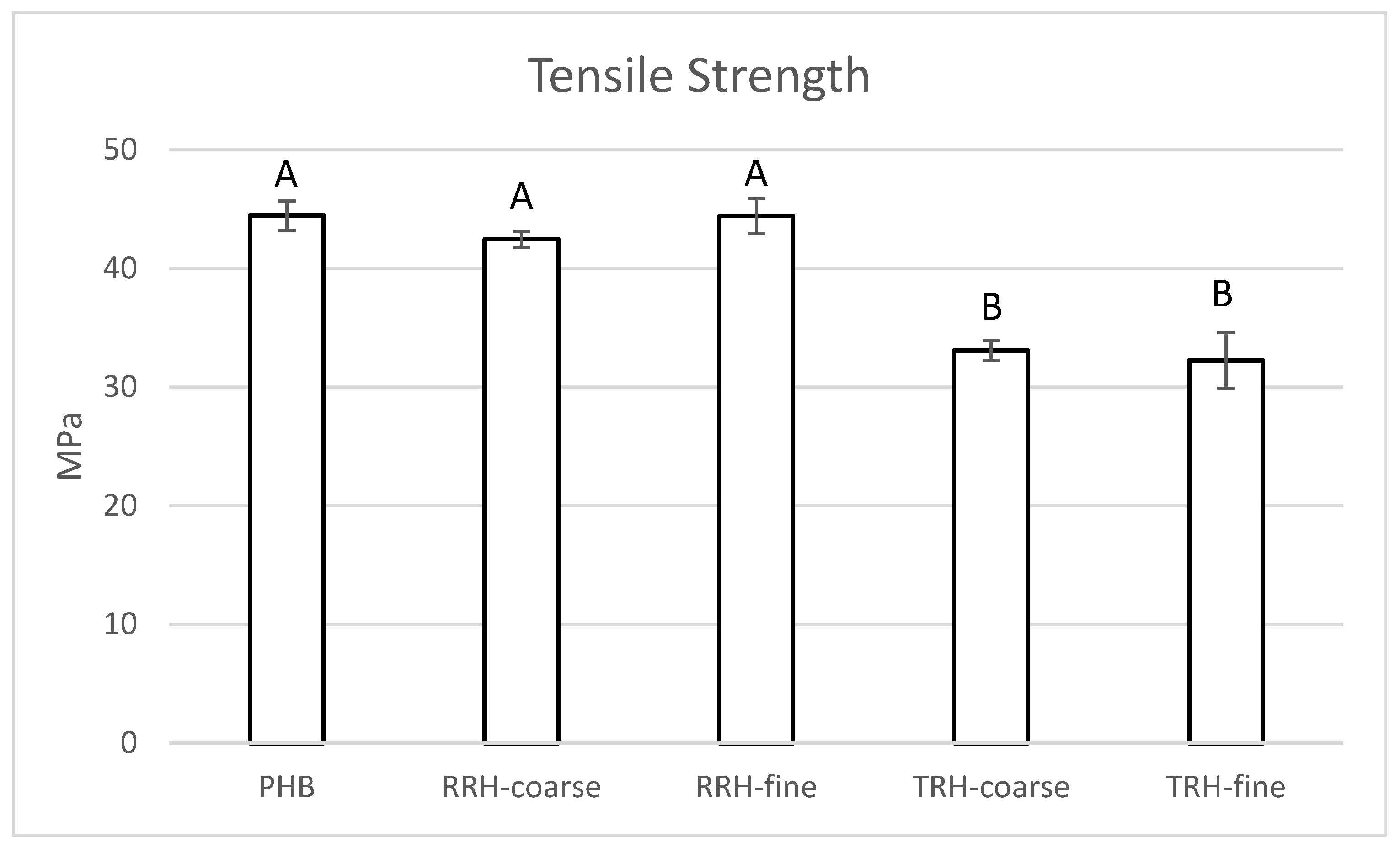
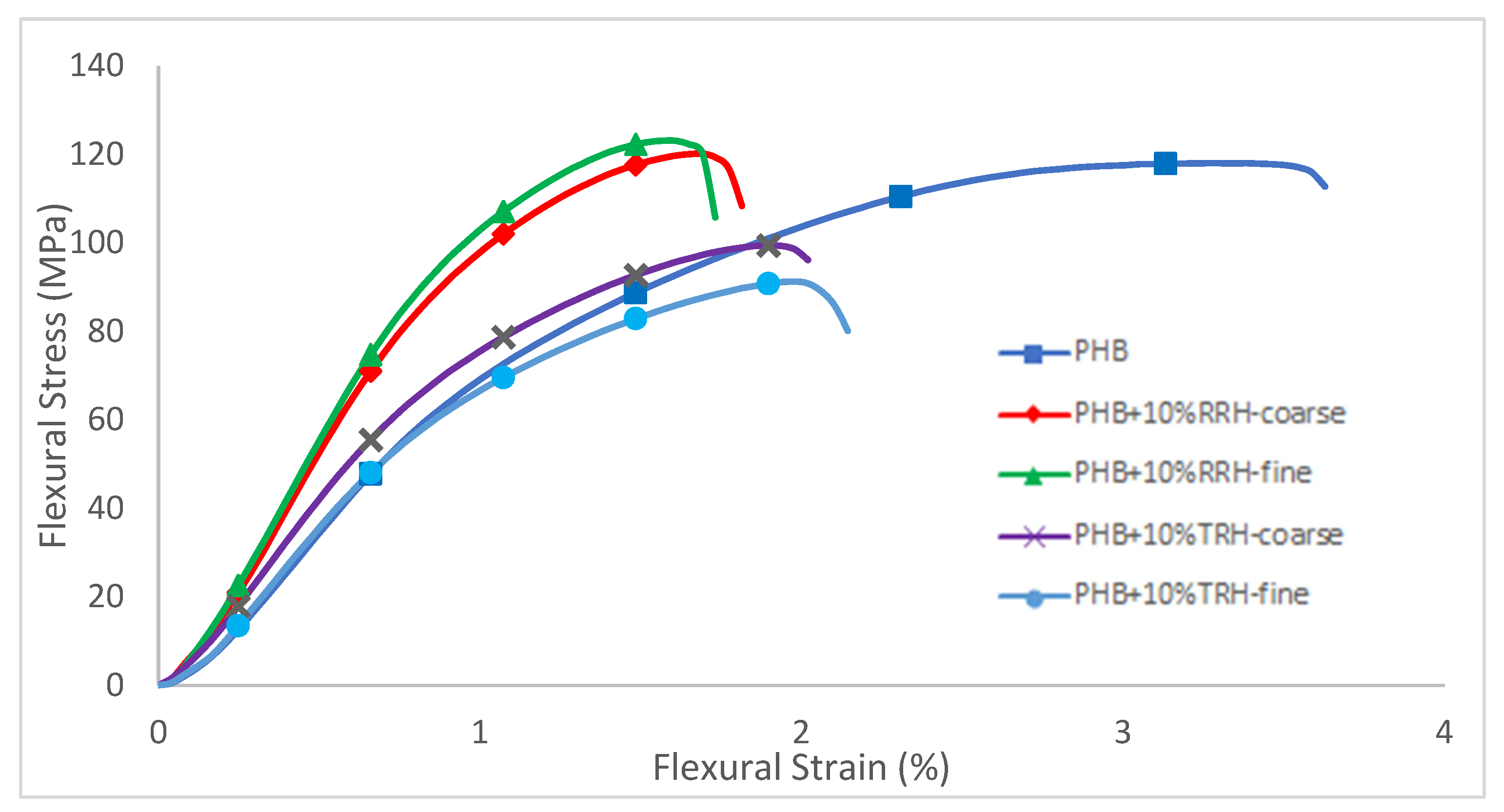
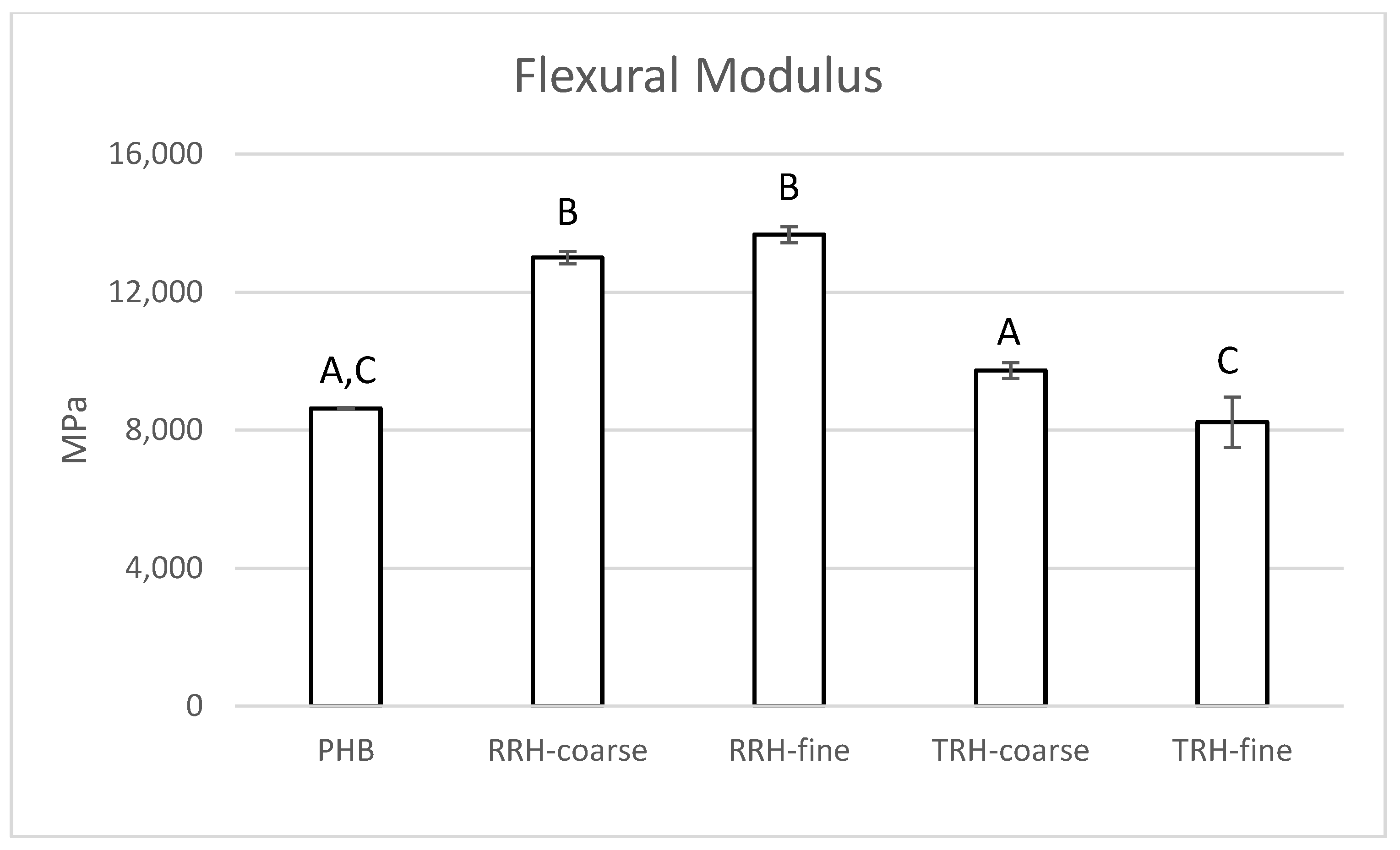
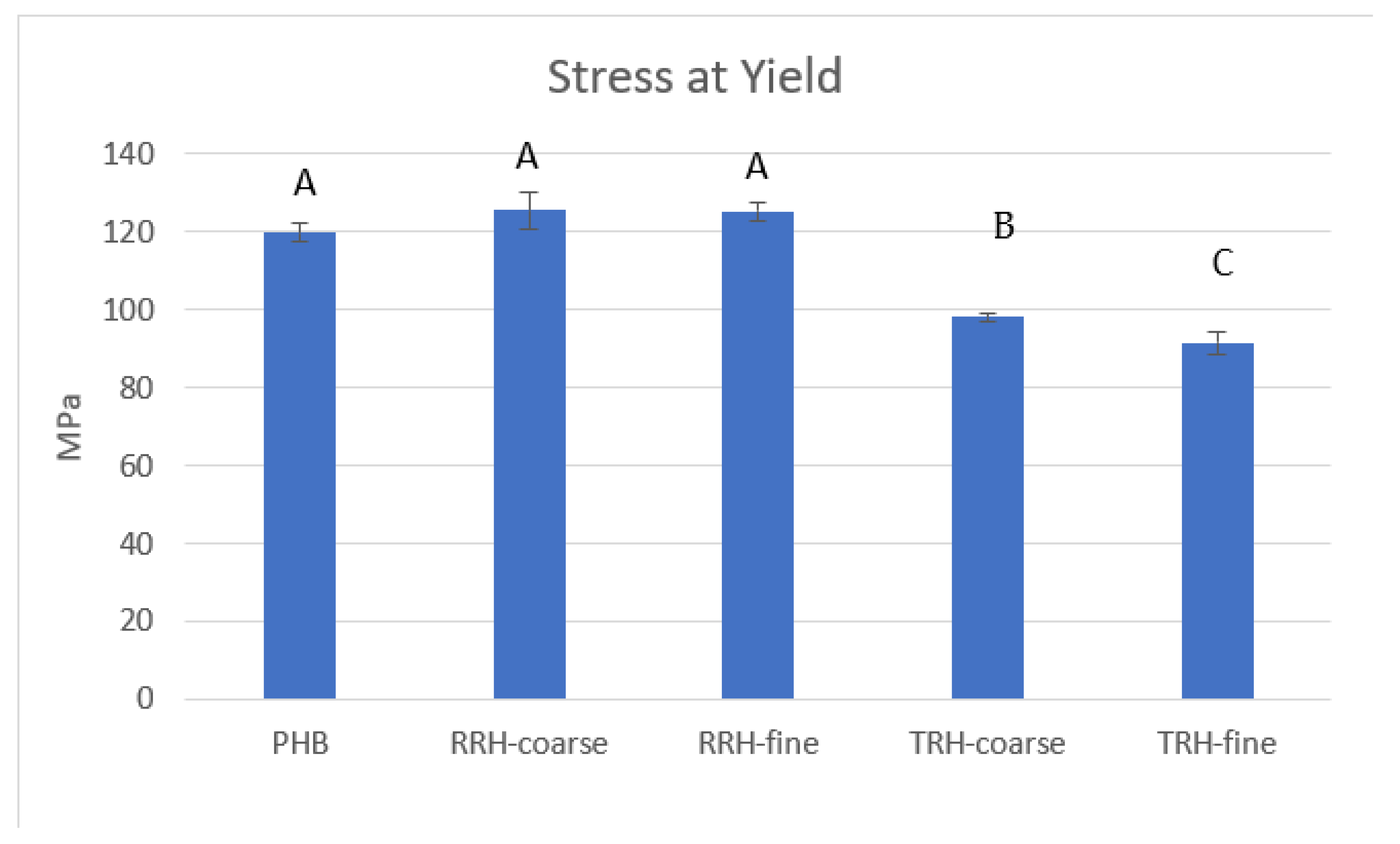
3.5. Mechanical Testing
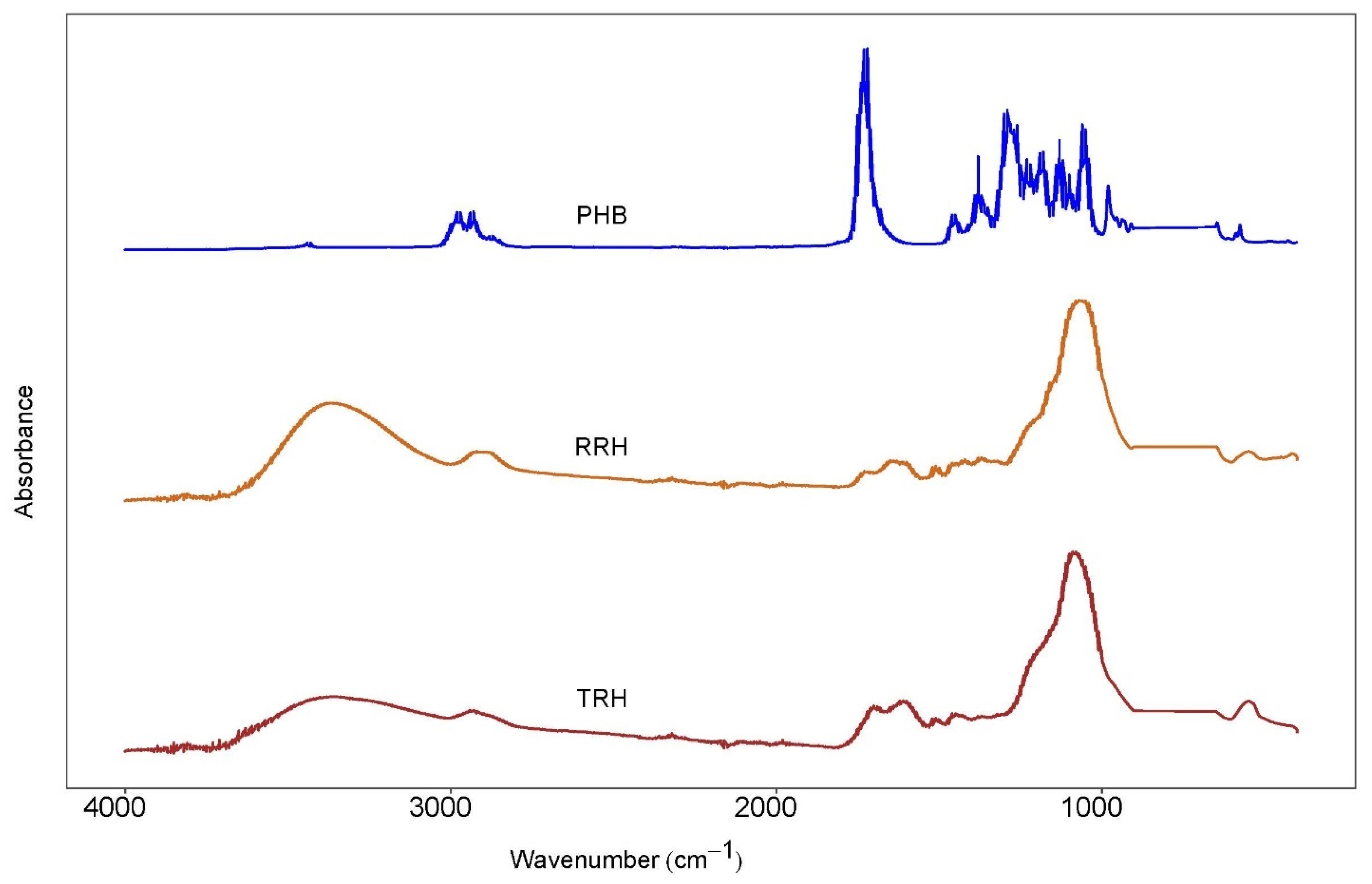
3.6. Fourier Transform Infrared (FTIR) Spectroscopy
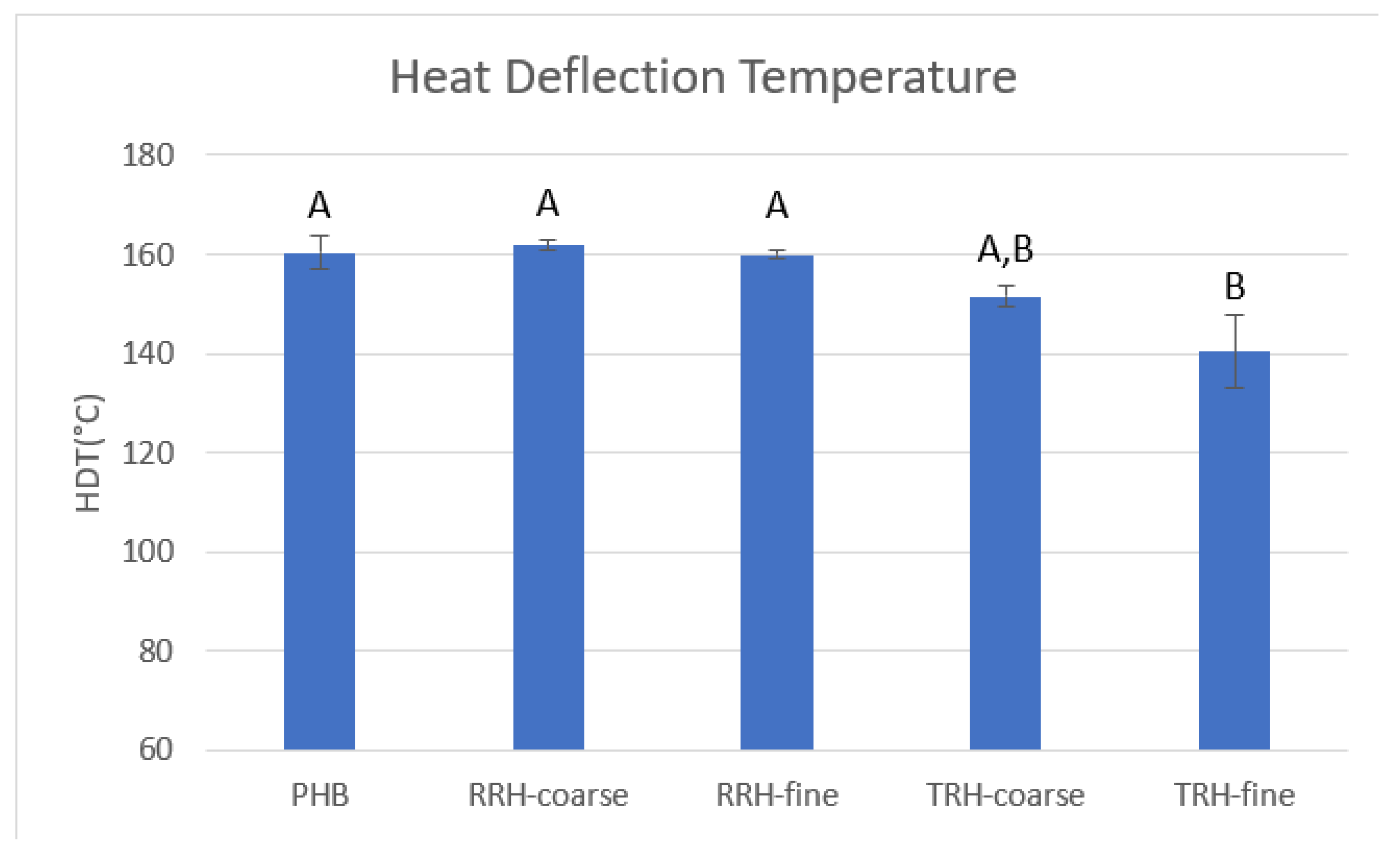
3.7. Thermomechanical Analysis (TMA)
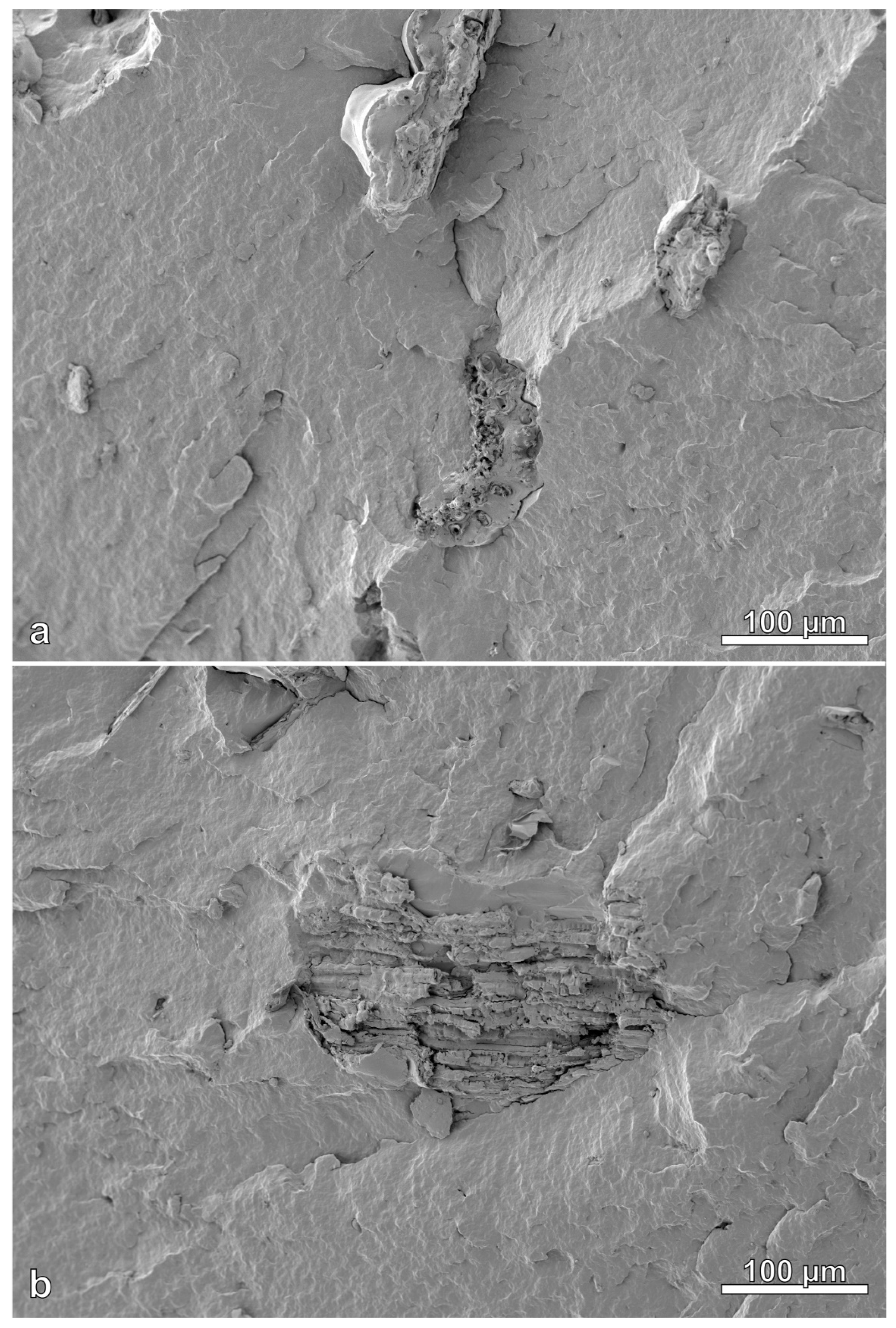
3.8. Scanning Electron Microscope
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
References
- Okoffo, E.; Donner, S.; McGrath, B.; Tscharke, J.; O’Brien, S.; O’Brien, F.; Ribeiro, S.; Burrows, T.; Toapanta, C.; Rauert, S.; et al. Plastics in biosolids from 1950 to 2016: A function of global plastic production and consumption. Water Res. 2021, 201, 117367. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Lebreton, L.C.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, E.; Wolverton, S. Plastic litter in streams: The behavioral archaeology of a pervasive environmental problem. Appl. Geogr. 2017, 84, 93–101. [Google Scholar] [CrossRef]
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 1–25. [Google Scholar] [CrossRef]
- Foekema, E.M.; De Gruijter, C.; Mergia, M.T.; van Franeker, J.A.; Murk, A.J.; Ko-elmans, A.A. Plastic in North Sea Fish. Environ. Sci. Technol. 2013, 47, 8818–8824. [Google Scholar] [CrossRef]
- Talsness, C.E.; Andrade, A.J.; Kuriyama, S.N.; Taylor, J.A.; vom Saal, F.S. Components of plastic: Experimental studies in animals and relevance for human health. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2079–2096. [Google Scholar] [CrossRef]
- Brahney, J.; Mahowald, N.; Prank, M.; Cornwell, G.; Klimont, Z.; Matsui, H.; Prather, K. Constraining the atmospheric limb of the plastic cycle. Proc. Natl. Acad. Sci. USA 2021, 118, e2020719118. [Google Scholar] [CrossRef]
- Kannan, K.; Vimalkumar, K. A Review of Human Exposure to Microplastics and Insights Into Microplastics as Obesogens. Front. Endocrinol. 2021, 12, 724989. [Google Scholar] [CrossRef]
- UNEP. Single Use Plastics: A Roadmap for Sustainability, 2018; (Rev. ed., pp. vi; 6). Available online: https://www.unep.org/resources/report/single-use-plastics-roadmap-sustainability (accessed on 12 September 2022).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Moshood, T.D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M.H.; AbdulGhani, A.; Kumar, S. Green product innovation: A means towards achieving global sustainable product within biodegradable plastic industry. J. Clean. Prod. 2022, 363, 132506. [Google Scholar] [CrossRef]
- Oliveira-Filho, E.R.; de Macedo, M.A.; Lemos, A.C.; Adams, F.; Merkel, O.M.; Taciro, M.K.; Gomez, J.G.C.; Silva, L.F. Engineering Burkholderia sacchari to enhance poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) [P(3HB-co-3HHx)] production from xylose and hexanoate. Int. J. Biol. Macromol. 2022, 213, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y. Bacterial polyhydroxyalkanoates. Biotechnol. Bioeng. 1996, 49, 1–14. [Google Scholar] [CrossRef]
- Acevedo, F.; Villegas, P.; Urtuvia, V.; Hermosilla, J.; Navia, R.; Seeger, M. Bacterial polyhydroxybutyrate for electrospun fiber production. Int. J. Biol. Macromol. 2018, 106, 692–697. [Google Scholar] [CrossRef]
- Poli, A.; Di Donato, P.; Abbamondi, G.R.; Nicolaus, B. Synthesis, Production, and Biotechnological Applications of Exopolysaccharides and Polyhydroxyalkanoates by Archaea. Archaea 2011, 2011, 693253. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Diaz-Pulido, G.; Won, B.; Cho, B. Phymatolithon atlanticum sp. nov. (Hapalidiales, Rhodophyta) from the northeast Atlantic Ocean. Phycologia 2021, 60, 200–209. [Google Scholar] [CrossRef]
- McAdam, B.; Brennan Fournet, M.; McDonald, P.; Mojicevic, M. Production of Polyhydroxybutyrate (PHB) and Factors Impacting Its Chemical and Mechanical Characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.; Lee, H.; Choi, Y.; Sung, S.; Choi, H.; Woo, H.M.; Sim, S.J. Improved CO2-derived polyhydroxybutyrate (PHB) production by engineering fast-growing cyanobacterium Synechococcus elongatus UTEX 2973 for potential utilization of flue gas. Bioresour. Technol. 2021, 327, 124789. [Google Scholar] [CrossRef]
- Goebel, L.; Bonten, C. Crystallization Behavior of Polyhydroxybutyrate (PHB). In Proceedings of the Regional Conference Graz; 2015. [Google Scholar]
- Pérez-Rivero, C.; López-Gómez, J.P.; Roy, I. A sustainable approach for the downstream processing of bacterial polyhydroxyalkanoates: State-of-the-art and latest developments. Biochem. Eng. J. 2019, 150, 107283. [Google Scholar] [CrossRef]
- Moreira, J.B.; Kuntzler, S.G.; da Silva Vaz, B.; da Silva, C.K.; Costa, J.A.V.; de Morais, M.G. Chapter 13—Polyhydroxybutyrate (PHB)-Based Blends and Composites, Biodegradable Polymers, Blends and Composites; Woodhead Publishing: Cambridge, UK, 2022. [Google Scholar]
- Childs, N.; LeBeau, B. Rice Outlook: May 2022, USDA, June 2022. Available online: https://www.ers.usda.gov/publications/pub-details/?pubid=103919 (accessed on 1 September 2022).
- Phonphuak, P. Chindaprasirt, Chapter 6—Types of Waste, Properties, and Durability of Pore-Forming Waste-Based Fired Masonry Bricks, Eco-Efficient Masonry Bricks and Blocks; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- Kalapathy, U.; Proctor, A.; Shultz, J. A simple method for production of pure silica from rice hull ash. Bioresour. Technol. 2000, 73, 257–262. [Google Scholar] [CrossRef]
- Simeone, A.; Bentancur, O.; Manasliski, E.; Rodríguez, F.; Félix, M.; Marizcurrena, M.; Beretta, V. Evaluation of wood chips and rice hulls as alternative fiber sources in rations self-fed to early-weaned calves. Anim. Feed. Sci. Technol. 2022, 291, 115351. [Google Scholar] [CrossRef]
- Hossain, S.S.; Bae, C.-J.; Roy, P.K. A replacement of traditional insulation refractory brick by a waste-derived lightweight refractory castable. Int. J. Appl. Ceram Technol. 2021, 18, 1783–1791. [Google Scholar] [CrossRef]
- Malasri, S.; Tiapradit, P.; Russell, S.; Poonpurmsiri, P.; Tarkarnviroj, C.; Pourhashemi, P.; Moats, R.; Hudson, B. Insulation Effectiveness of Rice Hull. Int. J. Adv. Packag. Technol. 2015, 3, 158–168. [Google Scholar] [CrossRef]
- Attia, N.; Afifi, H.; Hassan, M. Synergistic Study of Carbon Nanotubes, Rice Husk Ash and Flame Retardant Materials on the Flammability of Polystyrene Nanocomposites. Mater. Today Proc. 2015, 2, 3998–4005. [Google Scholar]
- Torres, L.F.; McCaffrey, Z.; Washington, W.; Williams, T.G.; Wood, D.F.; Orts, W.J.; McMahan, C.M. Torrefied agro-industrial residue as filler in natural rubber compounds. J. Appl. Polym. Sci. 2021, 138, e50684. [Google Scholar] [CrossRef]
- Lim, B.K.H.; Thian, E.S. Biodegradation of polymers in managing plastic waste—A review. Sci. Total Environ. 2022, 813, 151880. [Google Scholar] [CrossRef] [PubMed]
- Platnieks, O.; Barkane, A.; Ijudina, N.; Gaidukova, G.; Thakur, V.K.; Gaidukovs, S. Sustainable tetra pak recycled cellulose/Poly(Butylene succinate) based woody-like composites for a circular economy. J. Clean. Prod. 2020, 270, 122321. [Google Scholar] [CrossRef]
- Chiou, B.-S.; Valenzuela-Medina, D.; Wechsler, M.; Bilbao-Sainz, C.; Klamczynski, A.K.; Williams, T.G.; Wood, D.F.; Glenn, G.M.; Orts, W.J. Torrefied biomass-polypropylene composites. J. Appl. Polym. Sci. 2015, 132, 41582. [Google Scholar] [CrossRef]
- Peng, J.H.; Bi, H.T.; Sokhansanj, S.; Lim, J.C. A study of particle size effect on biomass torrefaction and densification. Energy Fuels 2012, 26, 3826–3839. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Zaghloul, M.Y.M.; Zaghloul, M.M.Y. Experimental and modeling analysis of mechanical-electrical behaviors of polypropylene composites filled with graphite and MWCNT fillers. Polym. Test. 2017, 63, 467–474. [Google Scholar] [CrossRef]
- Fu, S.Y.; Feng, X.Q.; Lauke, B.; Mai, Y.W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- McCaffrey, Z.; Torres, L.; Flynn, S.; Cao, T.; Chiou, B.-S.; Klamczynski, A.; Glenn, G.; Orts, W. Recycled polypropylene-polyethylene torrefied almond shell biocomposites. Ind. Crops Prod. 2018, 125, 425–432. [Google Scholar] [CrossRef]
- Berthet, M.A.; Commandré, J.M.; Rouau, X.; Gontard, N.; Angellier-Coussy, H. Torrefaction treatment of lignocellulosic fibres for improving fibre/matrix adhesion in a biocomposite. Mater. Des. 2016, 92, 223–232. [Google Scholar] [CrossRef]
- Haeldermans, T.; Samyn, P.; Cardinaels, R.; Vandamme, D.; Vanreppelen, K.; Cuypers, A.; Schreurs, S. Bio-based poly(3-hydroxybutyrate)/thermoplastic starch composites as a host matrix for biochar fillers. J. Polym. Environ. 2021, 29, 2478–2491. [Google Scholar] [CrossRef]
- Kane, S.; Van Roijen, E.; Ryan, C.; Miller, S. Reducing the environmental impacts of plastics while increasing strength: Biochar fillers in biodegradable, recycled, and fossil-fuel derived plastics. Compos. Part C 2022, 8, 100253. [Google Scholar] [CrossRef]
- Barham, P.J.; Keller, A.; Otun, E.L.; Holmes, P.A. Crystallization and morphology of a bacterial thermoplastic: Poly-3-hydroxybutyrate. J. Mater. Sci. 1984, 19, 2781–2794. [Google Scholar] [CrossRef]
- Kocaefe, D.; Poncsak, S.; Boluk, Y. Effect of Thermal Treatment on the Chemical Composition and Mechanical Properties of Birch and Aspen. BioResources 2008, 3, 517–537. [Google Scholar]
- Mayer-Laigle, C.; Rajaonarivony, R.K.; Blanc, N.; Rouau, X. Comminution of Dry Lignocellulosic Biomass: Part II. Technologies, Improvement of Milling Performances, and Security Issues. Bioengineering 2018, 5, 50. [Google Scholar] [CrossRef]
- Mburu, F.; Dumarçay, S.; Bocquet, J.F.; Petrissans, M.; Gérardin, P. Effect of chemical modifications caused by heat treatment on mechanical properties of grevillea robusta wood. Polym. Degrad. Stab. 2008, 93, 401–405. [Google Scholar] [CrossRef]
- Dhakate, M.; Joshi, I.; Khakhar, D. Analysis of grinding in a spiral jet mill. Part 1: Batch grinding. Chem. Eng. Sci. 2021, 231, 116310. [Google Scholar] [CrossRef]
- Nuamah, A.; Malmgren, G.; Riley, E. Lester, 5.05—Biomass Co-Firing. In Comprehensive Renewable Energy; Sayigh, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 55–73. [Google Scholar]
- Ronsse, F.; Nachenius, R.; Prins, W. Chapter 11—Carbonization of biomass. In Recent Advances in Thermo-Chemical Conversion of Biomass; Pandey, A., Bhaskar, T., Stöcker, M., Sukumaran, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 293–324. [Google Scholar]
- Gaur, S.; Reed, T.B. Thermal Data for Natural and Synthetic Fuels; Marcel Dekker: New City, NY, USA, 1998. [Google Scholar]
- Chen, D.; Zhou, J.; Zhang, Q.; Zhu, X.; Lu, Q. Upgrading of rice husk by torrefaction and its influence on the fuel properties. BioResources 2014, 9, 5893–5905. [Google Scholar] [CrossRef]
- Sanchez-Safont, E.L.; Aldureid, A.; Lagaron, J.M.; Gamez-Perez, J.; Cabedo, L. Biocomposites of different lignocellulosic wastes for sustainable food packaging applications. Compos. Part B 2018, 145, 215–225. [Google Scholar] [CrossRef]
- McCaffrey, Z.; Torres, L.; Chiou, B.-S.; Ferreira, S.; Silva, L.; Wood, D.; Orts, W. Torrefaction of Almond and Walnut Byproducts. Front. Energy Res. 2021, 9, 643306. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, S. The structural and thermal characteristics of wheat straw hemicellulose. J. Anal. Appl. Pyrolysis 2010, 88, 134–139. [Google Scholar] [CrossRef]
- Collard, F.-X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Díez, D.; Urueña, A.; Piñero, H.; Barrio, A.; Tamminen, T. Determination of Hemicellulose, Cellulose, and Lignin Content in Different Types of Biomasses by Thermogravimetric Analysis and Pseudocomponent Kinetic Model (TGA-PKM Method). Processes 2020, 8, 1048. [Google Scholar] [CrossRef]
- Moura, A.; Bolba, C.; Demori, R.; Lima, L.P.F.C.; Santana, R.M.C. Effect of rice husk treatment with hot water on mechanical performance in poly(hydroxybutyrate)/rice husk biocomposite. J. Polym. Environ. 2018, 26, 2632–2639. [Google Scholar] [CrossRef]
- Sanchez-Safont, E.L.; Aldureid, A.; Lagaron, J.M.; Cabedo, L.; Gamez-Perez, J. Study of the compatibilization effect of different reactive agents in PHB/natural fiber-based composites. Polymers 2020, 12, 1967. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Gao, A.; Ma, Z.; Fei, D.; Chang, Y.; Shen, C. In-depth study of rice husk torrefaction: Characterization of solid, liquid and gaseous products, oxygen migration and energy yield. Bioresour. Technol. 2018, 253, 148–153. [Google Scholar] [CrossRef]
- Kim, H.; Lee, B.; Lee, S.; Kim, H.; Dorgan, J.R. Enhanced interfacial adhesion, mechanical, and thermal properties of natural flour-filled biodegradable polymer bio-composites. J. Therm. Anal. 2011, 104, 331–338. [Google Scholar] [CrossRef]




| C [%] | H [%] | N [%] | S [%] | O [%] | Ash [%] | Total [%] | |
|---|---|---|---|---|---|---|---|
| RH | 38.70 (0.14) | 4.92 (0.01) | 0.47 (0.01) | 0.03 (0.01) | 37.15 (0.28) | 18.73 (0.41) | 100.00 |
| TRH | 42.60 (0.26) | 4.33 (0.04) | 0.57 (0.01) | 0.02 (0.00) | 17.34 (0.17) | 35.14 (0.47) | 100.00 |
| Crystallinity [%] | |
|---|---|
| PHB | 54.5% |
| PHB+10% RRH coarse | 54.3% |
| PHB+10% RRH fine | 54.8% |
| PHB+10% TRH coarse | 55.0% |
| PHB+10% TRH-fine | 54.2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCaffrey, Z.; Cal, A.; Torres, L.; Chiou, B.-S.; Wood, D.; Williams, T.; Orts, W. Polyhydroxybutyrate Rice Hull and Torrefied Rice Hull Biocomposites. Polymers 2022, 14, 3882. https://doi.org/10.3390/polym14183882
McCaffrey Z, Cal A, Torres L, Chiou B-S, Wood D, Williams T, Orts W. Polyhydroxybutyrate Rice Hull and Torrefied Rice Hull Biocomposites. Polymers. 2022; 14(18):3882. https://doi.org/10.3390/polym14183882
Chicago/Turabian StyleMcCaffrey, Zach, Andrew Cal, Lennard Torres, Bor-Sen Chiou, Delilah Wood, Tina Williams, and William Orts. 2022. "Polyhydroxybutyrate Rice Hull and Torrefied Rice Hull Biocomposites" Polymers 14, no. 18: 3882. https://doi.org/10.3390/polym14183882






