Facile Synthesis of Novel Disperse Dyes for Dyeing Polyester Fabrics: Demonstrating Their Potential Biological Activities
Abstract
:1. Introduction
2. Materials
3. Dyeing
Dye Uptake
4. Fastness Properties
5. In Vitro Cytotoxicity Screening
6. Antioxidant Activity (DPPH Radical Scavenging Activity)
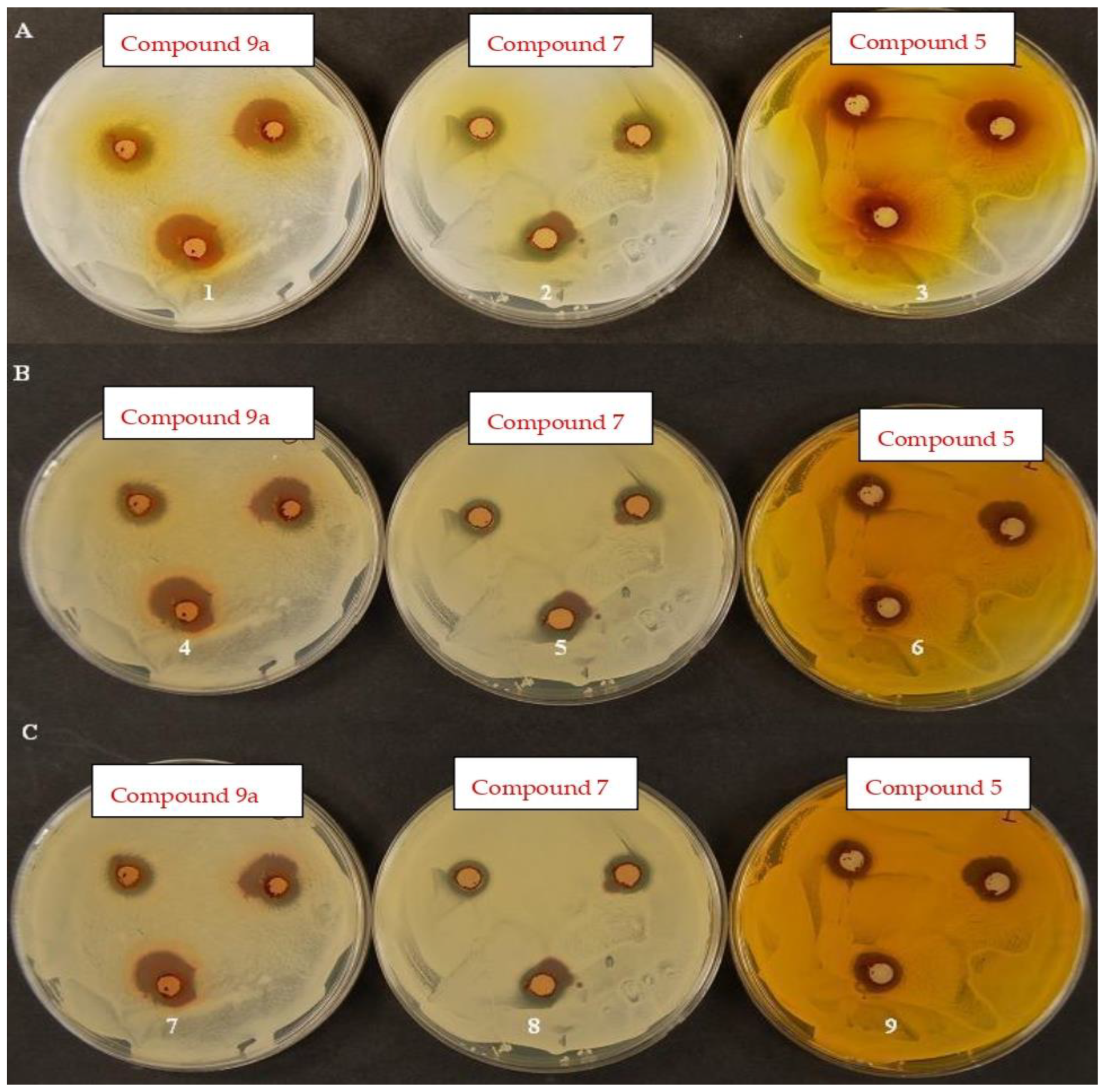
7. Antimicrobial Activities of Dyes 3, 5, 7, 9a–d, 22a–i and 26a,b

8. UV Protective Properties of Untreatedand Treated Polyester Fabrics with ZnO or TiO2 Nanoparticles NPs
9. Self-Cleaning of Untreatedand Treated Polyester Fabrics with ZnO or TiO2 NPs
10. Light Fastness of Untreated and Treated Polyester Fabrics with ZnO or TiO2 NPs
11. Antimicrobial Activity of Untreated and Treated Polyester Fabrics with ZnO or TiO2 NPs
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Etaibi, A.M.; El-Apasery, M.A. Ultrasonic Dyeing of Polyester Fabric with Azo Disperse Dyes Clubbed with Pyridonones and Its UV Protection Performance. Chemistry 2021, 3, 889–895. [Google Scholar] [CrossRef]
- Rehman, F.; Adeel, S.; Saif, M.J.; Khosa, M.K.; Anjum, M.N.; Kamran, M.; Zuber, M.; Asif, M. Ultrasonic Assisted Improvement in Dyeing Behaviour of Polyester Fabric Using Disperse Red343. Pol. J. Environ. Stud. 2020, 29, 261–265. [Google Scholar] [CrossRef]
- Adeel, S.; Shahid, S.; Khan, S.G.; Rehman, F.; Muneer, M.; Zube, M.; Akhtar, H. Eco-Friendly Disperse Dyeing of Ultraviolet-Treated Polyester Fabric Using DisperseYellow211. Pol. J. Environ. Stud. 2018, 27, 1935–1939. [Google Scholar] [CrossRef]
- Al-Etaibi, A.M.; El-Apasery, M.A. Microwave-Assisted Synthesis of Azo Disperse Dyes for Dyeing Polyester Fabrics: Our Contributions over the Past Decade. Polymers 2022, 14, 1703. [Google Scholar] [CrossRef] [PubMed]
- Ghaffar, A.; Adeel, S.; Habib, N.; Jalal, F.; Ul-Haq, A.; Munir, B.; Ahmad, A.; Jahangeer, M.; Jamil, Q. Effects of Microwave Radiation on Cotton Dyeing with Reactive Blue 21 Dye. Pol. J. Environ. Stud. 2019, 28, 1687–1691. [Google Scholar] [CrossRef]
- Gedye, R.; Smith, F.; Westaway, K.; Ali, H.; Baldisera, L.; Laberge, L.; Rousell, J. The Use of Microwave Ovens for Rapid Organic Synthesis. Tetrahedron Lett. 1986, 27, 279–282. [Google Scholar] [CrossRef]
- Stefanidis, G.D.; Muñoz, A.N.; Sturm, G.S.J.; Stankiewicz, A. A Helicopter View of Microwave Application to Chemical Processes: Reactions, Separations, and Equipment Concepts. Rev. Chem. Eng. 2014, 30, 233–259. [Google Scholar] [CrossRef]
- Bassyouni, F.A.; Abu-Bakr, S.M.; Abdel Rehim, A. Evolution of microwave irradiation and its application in green chemistry and biosciences. Res. Chem. Intermed. 2012, 38, 283–322. [Google Scholar] [CrossRef]
- Rana, K.K.; Rana, S. Microwave Reactors: A Brief Review on Its Fundamental Aspects and Applications. Open Access Libr. J. 2014, 1, 686. [Google Scholar] [CrossRef]
- Yoon, S.; Kim, H.; Oh, N.; Kim, S.; Kumar, S.; Koh, J. Synthesis and application of high-washability 4-amino-4′-fluorosulfonylazobenzene disperse dyes to cellulose diacetate for high color fastness. Fibers Polym. 2021, 22, 3075–3081. [Google Scholar] [CrossRef]
- Yoon, S.; Choi, B.; Rahman, M.M.; Kumar, S.; Kabir, S.M.M.; Koh, J. Dyeing of polyester with 4-arylazo-5-pyrazolone dyes containing fluoro sulfonyl group and application of environment-friendly after treatment for their high color fastness. Materials 2019, 12, 4209. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Ramanathan, S.; Basak, T. Microwave food processing—A review. Food Res. Int. 2013, 52, 243–261. [Google Scholar] [CrossRef]
- Crane, C.A.; Pantoya, M.L.; Weeks, B.L. Spatial observation and quantification of microwave heating in materials. Rev. Sci. Instrum. 2013, 84, 084705. [Google Scholar] [CrossRef]
- Rosa, R.; Veronesi, P.; Leonelli, C. A review on combustion synthesis intensification by means of microwave energy. Chem. Eng. Process. Process Intensif. 2013, 71, 2–18. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.; Yue, Q. Review on Microwave-Matter Interaction Fundamentals and Efficient Microwave-Associated Heating Strategies. Materials 2016, 9, 231. [Google Scholar]
- Orlik, K.; Lorgouilloux, Y.; Marchet, P.; Thuault, A.; Jean, F.; Rguiti, M.; Courtois, C. Influence of microwave sintering on electrical properties of BCTZ lead free piezoelectric ceramics. J. Eur. Ceram. Soc. 2020, 40, 1212–1216. [Google Scholar]
- Ramesh, S.; Zulkifli, N.; Tan, C.; Wong, Y.; Tarlochan, F.; Teng, W.; Sopyan, I.; Bang, L.; Sarhan, A.A.D. Comparison between microwave and conventional sintering on the properties and microstructural evolution of tetragonal zirconia. Ceram. Int. 2018, 44, 8922–8927. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, B.; Yang, B.; Ma, W.; Liu, G.; Li, H. Microwave sintering and fracture behavior of zirconia ceramics. Ceram. Int. 2019, 45, 17675–17680. [Google Scholar] [CrossRef]
- Huang, K.; Zheng, J.; Yuan, W.; Wang, X.; Song, Q.; Li, Y.; Crittenden, J.C.; Wang, L.; Wang, J. Microwave-assisted chemical recovery of glass fiber and epoxy resin from non-metallic components in waste printed circuit boards. Waste Manag. 2021, 124, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Serdar, G.; Demir, E.; Bayrak, S.; Sökmen, M. New Approaches for Effective Microwave Assisted Extraction of Caffeine and Catechins from Green Tea. Int. J. Second. Metab. 2016, 3, 3–13. [Google Scholar] [CrossRef]
- Bacsa, B.; Desai, B.; Dibó, G.; Kappe, C.O. Rapid solid-phase peptide synthesis using thermal and controlled microwave irradiation. J. Pept. Sci. 2006, 12, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.K.; Gellman, S.H. Application of microwave irradiation to the synthesis of 14-helical b-peptides. Org. Lett. 2005, 7, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Olivos, H.J.; Alluri, P.G.; Reddy, M.M.; Salony, D.; Kodadek, T. Microwave assisted solid-phase synthesis of peptoids. Org. Lett. 2002, 4, 4057–4059. [Google Scholar] [CrossRef]
- Collins, J.M.; Collins, M.J. Novel method for enhanced solid-phase peptide synthesis using microwave energy. Biopolymers 2003, 71, 361. [Google Scholar]
- Mallalcpour, S.E.; Hajipour, A.R.; Zamanlou, M.R. Synthesis of optically active poly(amide-imide)s derived from N,N′-(4,4′-carbonyldiphthaloyl)-bis-L-leucine diacid chloride and aromatic diamines by microwave radiation. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 177–186. [Google Scholar] [CrossRef]
- Mallakpour, S.E.; Hajipour, A.R.; Faghihi, K.; Foroughifar, N.; Bagheri, J. Novel Optically Active Poly(amideimide)s with Tetrahydropyrimidinone and Tetrahydro-2-Thioxopynmidine Moieties by Microwave-Assisted Polycondensation. J. Appl. Polym. Sci. 2001, 80, 2416–2421. [Google Scholar] [CrossRef]
- Mallakpour, S.E.; Hajipour, A.R.; Habibi, S. Facile synthesis of new optically active poly(amide-imide)s derived from N,N′-(pyromellitoy1)-bis-l-leucine diacid chloride and aromatic diamines under microwave irradiation. Eur. Polym. J. 2001, 37, 2435–2442. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, Q.; Zhu, X.; Wang, F. Microwave radiation copolymerization of dibutyltin maleate and allyl thiourea. J. Appl. Polym. Sci. 2001, 79, 312–318. [Google Scholar]
- Kappe, C.O.; Dallinger, R.D. The impact of microwave synthesis on drug discovery. Nat. Rev. Drug Discov. 2006, 5, 51–64. [Google Scholar] [CrossRef]
- Kappe, C.O.; Stadler, A.; Dallinger, D.; Strohmeier, G.; Perez, R.; Zbruyev, O.I.; Stiasni, N.; Walla, P.; Gorobets, N.; Yousefi, B.; et al. Adventures in microwave-assisted organic synthesis: Contributions from the Kappe laboratory 2000–2005. Nato Sci. Ser. Ii-Math. 2008, 246, 225–251. [Google Scholar]
- Yin, C. Microwave-assisted pyrolysis of biomass for liquid biofuels production. Bioresour. Technol. 2012, 120, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Wei, R.; Wang, P.; Zhang, G.; Wang, N.; Zheng, T. Microwave-responsive catalysts for wastewater treatment: A review. Chem. Eng. J. 2020, 382, 122781. [Google Scholar] [CrossRef]
- Remya, N.; Lin, J.-G. Current status of microwave application in wastewater treatment—A review. Chem. Eng. J. 2011, 166, 797–813. [Google Scholar] [CrossRef]
- Mudhoo, A.; Sharma, S.K.; Sharma, S.K. Microwave Irradiation Technology in Waste Sludge and Wastewater Treatment Research. Crit. Rev. Environ. Sci. Technol. 2011, 41, 999–1066. [Google Scholar] [CrossRef]
- Vialkova, E.; Obukhova, M.; Belova, L. Microwave Irradiation in Technologies of Wastewater and Wastewater Sludge Treatment: A Review. Water 2021, 13, 1784. [Google Scholar] [CrossRef]
- Dominguez, A.; Menendez, J.A.; Inguanzo, M.; Pis, J.J. Investigations into the characteristics of oils produced from microwave pyrolysis of sewage sludge. Fuel Process. Technol. 2005, 86, 1007–1020. [Google Scholar] [CrossRef]
- Dominguez, A.; Menendez, J.A.; Inguanzo, M.; Pís, J.J. Production of bio-fuels by high temperature pyrolysis of sewage sludge using conventional and microwave heating. Bioresour. Technol. 2006, 97, 1185–1193. [Google Scholar] [CrossRef]
- El-Apasery, M.A. Synthesis of some azo disperse dyes by the use of focused microwave heating. Pol. J. Appl. Chem. 2006, 50, 75–81. [Google Scholar]
- El-Apasery, M.A. Solvent-free one-pot synthesis of some azo disperse dyes under microwave irradiation: Dyeing of polyester fabrics. J. Appl. Polym. Sci. 2008, 109, 695–699. [Google Scholar] [CrossRef]
- Al-Zaydi, K.M.; Borik, R.M.; Elnagdi, M.H. Studies with arylhydrazonopyridinones: Synthesis of new arylhydrazono thieno[3,4-c]pyridinones as novel D2T2 dye class; classical verse green methodologies. Ultrason. Sonochem. 2009, 16, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Devi, A.S.; Kaping, S.; Vishwakarma, J.N. A facile environment-friendly one-pot two-step regioselective synthetic strategy for 3,7-diarylpyrazolo[1,5-a]pyrimidines related to zaleplon and 3,6-diarylpyrazolo[1,5-a]pyrimidine-7-amines assisted by KHSO4 in aqueous media. Mol. Divers. 2015, 19, 759–771. [Google Scholar] [CrossRef] [PubMed]
- El-Bayouki, M.; Abdel Hameed, K.; Asyouni, W.M.; Mohamed, Y.A.; Aly, M.M.; Abbas, S.Y. Novel 4(3H)-quinazolinones containing biologically active thiazole, pyridinone and chromene of expected antitumor and antifungal activities. Eur. J. Chem. 2011, 2, 455–462. [Google Scholar] [CrossRef]
- Okada, Y.; Hihara, T.; Morita, Z. Analysis of the photofading of phenylazo-aniline and phenylazo-pyridone disperse dyes on poly(ethylene terephthalate) substrate using the semiempirical molecular orbital PM5 method. Dye. Pigment. 2008, 79, 111–125. [Google Scholar] [CrossRef]
- Okada, Y.; Hihara, T.; Morita, Z. Photofading of phenylazo-aniline, -pyridone and -quinolone disperse dyes on a nylon 6 substrate. Color. Technol. 2009, 125, 86–98. [Google Scholar] [CrossRef]
- Darwish, E.; Mahmoud, F.F.; Altalbawy, F.M.A. Synthesis and Antimicrobial Evaluation of Some New Pyrazole, Fused Pyrazolo [1,5-a]-pyrimidine and Pyrazolo[1,5-d]pyrimido[4,5-d][1,2,3]triazine Derivatives. Asian J. Chem. 2012, 24, 2997–3002. [Google Scholar]
- Okada, Y.; Hihara, T.; Morita, Z. Analysis of the catalytic fading of pyridone-azo disperse dyes on polyester using the semi-empirical, molecular orbital PM5 method. Dye. Pigment. 2008, 78, 179–198. [Google Scholar] [CrossRef]
- Jang, H.K.; Doh, S.J.; Lee, J.J. Eco-friendly dyeing of poly(trimethylene terephthalate) with temporarily solubilized azo disperse dyes based on pyridone derivatives. Fibers Polym. 2009, 10, 315–319. [Google Scholar] [CrossRef]
- Gadre, J.N.; Periaswamy, R.M.S.; Mulay, M.; Vaze, C.S. Synthesis of pyridone based azo disperse dyes. Indian J. Heterocycl. Chem. 2006, 16, 43–46. [Google Scholar]
- Tsai, P.C.; Wang, I.J. Synthesis and solvatochromic properties of some disazo dyes derived from pyrazolo[1,5-a]pyrimidine derivatives. Dye. Pigment. 2005, 64, 259–264. [Google Scholar] [CrossRef]
- Tsai, P.C.; Wang, I.J. A facile synthesis of some new pyrazolo[1,5-a]pyrimidine heterocyclic disazo dyes and an evaluation of their solvatochromic behaviour. Dye. Pigment. 2007, 74, 578–584. [Google Scholar] [CrossRef]
- Balalaie, S.; Kowsari1, E.; Hashtroudi, M.S. An Efficient Method for the Synthesis of 3-Cyano-6-hydroxy-2(1H)-pyridinones under Microwave Irradiation and Solvent-free Conditions. Mon. Chem. 2003, 134, 453–456. [Google Scholar] [CrossRef]
- El-Adasy, A.A.A.M.; Kamel, M.M.; Saleh, M.O.; Hussein, A.M.; El-Apasery, M.A. Disperse Dyes Based on Pyrazolopyrimidinones I: Their Dyeing Applications and Antimicrobial Activities. Int. J. ChemTech Res. 2016, 9, 31–38. [Google Scholar]
- Brittany, H. Microwave Synthesis: Chemistry at the Speed of Light; CEM Publishing: Matthews, NC, USA, 2002; pp. 11–27. [Google Scholar]
- Díaz-Ortiz, Á.; Prieto, P.; De la Hoz, A. A Critical Overview on the Effect of Microwave Irradiation in Organic Synthesis. Chem. Rec. 2019, 19, 85. [Google Scholar]
- El-Apasery, M.A.; Hussein, A.M.; El-Adasy, A.A.M.; Saleh, M.O.; Kamel, M.M. Microwave Assisted Synthesis of Some Azo Disperse Dyes with Antibacterial Activities. Part 1. Egypt. J. Chem. 2019, 62, 1253–1259. [Google Scholar]
- Al-Etaibi, A.; El-Apasery, M.A.; Al-Awadi, N. The effect of dispersing agent on the dyeing of polyester fabrics with disperse dyes derived from 1,4-diethyl-2,6-dioxo-1,2,5,6-tetrahydropyridine-3-carbonitrile. Eur. J. Chem. 2013, 4, 240–244. [Google Scholar] [CrossRef]
- Al-Etaibi, A.; Al-Awadi, N.A.; El-Apasery, M.A.; Ibrahim, M.R. Synthesis of some novel pyrazolo [1, 5-a] pyrimidine derivatives and their application as disperse dyes. Molecules 2011, 16, 5182–5193. [Google Scholar] [CrossRef] [PubMed]
- Al-Etaibi, A.; El-Apasery, M.A.; Ibrahim, M.R.; Al-Awadi, N.A. A facile synthesis of new monoazo disperse dyes derived from 4-hydroxyphenylazopyrazole-5-amines: Evaluation of microwave assisted dyeing behavior. Molecules 2012, 17, 13891–13909. [Google Scholar] [CrossRef]
- Al-Etaibi, A.; El-Apasery, M.A.; Mahmoud, H.M.; Al-Awadi, N.A. One-pot synthesis of disperse dyes under microwave irradiation: Dyebath reuse in dyeing of polyester fabrics. Molecules 2012, 17, 4266–4280. [Google Scholar] [CrossRef]
- Al-Etaibi, A.; El-Apasery, M.A.; Mahmoud, H.; Al-Awadi, N. Synthesis, characterization and antimicrobial activity, and applications of new azo pyridone disperse dyes on polyester fabric. Eur. J. Chem. 2014, 5, 321–327. [Google Scholar]
- Al-Etaibi, A.M. Synthesis and Antimicrobial Activity of some Disperse Dyes derived from Pyridones. Int. J. ChemTech Res. 2019, 12, 129–133. [Google Scholar] [CrossRef]
- Al-Etaibi, A.M.; El-Apasery, M.A. Dyeing Performance of Disperse Dyes on Polyester Fabrics Using Eco-Friendly Carrier and Their Antioxidant and Anticancer Activities. Int. J. Environ. Res. Public Health 2019, 16, 4603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Etaibi, A.M.; El-Apasery, M.A. Nano TiO2 Imparting Multifunctional Performance on Dyed Polyester Fabrics with some Disperse Dyes Using High Temperature Dyeing as an Environmentally Benign Method. Int. J. Environ. Res. Public Health 2020, 17, 1377. [Google Scholar] [CrossRef] [PubMed]
- Mashaly, H.M.; Abdelghaffar, R.A.; Kamel, M.M.; Youssef, B.M. Dyeing of polyester fabric using nano disperse dyes and improving their light fastness using ZnOnano powder. Ind. J. Sci. Technol. 2012, 7, 960–967. [Google Scholar] [CrossRef]
- Al-Etaibi, A.M.; El-Apasery, M.A. A comprehensive review on the synthesis and versatile applications of biologically active pyridone-based disperse dyes. Int. J. Environ. Res. Public Health 2020, 17, 4714. [Google Scholar] [CrossRef]
- Dostanić, J.; Valentić, N.V.; Ušćumlić, G.; Mijin, D. Synthesis of 5-(substituted phenylazo)-6-hydroxy-4-methyl-3–cyano-2-pyridones from ethyl 3-oxo-2-(substituted phenylazo)butanoates. J. Serb. Chem. Soc. 2011, 76, 499–504. [Google Scholar] [CrossRef]
- Mijin, D.Ž.; Baghbanzadeh, M.; Reidlinger, C.; Kappe, C.O. The microwave-assisted synthesis of 5-arylazo-4,6-disubstituted-3-cyano-2-pyridone dyes. Dyes Pigm. 2010, 85, 73–78. [Google Scholar] [CrossRef]
- Gaffer, H.E.; Shkra, S.; Abbas, D.; Allam, E.A. Synthesis and antimicrobial activity of some new sulphonamide disperse dyes and their applications to polyester fibres. J. Appl. Polym. Res. 2013, 9, 4051–4058. [Google Scholar]
- Ahmed, K.A.; Elhennawy, H.M.; Elkashouti, M.A. Microwave Assists the Synthesis of Pyridone azo Dyes and their Application in Polyester Printing. Res. J. Chem. Sci. 2012, 2, 14–19. [Google Scholar]
- Mijin, D.; Ušćumlić, G.; Perišić-Janjić, N.; Trkulja, I.; Radetić, M.; Jovančić, P. Synthesis, properties and color assessment of some new 5-(3-and 4-substituted phenylazo)-4, 6-dimethyl-3-cyano-2-pyridones. J. Serb. Chem. Soc. 2006, 71, 435–444. [Google Scholar] [CrossRef]
- Al-Etaibi, A.M.; El-Apasery, M.A.; Kamel, M.M. Dyeing of polyester with disperse dyes: Part 1. Antimicrobial activity and dyeing performance of some disperse dyes. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 923–928. [Google Scholar]
- Al-Etaibi, A.M.; El-Apasery, M.A. Dyeing of polyester with disperse dyes: Part 3. Characterization of ZnO nanoparticles treated polyester fabrics for antibacterial, self-cleaning and UV protective. Int. J. ChemTech Res. 2016, 9, 162–169. [Google Scholar]
- Al-Etaibi, A.M.; Alnassar, H.S.; El-Apasery, M.A. Dyeing of polyester with disperse dyes: Part 2. Synthesis and dyeing characteristics of some azo disperse dyes for polyester fabrics. Molecules 2016, 21, 855. [Google Scholar] [CrossRef] [PubMed]
- Al-Etaibi, A.M.; Kamel, M.M.; El-Apasery, M.A. Synthesis and applications of new aminothienopyridazines disperse dyes on polyester fabric. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 826–832. [Google Scholar]















| Dye | Color Shade on Polyester | Color Strength (K/S) | Ref. |
|---|---|---|---|
| 5 | Yellowish-orange | 2.12 | [58] |
| 7 | Pale orange | 3.79 | |
| 9a | Dark orange | 5.95 | |
| 9b | Dark orange | 5.81 | |
| 9c | Orange | 4.64 | |
| 9d | Orange | 4.73 | |
| 14 | Yellowish brown | 0.84 | [60] |
| 15 | Pale brown | 4.35 | |
| 16a | Yellow | 9.78 | |
| 16b | Yellow | 15.72 | |
| 16c | Yellow | 14.92 | |
| 16d | Yellowish brown | 21.08 | |
| 16f | Yellowish orange | 23.55 | |
| 16g | Orange | 20.93 | |
| 16h | Orange | 16.52 | |
| 22a | Yellow | 27.39 | [61] |
| 22b | Dark orange | 30.29 | |
| 22c | Orange | 30.28 | |
| 22d | Orange | 8.88 | |
| 22e | Yellow | 28.91 | |
| 22f | Dark Yellow | 28.09 | |
| 22g | Very dark yellow | 27.31 | |
| 22h | Deep greenish yellow | 19.38 | |
| 22i | Orange yellow | 12.63 | |
| 26a | Greenish-yellow | 17.59 | [72] |
| 26b | Yellowish-orange | 16.69 | |
| 28a | Reddish-orange | 10.05 | [75] |
| 28b | Red | 16.03 | |
| 28c | Pink | 7.84 | |
| 28d | Violet | 9.79 |
| Dye No. | K/S | λmax | L* | a* | b* | c | h | Ref. |
|---|---|---|---|---|---|---|---|---|
| High temperature dyeing at 130 °C | ||||||||
| 22h | 19.38 | 445 | 76.33 | 4.47 | 93.78 | 93.88 | 87.27 | [63] |
| 22i | 12.63 | 450 | 78.41 | −0.56 | 87.34 | 87.34 | 90.37 | |
| 26a | 17.59 | 410 | 77.22 | −20.37 | 53.94 | 57.66 | 110.69 | [72] |
| 26b | 16.69 | 405 | 67.58 | 9.29 | 61.34 | 62.04 | 81.39 | |
| Low temperature dyeing at 100 °C | ||||||||
| 22h | 4.74 | 445 | 80.06 | −4.58 | 68.35 | 68.51 | 93.83 | [63] |
| 22i | 3.46 | 450 | 80.35 | −5.20 | 61.82 | 62.04 | 94.80 | |
| 26a | 12.21 | 410 | 84.81 | −14.26 | 52.16 | 54.08 | 105.29 | [73] |
| 26b | 8.97 | 405 | 78.08 | −0.21 | 51.20 | 51.21 | 90.23 | |
| US dyeing at 80 °C | ||||||||
| 22h | 9.07 | 445 | 80.58 | −3.80 | 78.86 | 78.92 | 92.24 | [1] |
| Dye No. | Light Fastness | Washing Fastness | Perspiration Fastness | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Alkaline | Acidic | |||||||
| SC | SW | SC | SW | SC | SW | |||
| 5 | 4 | 5 | 5 | 5 | 5 | 4 | 5 | [58] |
| 7 | 2 | 5 | 5 | 5 | 5 | 5 | 5 | |
| 9a | 2–3 | 5 | 5 | 5 | 5 | 5 | 5 | |
| 9b | 3 | 5 | 5 | 5 | 5 | 4 | 5 | |
| 9c | 2–3 | 4 | 4–5 | 5 | 5 | 4–5 | 4–5 | |
| 9d | 2–3 | 4–5 | 4–5 | 5 | 5 | 5 | 5 | |
| 14 | 3–4 | 5 | 5 | 5 | 5 | 4 | 5 | [60] |
| 15 | 5–6 | 5 | 5 | 5 | 5 | 5 | 5 | |
| 16a | 3–4 | 5 | 5 | 5 | 5 | 5 | 5 | |
| 16b | 3 | 5 | 5 | 5 | 5 | 5 | 5 | |
| 16c | 3 | 5 | 5 | 5 | 5 | 4 | 5 | |
| 16d | 3 | 4 | 4–5 | 5 | 5 | 5 | 5 | |
| 16f | 2–3 | 5 | 4–5 | 5 | 5 | 5 | 5 | |
| 16g | 3 | 5 | 4–5 | 5 | 5 | 5 | 5 | |
| 16h | 6 | 5 | 5 | 5 | 5 | 5 | 5 | |
| 22a | 4 | 5 | 5 | 5 | 5 | 5 | 5 | [61] |
| 22b | 2 | 5 | 5 | 5 | 5 | 5 | 5 | |
| 22c | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| 22d | 2 | 5 | 5 | 5 | 5 | 5 | 5 | |
| 22e | 3–4 | 5 | 5 | 4–5 | 5 | 5 | 5 | |
| 22f | 3–4 | 5 | 5 | 3 | 5 | 3–4 | 5 | |
| 22g | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| 22h | 4–5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| 22i | 4 | 5 | 5 | 5 | 5 | 5 | 5 | |
| 26a | 3 | 4–5 | 4–5 | 5 | 5 | 5 | 5 | [72] |
| 26b | 3–4 | 4–5 | 4–5 | 5 | 5 | 5 | 5 | |
| 28a | 3–4 | 5 | 5 | 5 | 5 | 5 | 5 | [75] |
| 28b | 4 | 4–5 | 4 | 5 | 5 | 5 | 5 | |
| 28c | 3–4 | 5 | 5 | 5 | 5 | 5 | 5 | |
| 28d | 4 | 5 | 5 | 5 | 5 | 5 | 5 | |
| Dye No. | Antioxidant Activity (IC50) | Cytotoxic Activity (IC50) | Ref. | |||
|---|---|---|---|---|---|---|
| MCF-7 | HepG-2 | A-549 | HCT-116 | |||
| 22h | 64.5 | 62.2 ± 4.1 | 23.4 ± 1.2 | 53.6 ± 5.8 | 28 ± 1.9 | [64] |
| 22i | 191.6 | 482 ± 8.9 | 196 ± 3.2 | 456 ± 7.3 | 242 ±3.6 | |
| Ascorbic acid | 14.2 | |||||
| Imatinib | 24.6 | 9.7 | ||||
| Cisplatin | 18.4 ± 0.9 | 19.3 ± 0.8 | ||||
| Dye No. | Inhibition Zone Diameter (Nearest mm) | ||
|---|---|---|---|
| G− Bacteria | G+ Bacteria | Yeast | |
| E. coli | B. subtilus | C. albicans | |
| 3 | NI | 11(0.1) | 8(0.3) |
| 5 | NI | 11(0.2) | 1(0.2) |
| 7 | NI | 4(0.2) | 4(0.1) |
| 9a | NI | 7(0.2) | 1(0.1) |
| 9b | NI | 2(0) | 2(0.1) |
| 9c | NI | 2(0) | 3(0.2) |
| 9d | NI | 2(0) | 3(0.2) |
| 22a | 14 (1) | 12.2 (0.5) | 14 (0.3) |
| 22b | 10.8 (1.5) | 9 (0.3) | 12 (1) |
| 22c | 12.1 (0.7) | 10.8 (0.5) | 19.1 (1.3) |
| 22d | 15 (0.5) | 14.6 (0.5) | 10.1 (0.5) |
| 22e | 10 (0.6) | 9 (0.2) | 12.7 (0.6) |
| 22f | 10 (0.4) | NI | 20.4 (0.7) |
| 22g | 11 (0.6) | 9.7 (0.5) | 16.2 (1) |
| 22h | 8 | NI | NI |
| 22i | NI | NI | 8 |
| 26a | 9 | NC | NI |
| 26b | 10 | NC | 20 |
| Dye No. | ZnO Treatment | UPF | Dye No. | TiO2 Treatment | UPF |
|---|---|---|---|---|---|
| Blank | 19.42 | Blank | 8.2 | ||
| 26a | Untreated | 141.88 | 22h | Untreated | 236.2 |
| Treated | 173.25 | Treated | 283.60 | ||
| 26b | Untreated | 122.37 | 22i | Untreated | 25.5 |
| Treated | 190.59 | Treated | 34.9 |
| Dye No. | ZnO% Treatment | Methyl Red Stain ∆E* | Light Fastness | Dye No. | TiO2% Treatment | Methylene Blue Stain | Light Fastness |
|---|---|---|---|---|---|---|---|
| Untreated | 67.23 | 3–4 | Untreated | No removal | 5–6 | ||
| 26a | 0.5 | 60.20 | 3 | 22h | 1 | 80% | 5–6 |
| 1.0 | 59.41 | 3 | 2 | 80% | 6 | ||
| 1.5 | 60.88 | 3 | 3 | 75% | 6 | ||
| 2.0 | 55.44 | 3 | 4 | 80% | 6 | ||
| 2.5 | 60.36 | 3 | 5 | 60% | 6 | ||
| Untreated | 50.02 | 4 | 22i | Untreated | 10% | 5 | |
| 26b | 0.5 | 54.42 | 5 | 1 | 80% | 5 | |
| 1.0 | 55.32 | 5 | 2 | 75% | 5 | ||
| 1.5 | 54.37 | 4 | 3 | 80% | 5 | ||
| 2.0 | 53.73 | 4–5 | 4 | 70% | 5 | ||
| 2.5 | 60.18 | 4–5 | 5 | 65% | 5–6 |
| Dye No. | ZnO% Treatment | Inhibition Zone Diameter (Nearest mm) | Dye No. | TiO2% Treatment | Inhibition Zone Diameter (Nearest mm) Fungi | ||
|---|---|---|---|---|---|---|---|
| G+ Bacteria Bacillus subtilis | G− Bacteria Klebsiella pneumoniae | Aspergillus flavus | Penicillium chrysogenum | ||||
| 26a | Untreated | NI | NI | 22h | Untreated | NI | NI |
| Treated | 11 | NI | Treated | 21 | 19 | ||
| 26b | Untreated | NI | NI | 22i | Untreated | NI | NI |
| Treated | 8 | 10 | Treated | NI | NI | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Etaibi, A.M.; El-Apasery, M.A. Facile Synthesis of Novel Disperse Dyes for Dyeing Polyester Fabrics: Demonstrating Their Potential Biological Activities. Polymers 2022, 14, 3966. https://doi.org/10.3390/polym14193966
Al-Etaibi AM, El-Apasery MA. Facile Synthesis of Novel Disperse Dyes for Dyeing Polyester Fabrics: Demonstrating Their Potential Biological Activities. Polymers. 2022; 14(19):3966. https://doi.org/10.3390/polym14193966
Chicago/Turabian StyleAl-Etaibi, Alya M., and Morsy Ahmed El-Apasery. 2022. "Facile Synthesis of Novel Disperse Dyes for Dyeing Polyester Fabrics: Demonstrating Their Potential Biological Activities" Polymers 14, no. 19: 3966. https://doi.org/10.3390/polym14193966
APA StyleAl-Etaibi, A. M., & El-Apasery, M. A. (2022). Facile Synthesis of Novel Disperse Dyes for Dyeing Polyester Fabrics: Demonstrating Their Potential Biological Activities. Polymers, 14(19), 3966. https://doi.org/10.3390/polym14193966






