Comparative Assessment of Different Pre-Treatment Bonding Strategies to Improve the Adhesion of Self-Adhesive Composites to Dentin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Acquisition and Materials
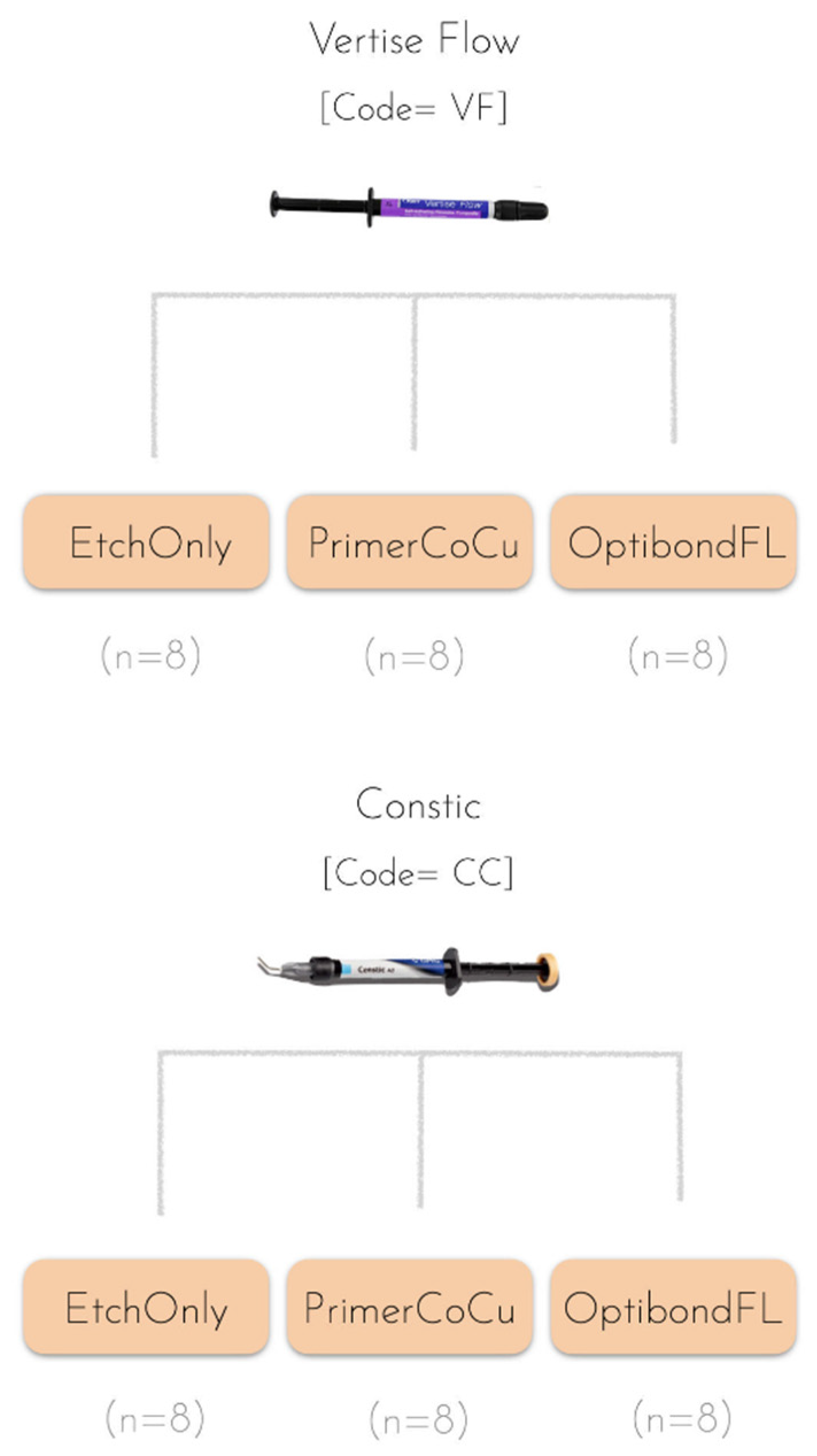
2.2. Experimental Design
2.3. Sample Preparation and Restorative Procedure
2.4. Microtensile Bond Strength of Resin Composite–Dentin Interfaces
2.5. Masson’s Trichrome–Light Microscopy
2.6. Statistical Analysis
3. Results
3.1. Microtensile Bond Strength of Composite–Dentin Interfaces
3.2. Bond Failure Mode Analysis
3.3. Masson’s Trichrome–Light Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moszner, N.; Hirt, T. New Polymer-Chemical Developments in Clinical Dental Polymer Materials: Enamel-Dentin Adhesives and Restorative Composites. J. Polym. Sci. A Polym. Chem. 2012, 50, 4369–4402. [Google Scholar] [CrossRef]
- Magne, P. Composite Resins and Bonded Porcelain: The Postamalgam Era? J. Calif. Dent. Assoc. 2006, 34, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, M. Polymerization Mechanics of Dental Composites-Advantages and Disadvantages. Proc. Procedia Eng. 2016, 149, 13–320. [Google Scholar] [CrossRef]
- Drummond, J.L. Degradation, Fatigue and Failure of Resin Dental Composite Materials. J. Dent. Res. 2008, 87, 710. [Google Scholar] [CrossRef] [PubMed]
- al Sunbul, H.; Silikas, N.; Watts, D.C. Polymerization Shrinkage Kinetics and Shrinkage-Stress in Dental Resin-Composites. Dent. Mater. 2016, 32, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Nagano, F.; Endo, K.; Ohno, H. A Review: Biodegradation of Resin-Dentin Bonds. Jpn. Dent. Sci. Rev. 2011, 47, 5–12. [Google Scholar] [CrossRef]
- van Landuyt, K.L.; Snauwaert, J.; de Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; van Meerbeek, B. Systematic Review of the Chemical Composition of Contemporary Dental Adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef]
- Ozel Bektas, O.; Eren, D.; Akin, E.G.; Akin, H. Evaluation of a Self-Adhering Flowable Composite in Terms of Micro-Shear Bond Strength and Microleakage. Acta Odontol. Scand. 2013, 71, 541–546. [Google Scholar] [CrossRef]
- Poitevin, A.; de Munck, J.; van Ende, A.; Suyama, Y.; Mine, A.; Peumans, M.; van Meerbeek, B. Bonding Effectiveness of Self-Adhesive Composites to Dentin and Enamel. Dent. Mater. 2013, 29, 221–230. [Google Scholar] [CrossRef]
- Peterson, J.; Rizk, M.; Hoch, M.; Wiegand, A. Bonding Performance of Self-Adhesive Flowable Composites to Enamel, Dentin and a Nano-Hybrid Composite. Odontology 2018, 106, 171–180. [Google Scholar] [CrossRef]
- van Meerbeek, B.; Frankenberger, R. On Our Way towards Self-Adhesive Restorative Materials? J. Adhes. Dent. 2019, 21, 295–296. [Google Scholar] [CrossRef]
- Maj, A.; Trzcionka, A.; Twardawa, H.; Tanasiewicz, M. A Comparative Clinical Study of the Self-Adhering Flowable Composite Resin Vertise Flow and the Traditional Flowable Composite Resin Premise Flowable. Coatings 2020, 10, 800. [Google Scholar] [CrossRef]
- David, C.; Cardoso de Cardoso, G.; Isolan, C.P.; Piva, E.; Moraes, R.R.; Cuevas-Suarez, C.E. Bond Strength of Self-Adhesive Flowable Composite Resins to Dental Tissues: A Systematic Review and Meta-Analysis of in Vitro Studies. J. Prosthet. Dent. 2021. [Google Scholar] [CrossRef]
- Delgado, A.H.S.; Almuusa, A.; Eshmawi, Y.; Xia, W.; Ashley, P.; Young, A.M. Novel Self-Bonding Composites: Resin-Dentin Interfacial Chemistry. Ann. Med. 2019, 51, 97. [Google Scholar] [CrossRef]
- van Dijken, J.W.V.; Pallesen, U.; Benetti, A. A Randomized Controlled Evaluation of Posterior Resin Restorations of an Altered Resin Modified Glass-Ionomer Cement with Claimed Bioactivity. Dent. Mater. 2019, 35, 335–343. [Google Scholar] [CrossRef]
- Meharry, M.R.; Schwartz, J.; Montalvo, A.; Mueller, D.; Mitchell, J.C. Comparison of 2 Self-Adhesive Resin Cements with or without a Self-Etching Primer. Gen. Dent. 2020, 68, 22–28. [Google Scholar]
- Kim, B.N.; Son, S.A.; Park, J.K. Effect of Exclusive Primer and Adhesive on Microtensile Bond Strength of Self-Adhesive Resin Cement to Dentin. Materials 2020, 13, 2353. [Google Scholar] [CrossRef]
- van Meerbeek, B.; Yoshihara, K.; van Landuyt, K.; Yoshida, Y.; Peumans, M. From Buonocore’s Pioneering Acid-Etch Technique to Self-Adhering Restoratlves. A Status Perspective of Rapidly Advancing Dentai Adheslve Technology. J. Adhes. Dent. 2020, 22, 7–34. [Google Scholar] [CrossRef]
- Frankenberger, R.; Lopes, M.; Perdigão, J.; Ambrose, W.W.; Rosa, B.T. The Use of Flowable Composites as Filled Adhesives. Dent. Mater. 2002, 18, 227–238. [Google Scholar] [CrossRef]
- Al-Nabulsi, M.; Daud, A.; Yiu, C.; Omar, H.; Sauro, S.; Fawzy, A.; Daood, U. Co-Blend Application Mode of Bulk Fill Composite Resin. Materials 2019, 12, 2504. [Google Scholar] [CrossRef]
- Viswanathan, R.; Shashibhushan, K.K.; Subba Reddy, V.V. Short Communication: Pre- and Co-Curing Effect of Adhesives on Shear Bond Strengths of Composite Resins to Primary Enamel and Dentine: An in Vitro Study. Eur. Arch. Paediatr. Dent. 2011, 12, 308–311. [Google Scholar] [CrossRef]
- Knight, G.M.; McIntyre, J.M. Bond Strengths between Composite Resin and Auto Cure Glass Ionomer Cement Using the Co-Cure Technique. Aust. Dent. J. 2006, 51, 175–179. [Google Scholar] [CrossRef]
- Vukelja, J.; Sever, E.K.; Sever, I.; Krmek, S.J.; Tarle, Z. Effect of Conventional Adhesive Application or Co-Curing Technique on Dentin Bond Strength. Materials 2021, 14, 7664. [Google Scholar] [CrossRef]
- Armstrong, S.; Breschi, L.; Özcan, M.; Pfefferkorn, F.; Ferrari, M.; van Meerbeek, B. Academy of Dental Materials Guidance on in Vitro Testing of Dental Composite Bonding Effectiveness to Dentin/Enamel Using Micro-Tensile Bond Strength (ΜTBS) Approach. Dent. Mater. 2017, 33, 133–143. [Google Scholar] [CrossRef]
- Monticelli, F.; Osorio, R.; Mazzitelli, C.; Ferrari, M.; Toledano, M. Limited Decalcification/Diffusion of Self-Adhesive Cements into Dentin. J. Dent. Rest 2008, 87, 974–979. [Google Scholar] [CrossRef]
- Yao, C.; Ahmed, M.H.; Okazaki, Y.; van Landuyt, K.L.; Huang, C.; van Meerbeek, B. Bonding Efficacy of a New Self-Adhesive Restorative onto Flat Dentin vs Class-I Cavity-Bottom Dentin. J. Adhes. Dent. 2020, 22, 65–77. [Google Scholar] [CrossRef]
- Latta, M.A.; Radniecki, S.M. Bond Strength of Self-Adhesive Restorative Materials Affected by Smear Layer Thickness but Not Dentin Desiccation. J. Adhes. Dent. 2020, 22, 79–84. [Google Scholar] [CrossRef]
- Brueckner, C.; Schneider, H.; Haak, R. Shear Bond Strength and Tooth-Composite Interaction with Self-Adhering Flowable Composites. Oper Dent. 2017, 42, 90–100. [Google Scholar] [CrossRef]
- Nikaido, T.; Inoue, G.; Takagaki, T.; Waidyasekera, K.; Iida, Y.; Shinohara, M.S.; Sadr, A.; Tagami, J. New Strategy to Create “Super Dentin” Using Adhesive Technology: Reinforcement of Adhesive-Dentin Interface and Protection of Tooth Structures. Jpn. Dent. Sci. Rev. 2011, 47, 31–42. [Google Scholar] [CrossRef]
- Latta, M.A.; Tsujimoto, A.; Takamizawa, T.; Barkmeier, W.W. Enamel and Dentin Bond Durability of Self-Adhesive Restorative Materials. J. Adhes. Dent. 2020, 22, 99–105. [Google Scholar] [CrossRef]
- Valizadeh, S.; Hashemi, S.F.; Hashemikamangar, S.S.; Kharazifard, M.J. Microleakage of a Self-Adhesive Composite of Class V Cavities: Effect of Surface Treatment and Thermocycling. J. Contemp. Dent. Pract. 2020, 21, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, M.G. A Simple Method of Increasing the Adhesion of Acrylic Filling Materials to Enamel Surfaces. J. Dent. Res. 1955, 34, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Perdigão, J.; Araujo, E.; Ramos, R.Q.; Gomes, G.; Pizzolotto, L. Adhesive Dentistry: Current Concepts and Clinical Considerations. J. Esthet. Restor. Dent. 2021, 33, 51–68. [Google Scholar] [CrossRef] [PubMed]
- van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; de Munck, J.; van Landuyt, K.L. State of the Art of Self-Etch Adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef]
- Fugolin, A.P.P.; Pfeifer, C.S. New Resins for Dental Composites. J. Dent. Res. 2017, 96, 1085–1091. [Google Scholar] [CrossRef]
- Breschi, L.; Maravic, T.; Cunha, S.R.; Comba, A.; Cadenaro, M.; Tjäderhane, L.; Pashley, D.H.; Tay, F.R.; Mazzoni, A. Dentin Bonding Systems: From Dentin Collagen Structure to Bond Preservation and Clinical Applications. Dent. Mater. 2018, 34, 78–96. [Google Scholar] [CrossRef]
- Chapman, J.L.; Burgess, J.O.; Holst, S.; Sadan, A.; Blatz, M.B. Precuring of Self-Etching Bonding Agents and Its Effect on Bond Strength of Resin Composite to Dentin and Enamel. Quintessence Int. 2007, 38, 637–641. [Google Scholar]
- Abdelaziz, K.M.; Saleh, A.A. Influence of Adhesive-Composite Application Modalities on Their Bonding to Tooth Structure and Resistance of the Performed Restorations to Failure. J. Dent. Sci. 2018, 13, 378–385. [Google Scholar] [CrossRef]
- Armstrong, S.R.; Keller, J.C.; Boyer, D.B. The Influence of Water Storage and C-Factor on the Dentin-Resin Composite Microtensile Bond Strength and Debond Pathway Utilizing a Filled and Unfilled Adhesive Resin. Dent. Mater. 2001, 17, 268–276. [Google Scholar] [CrossRef]
- Kumar, D.; Bolskar, R.D.; Mutreja, I.; Jones, R.S. Methacrylate Polymers With “Flipped External” Ester Groups: A Review. Front. Dent. Med. 2022, 0, 41. [Google Scholar] [CrossRef]
- Hashimoto, M.; Ohno, H.; Kaga, M.; Sano, H.; Tay, F.R.; Oguchi, H.; Araki, Y.; Kubota, M. Over-Etching Effects on Micro-Tensile Bond Strength and Failure Patterns for Two Dentin Bonding Systems. J. Dent. 2002, 30, 99–105. [Google Scholar] [CrossRef]
- Moosavi, H.; Kimyai, S.; Forghani, M.; Khodadadi, R. The Clinical Effectiveness of Various Adhesive Systems: An 18-Month Evaluation. Oper. Dent. 2013, 38, 134–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peumans, M.; Wouters, L.; de Munck, J.; van Meerbeek, B.; van Landuyt, K. Nine-Year Clinical Performance of a HEMA-Free One-Step Self-Etch Adhesive in Noncarious Cervical Lesions. J. Adhes. Dent. 2018, 20, 195–203. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, L.; Chen, L.; Qian, F.; Vargas, M.A. Effect of Adhesive Filler Content on Marginal Adaptation of Class II Composite Resin Restorations. J. Oper. Esthet. Dent. 2017, 2, 1–7. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Q.; Yang, Z. Effect of Loading Chemically and Mechanically Pre-Treated Fumed Silica as Filler on an Etch & Rinse Model Dental Adhesive. J. Adhes. Sci. Technol. 2018, 32, 527–541. [Google Scholar] [CrossRef]
- Azad, E.; Atai, M.; Zandi, M.; Shokrollahi, P.; Solhi, L. Structure–Properties Relationships in Dental Adhesives: Effect of Initiator, Matrix Monomer Structure, and Nano-Filler Incorporation. Dent. Mater. 2018, 34, 1263–1270. [Google Scholar] [CrossRef]
- Silva e Sousa Junior, M.H.; Carneiro, K.G.K.; Lobato, M.F.; Silva e Souza, P.d.A.R.; Góes, M.F. Adhesive Systems: Important Aspects Related to Their Composition and Clinical Use. J. Appl. Oral. Sci. 2010, 18, 207–214. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H. Have Dentin Adhesives Become Too Hydrophilic? J. Can. Dent. Assoc. 2003, 69, 729–731b. [Google Scholar]
- Eliades, A.; Birpou, E.; Eliades, T.; Eliades, G. Self-Adhesive Restoratives as Pit and Fissure Sealants: A Comparative Laboratory Study. Dent. Mater. 2013, 29, 752–762. [Google Scholar] [CrossRef]
- Peumans, M.; de Munck, J.; van Landuyt, K.L.; Poitevin, A.; Lambrechts, P.; van Meerbeek, B. A 13-Year Clinical Evaluation of Two Three-Step Etch-and-Rinse Adhesives in Non-Carious Class-V Lesions. Clin. Oral. Investig. 2012, 16, 129–137. [Google Scholar] [CrossRef]
- Perdigão, J.; Dutra-Corrêa, M.; Saraceni, C.H.C.; Ciaramicoli, M.T.; Kiyan, V.H.; Queiroz, C.S. Randomized Clinical Trial of Four Adhesion Strategies: 18-Month Results. Oper. Dent. 2012, 37, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Stavridakis, M.; Krejci, I.; Magne, P. Immediate Dentin Sealing of Onlay Preparations: Thickness of Pre-Cured Dentin Bonding Agent and Effect of Surface Cleaning. Oper. Dent. 2005, 30, 747–757. [Google Scholar] [PubMed]
- Yuan, H.; Li, M.; Guo, B.; Gao, Y.; Liu, H.; Li, J. Evaluation of Microtensile Bond Strength and Microleakage of a Self-Adhering Flowable Composite. J. Adhes. Dent. 2015, 17, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Chowdhury, A.F.M.A.; Saikaew, P.; Matsumoto, M.; Hoshika, S.; Yamauti, M. The Microtensile Bond Strength Test: Its Historical Background and Application to Bond Testing. Jpn. Dent. Sci. Rev. 2020, 56, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.J.; Faria-E-Silva, A.L.; Rodrigues, M.d.P.; Fernandes Vilela, A.B.; Pfeifer, C.S.; Tantbirojn, D.; Versluis, A. Polymerization Shrinkage Stress of Composite Resins and Resin Cements—What Do We Need to Know? Braz. Oral. Res. 2017, 31, 49–63. [Google Scholar] [CrossRef]
- Dickens, S.H.; Cho, B.H. Interpretation of Bond Failure through Conversion and Residual Solvent Measurements and Weibull Analyses of Flexural and Microtensile Bond Strengths of Bonding Agents. Dent. Mater. 2005, 21, 354–364. [Google Scholar] [CrossRef]
- Toledano, M.; Aguilera, F.S.; Sauro, S.; Cabello, I.; Osorio, E.; Osorio, R. Load Cycling Enhances Bioactivity at the Resin-Dentin Interface. Dent. Mater. 2014, 30, e169–e188. [Google Scholar] [CrossRef]
- Osorio, R.; Cabello, I.; Medina-Castillo, A.L.; Osorio, E.; Toledano, M. Zinc-Modified Nanopolymers Improve the Quality of Resin–Dentin Bonded Interfaces. Clin. Oral. Investig. 2016, 20, 2411–2420. [Google Scholar] [CrossRef]
- Stape, T.H.S.; Tjäderhane, L.; Marques, M.R.; Aguiar, F.H.B.; Martins, L.R.M. Effect of Dimethyl Sulfoxide Wet-Bonding Technique on Hybrid Layer Quality and Dentin Bond Strength. Dent. Mater. 2015, 31, 676–683. [Google Scholar] [CrossRef]
- Apolonio, F.M.; de Souza, L.C.; E Silva, F.C.F.A.; Yamauti, M.; Breschi, L.; Saboia, V.D.P.A. Evaluation of Resin/Dentin Bonded Interfaces Formed by Different Adhesive Strategies and Exposed to NaOCl Challenge. Int. J. Adhes. Adhes. 2015, 59, 21–26. [Google Scholar] [CrossRef]
- Spencer, P.; Ye, Q.; Park, J.; Topp, E.M.; Misra, A.; Marangos, O.; Wang, Y.; Bohaty, B.S.; Singh, V.; Sene, F.; et al. Adhesive/Dentin Interface: The Weak Link in the Composite Restoration. Ann. Biomed. Eng. 2010, 38, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Spencer, P.; Yuca, E.; Tamerler, C. Engineered Peptide Repairs Defective Adhesive–Dentin Interface. Macromol. Mater. Eng. 2017, 302, 1600487. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, N.G.; Lima, A.S.L.C.; da Silveira, M.T.; de Souza Araújo, P.R.; de Melo Monteiro, G.Q.; de Vasconcelos Carvalho, M. Evaluation of Postoperative Sensitivity in Restorations with Self-Adhesive Resin: A Randomized Split-Mouth Design Controlled Study. Clin. Oral. Investig. 2020, 24, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.H. Characterization of Dental Bonding Systems and Commercial versus Novel Self-Adhesive Restoratives; Eastman Dental Institute: London, UK, 2021. [Google Scholar]
- Hanabusa, M.; Mine, A.; Kuboki, T.; Momoi, Y.; van Ende, A.; van Meerbeek, B.; de Munck, J. Bonding Effectiveness of a New “multi-Mode” Adhesive to Enamel and Dentine. J. Dent. 2012, 40, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Bertassoni, L.E.; Orgel, J.P.R.; Antipova, O.; Swain, M.V. The Dentin Organic Matrix—Limitations of Restorative Dentistry Hidden on the Nanometer Scale. Acta Biomater. 2012, 8, 2419–2433. [Google Scholar] [CrossRef]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the Art Etch-and-Rinse Adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef]
- Fusayama, T.; Nakamura, M.; Kurosaki, N.; Iwaku, M. Non-Pressure Adhesion of a New Adhesive Restorative Resin. J. Dent. Res. 1979, 58, 1364–1370. [Google Scholar] [CrossRef]
- Nakahayashi, N.; Nakamura, M.; Nohoroir, Y. Hybrid Layer as a Dentin-Bonding Mechanism. J. Esthet Dent. 1991, 3, 133–138. [Google Scholar] [CrossRef]
- HS Delgado, A.; Belmar Da Costa, M.; Polido, M.C.; Mano Azul, A.; Sauro, S. Collagen-Depletion Strategies in Dentin as Alternatives to the Hybrid Layer Concept and Their Effect on Bond Strength: A Systematic Review. Sci. Rep. 2022, 12, 13028. [Google Scholar] [CrossRef]







| Material | Type | Manufacturer and Details | Composition |
|---|---|---|---|
| VertiseTM Flow | Self-adhesive composite | Kerr Italia, S.r.I.; Scafati, Italy Shade: A2 Batch number: 8755817 | Organic matrix: 5–10% HEMA, N/A% Bis-GMA, 5–10% UDMA, and 1–5% GPDM Filler particles: Ytterbium fluoride and barium aluminosilicate Filler load: 70 vol% |
| Constic | Self-adhesive composite | DMG Chem. -Pharm. Fabrik GmbH; Hamburg, Germany Shade: A2 Batch number: 256266 | Organic matrix: 15–35% Bis-GMA, <45% TEGDMA, and N/A% 10-MDP Filler particles: Barium aluminosilicate Filler load: 66 vol% |
| OptiBondTM FL Primer | Commercial etch-and-rinse dental adhesive (part I) | Kerr Italia, S.r.I.; Scafati, Italy Batch number: 7887502 | 10–30% HEMA, 5–10% GPDM, BHT, PAMA, CQ Solvent: Ethanol/Water |
| OptiBondTM FL Bond | Commercial etch-and-rinse dental adhesive (part II) | Kerr Italia, S. r. I.; Scafati, Italy Batch number: 7517622 | Organic matrix: 10–30% HEMA, N/A% Bis-GMA, GDMA Filler particles: Barium aluminosilicate, sodium hexafluorosilicate and fumed silica |
| Bonding Strategies | ||
|---|---|---|
| Etch-Only | Primer Co-Curing | Optibond FL |
|
|
|
| Reagents | Protocol |
|---|---|
| Solute A Weigert’s hematoxylin A + B in equal parts Solute B 2cc ponceau of 1% xylidine in 1% acetic acid or 1cc of 1% acid fuchsin in 1% acetic acid Solute C 1% phosphomolybdic acid Solute D 2% Light Green in 1% acetic acid |
|
| Type III Sum of Squares | df | Mean Square | Z | Sig. | |
|---|---|---|---|---|---|
| Model | 3302.921 | 5 | 660.584 | 179.386 | <0.001 |
| Intercept | 5315.125 | 1 | 5315.125 | 1443.359 | <0.001 |
| Composite | 22.277 | 1 | 22.277 | 6.049 | 0.018 |
| Bonding Strategy | 3271.365 | 2 | 1635.683 | 444.181 | <0.001 |
| Composite * Bonding Strategy | 9.279 | 2 | 4.639 | 1.260 | 0.294 |
| Pattern | 154.664 | 42 | 3.682 | ||
| Total | 8772.710 | 48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inglês, M.; Vasconcelos e Cruz, J.; Mano Azul, A.; Polido, M.; Delgado, A.H.S. Comparative Assessment of Different Pre-Treatment Bonding Strategies to Improve the Adhesion of Self-Adhesive Composites to Dentin. Polymers 2022, 14, 3945. https://doi.org/10.3390/polym14193945
Inglês M, Vasconcelos e Cruz J, Mano Azul A, Polido M, Delgado AHS. Comparative Assessment of Different Pre-Treatment Bonding Strategies to Improve the Adhesion of Self-Adhesive Composites to Dentin. Polymers. 2022; 14(19):3945. https://doi.org/10.3390/polym14193945
Chicago/Turabian StyleInglês, Magali, Joana Vasconcelos e Cruz, Ana Mano Azul, Mário Polido, and António H. S. Delgado. 2022. "Comparative Assessment of Different Pre-Treatment Bonding Strategies to Improve the Adhesion of Self-Adhesive Composites to Dentin" Polymers 14, no. 19: 3945. https://doi.org/10.3390/polym14193945





