Modeling Solution Drying by Moving a Liquid-Vapor Interface: Method and Applications
Abstract
:1. Introduction
2. Model and Simulation Methodology
2.1. Langevin Dynamics
2.2. Moving Interface Method
3. Applications of Moving Interface Method
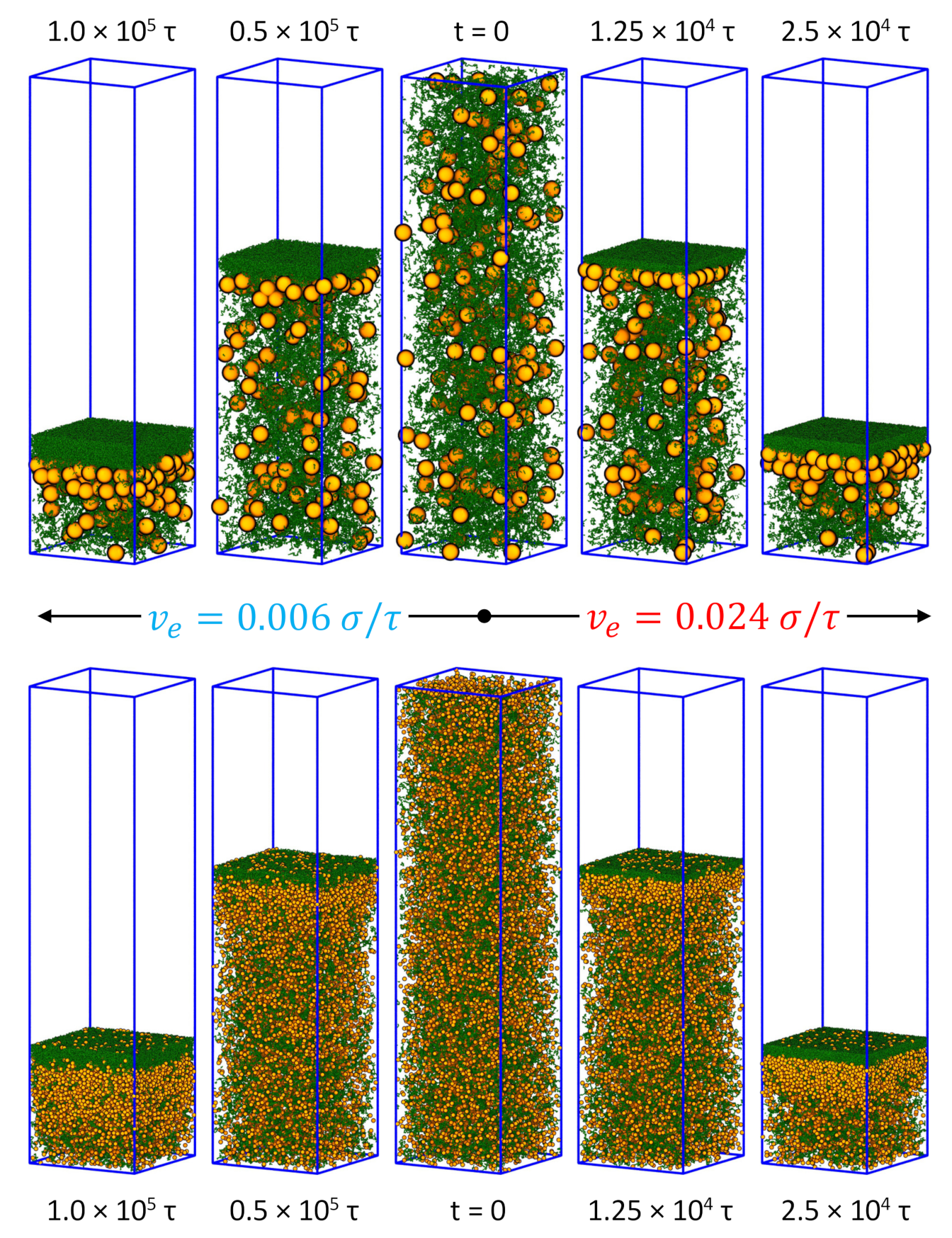
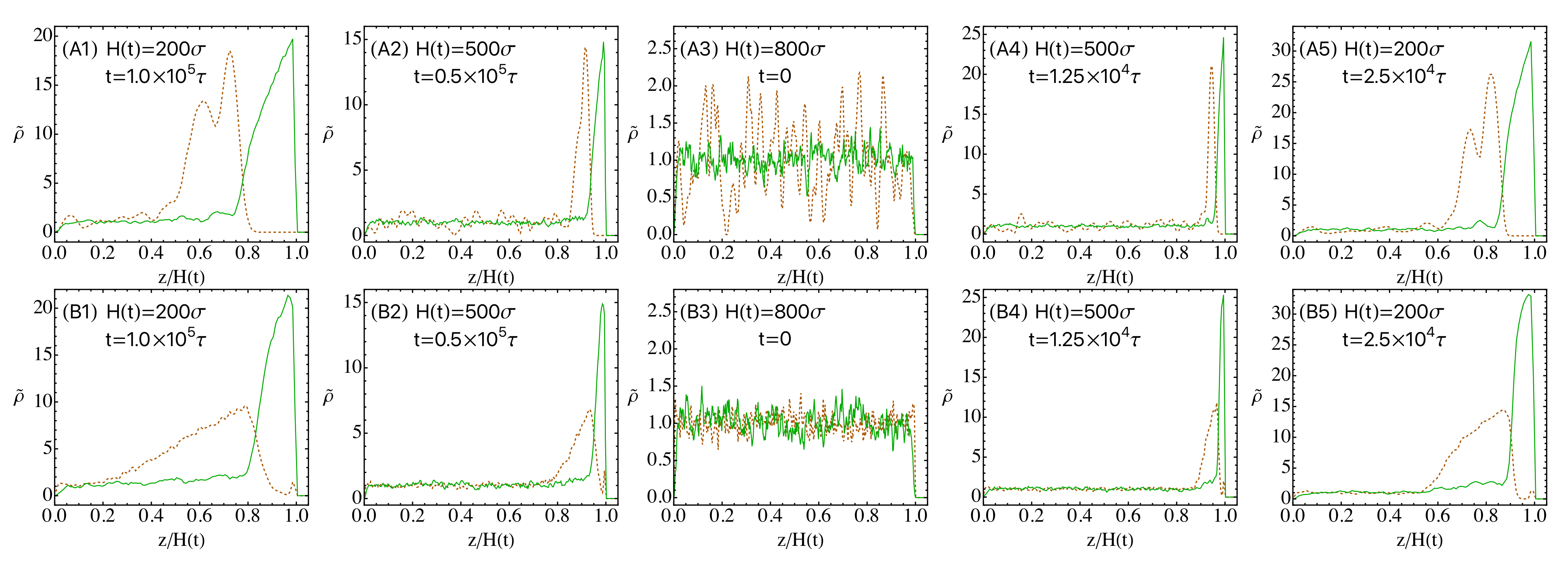
3.1. Drying of Solution Films of Polymer-Nanoparticle Mixtures
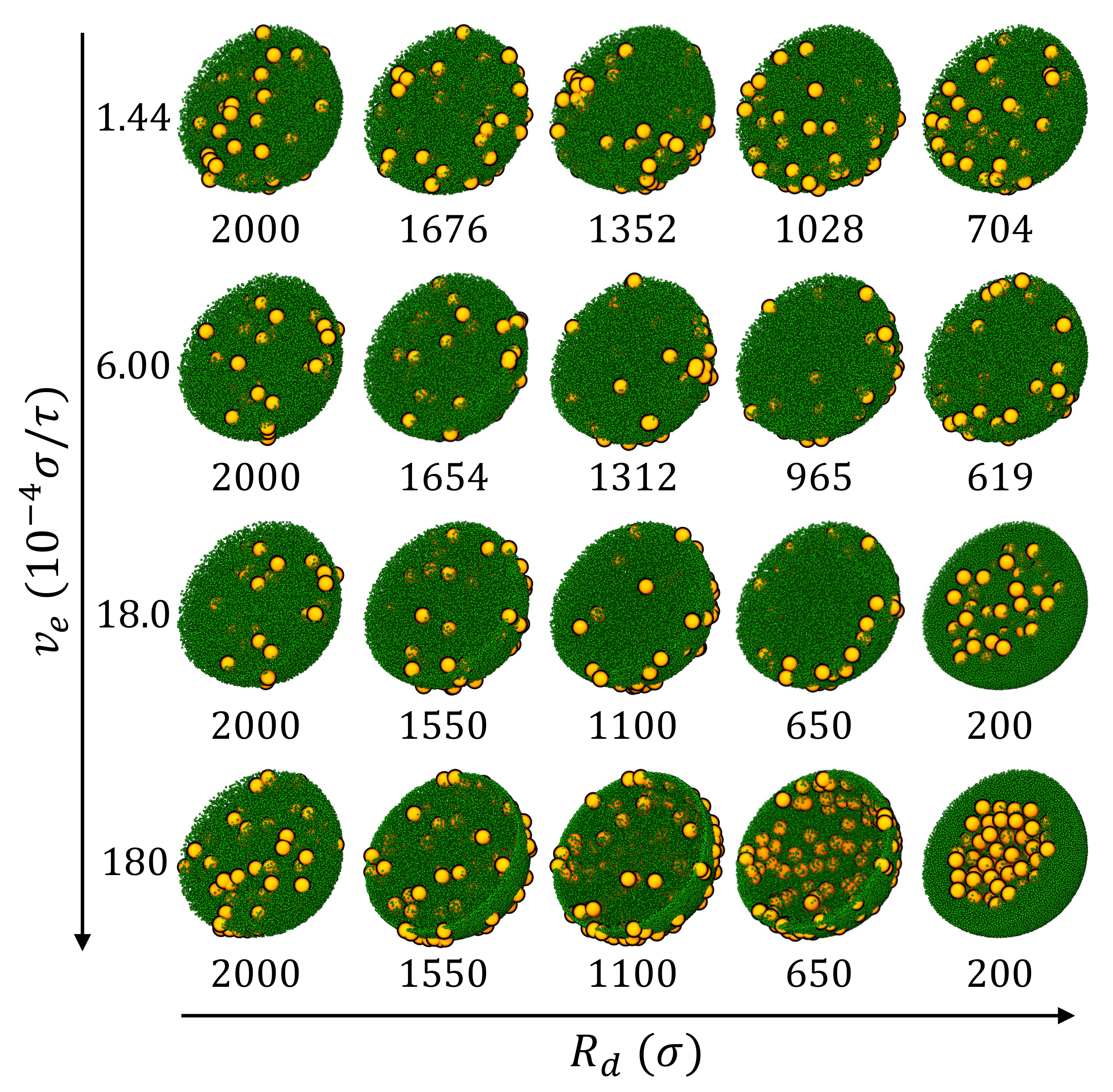
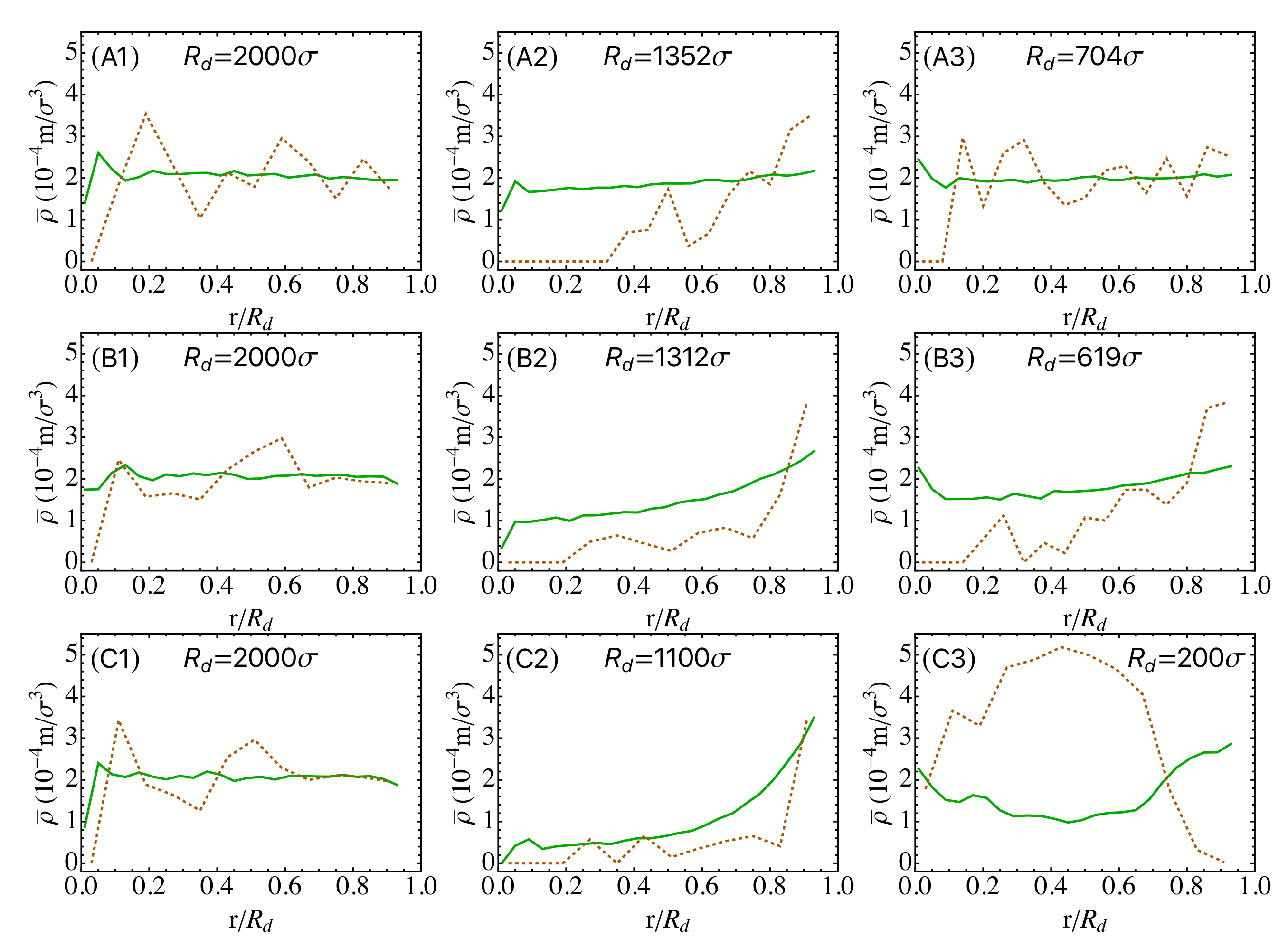
3.2. Drying of Suspension Droplets of Bidisperse Mixtures of Nanoparticles
3.3. Drying of Solution Droplets of a Polymer Blend
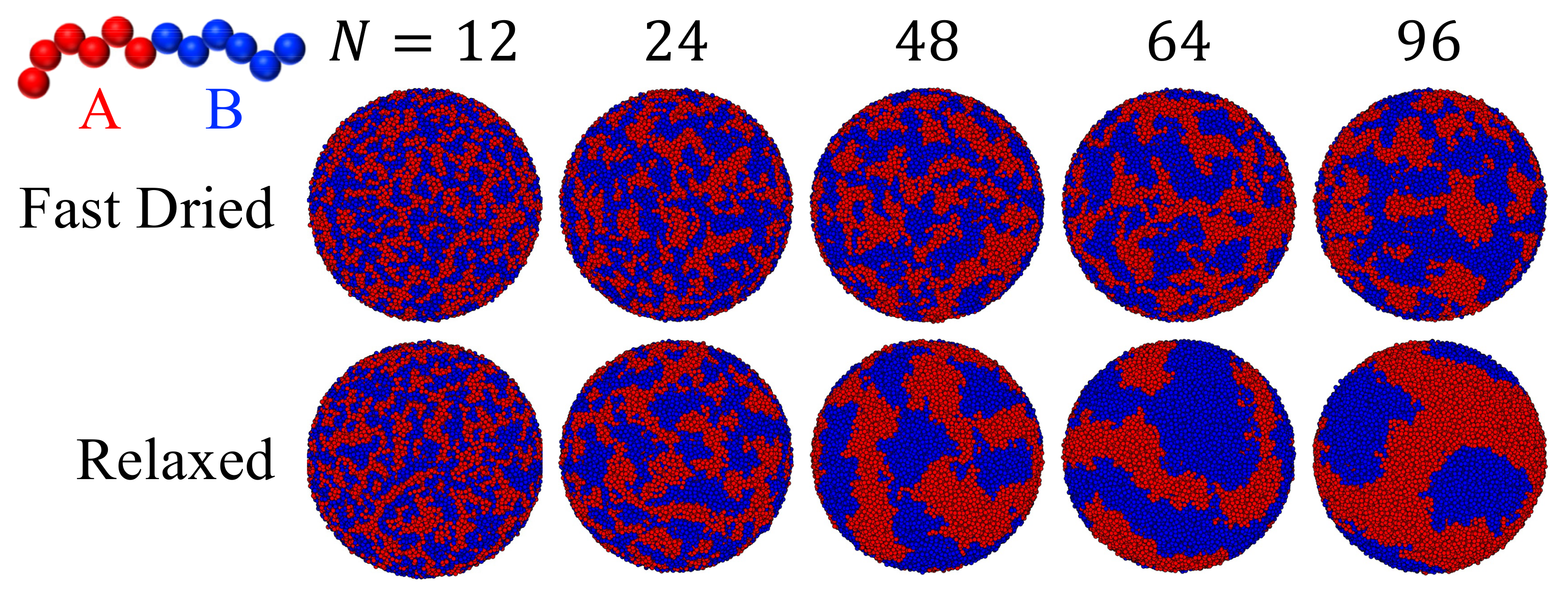
3.4. Drying of Solution Droplets of Diblock Copolymers
4. Summary and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COVID | Coronavirus disease |
| ODT | Oder-disorder transition |
| MD | Molecular dynamics |
| SNP | Small nanoparticle |
| MNP | Medium nanoparticle |
| LNP | Large nanoparticle |
| FENE | Finite extensible nonlinear elastic |
| LJ | Lennard-Jones |
| FNP | Flash nanoprecipitation |
References
- Dinçer, Í.; Zamfirescu, C. Drying Phenomena: Theory and Applications; Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Brinker, C.J. Evaporation-induced self-assembly: Functional nanostructures made easy. MRS Bull. 2004, 29, 631–640. [Google Scholar] [CrossRef]
- Zhou, J.; Man, X.; Jiang, Y.; Doi, M. Structure formation in soft-matter solutions induced by solvent evaporation. Adv. Mater. 2017, 29, 1703769. [Google Scholar] [CrossRef]
- Keddie, J.L.; Routh, A.F. Fundamentals of Latex Film Formation: Processes and Properties; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Keddie, J.L. Film formation of latex. Mater. Sci. Eng. R Rep. 1997, 21, 101–170. [Google Scholar] [CrossRef]
- Luo, H.; Cardinal, C.M.; Scriven, L.E.; Francis, L.F. Ceramic nanoparticle/monodisperse latex coatings. Langmuir 2008, 24, 5552–5561. [Google Scholar] [CrossRef]
- Van der Kooij, H.M.; Sprakel, J. Watching paint dry: More exciting than it seems. Soft Matter 2015, 11, 6353–6359. [Google Scholar] [CrossRef]
- Strawhecker, K.E.; Kumar, S.K.; Douglas, J.F.; Karim, A. The critical role of solvent evaporation on the roughness of spin-cast polymer films. Macromolecules 2001, 34, 4669–4672. [Google Scholar] [CrossRef]
- Luo, S.C.; Craciun, V.; Douglas, E.P. Instabilities during the formation of electroactive polymer thin films. Langmuir 2005, 21, 2881–2886. [Google Scholar] [CrossRef]
- Sousa, S.; Costa, A.; Silva, A.; Simões, R. Poly(lactic acid)/cellulose films produced from composite spheres prepared by emulsion-solvent evaporation method. Polymers 2019, 11, 66. [Google Scholar] [CrossRef]
- Shin, J.M.; Kim, Y.; Yun, H.; Yi, G.R.; Kim, B.J. Morphological evolution of block copolymer particles: Effect of solvent evaporation rate on particle shape and morphology. ACS Nano 2017, 11, 2133–2142. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, H.; Lu, H. Preparation of polyetherimide nanoparticles by a droplet evaporation-assisted thermally induced phase-separation method. Polymers 2021, 13, 1548. [Google Scholar] [CrossRef]
- Jouault, N.; Zhao, D.; Kumar, S.K. Role of casting solvent on nanoparticle dispersion in polymer nanocomposites. Macromolecules 2014, 47, 5246–5255. [Google Scholar] [CrossRef]
- Imel, A.E.; Dadmun, M.D. The impact of fullerenes on the ordering of polyacrylonitrile during nanocomposites formation. Polymer 2015, 75, 134–140. [Google Scholar] [CrossRef]
- Cheng, S.; Grest, G.S. Dispersing nanoparticles in a polymer film via solvent evaporation. ACS Macro Lett. 2016, 5, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Ganesan, V.; Riggleman, R.A. Perspective: Outstanding theoretical questions in polymer-nanoparticle hybrids. J. Chem. Phys. 2017, 147, 020901. [Google Scholar] [CrossRef]
- Ge, T.; Cheng, S. Physicochemical properties of respiratory droplets and their role in COVID-19 pandemics: A critical review. Biomater. Transl. 2021, 2, 10–18. [Google Scholar] [CrossRef]
- Leung, N.H.L. Transmissibility and transmission of respiratory viruses. Nat. Rev. Microbiol. 2021, 19, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Routh, A.F. Drying of thin colloidal films. Rep. Prog. Phys. 2013, 76, 046603. [Google Scholar] [CrossRef]
- Schulz, M.; Keddie, J.L. A critical and quantitative review of the stratification of particles during the drying of colloidal films. Soft Matter 2018, 14, 6181–6197. [Google Scholar] [CrossRef]
- Tsige, M.; Grest, G.S. Molecular dynamics study of the evaporation process in polymer films. Macromolecules 2004, 37, 4333–4335. [Google Scholar] [CrossRef]
- Tsige, M.; Mattsson, T.R.; Grest, G.S. Morphology of evaporated multiblock copolymer membranes studied by molecular dynamics simulations. Macromolecules 2004, 37, 9132–9138. [Google Scholar] [CrossRef]
- Tsige, M.; Grest, G.S. Solvent evaporation and interdiffusion in polymer films. J. Phys. Conden. Matt. 2005, 17, S4119. [Google Scholar] [CrossRef]
- Cheng, S.; Grest, G.S. Molecular dynamics simulations of evaporation-induced nanoparticle assembly. J. Chem. Phys. 2013, 138, 064701. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Stevens, M.J.; Grest, G.S. Ordering nanoparticles with polymer brushes. J. Chem. Phys. 2017, 147, 224901. [Google Scholar] [CrossRef] [PubMed]
- Fortini, A.; Martín-Fabiani, I.; De La Haye, J.L.; Dugas, P.Y.; Lansalot, M.; D’Agosto, F.; Bourgeat-Lami, E.; Keddie, J.L.; Sear, R.P. Dynamic stratification in drying films of colloidal mixtures. Phys. Rev. Lett. 2016, 116, 118301. [Google Scholar] [CrossRef] [PubMed]
- Fortini, A.; Sear, R.P. Stratification and size segregation of ternary and polydisperse colloidal suspensions during drying. Langmuir 2017, 33, 4796–4805. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.P.; Nikoubashman, A.; Panagiotopoulos, A.Z. Stratification dynamics in drying colloidal mixtures. Langmuir 2017, 33, 3685–3693. [Google Scholar] [CrossRef]
- Howard, M.P.; Nikoubashman, A.; Panagiotopoulos, A.Z. Stratification in drying polymer-polymer and colloid-polymer mixtures. Langmuir 2017, 33, 11390–11398. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.P.; Reinhart, W.F.; Sanyal, T.; Shell, M.S.; Nikoubashman, A.; Panagiotopoulos, A.Z. Evaporation-induced assembly of colloidal crystals. J. Chem. Phys. 2018, 149, 094901. [Google Scholar] [CrossRef]
- Reinhart, W.F.; Long, A.W.; Howard, M.P.; Ferguson, A.L.; Panagiotopoulos, A.Z. Machine learning for autonomous crystal structure identification. Soft Matter 2017, 13, 4733–4745. [Google Scholar] [CrossRef]
- Reinhart, W.F.; Panagiotopoulos, A.Z. Automated crystal characterization with a fast neighborhood graph analysis method. Soft Matter 2018, 14, 6083–6089. [Google Scholar] [CrossRef]
- Statt, A.; Howard, M.P.; Panagiotopoulos, A.Z. Solvent quality influences surface structure of glassy polymer thin films after evaporation. J. Chem. Phys. 2017, 147, 184901. [Google Scholar] [CrossRef]
- Statt, A.; Howard, M.P.; Panagiotopoulos, A.Z. Influence of hydrodynamic interactions on stratification in drying mixtures. J. Chem. Phys. 2018, 149, 024902. [Google Scholar] [CrossRef]
- Tatsumi, R.; Iwao, T.; Koike, O.; Yamaguchi, Y.; Tsuji, Y. Effects of the evaporation rate on the segregation in drying bimodal colloidal suspensions. Appl. Phys. Lett. 2018, 112, 053702. [Google Scholar] [CrossRef]
- Tang, Y.; Grest, G.S.; Cheng, S. Stratification in drying films containing bidisperse mixtures of nanoparticles. Langmuir 2018, 34, 7161–7170. [Google Scholar] [CrossRef]
- Tang, Y.; Grest, G.S.; Cheng, S. Control of stratification in drying particle suspensions via temperature gradients. Langmuir 2019, 35, 4296–4304. [Google Scholar] [CrossRef]
- Tang, Y.; Grest, G.S.; Cheng, S. Stratification of drying particle suspensions: Comparison of implicit and explicit solvent simulations. J. Chem. Phys. 2019, 150, 224901. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Mitov, Z.; Kumacheva, E. Convection-induced patterns in phase-separating polymeric fluids. Phys. Rev. Lett. 1998, 81, 3427–3430. [Google Scholar] [CrossRef]
- Bassou, N.; Rharbi, Y. Role of Bénard-Marangoni instabilities during solvent evaporation in polymer surface corrugations. Langmuir 2009, 25, 624–632. [Google Scholar] [CrossRef]
- Chapman, N.; Chapman, M.; Euler, W.B. Evolution of surface morphology of spin-coated poly(methyl methacrylate) thin films. Polymers 2021, 13, 2184. [Google Scholar] [CrossRef]
- Cheng, S.; Grest, G.S. Structure and diffusion of nanoparticle monolayers floating at liquid/vapor interfaces: A molecular dynamics study. J. Chem. Phys. 2012, 136, 214702. [Google Scholar] [CrossRef]
- Grest, G.S.; Wang, Q.; in ’t Veld, P.; Keffer, D.J. Effective potentials between nanoparticles in suspension. J. Chem. Phys. 2011, 134, 144902. [Google Scholar] [CrossRef]
- Tang, Y.; Cheng, S. Capillary forces on a small particle at a liquid-vapor interface: Theory and simulation. Phys. Rev. E 2018, 98, 032802. [Google Scholar] [CrossRef]
- Schneider, T.; Stoll, E. Molecular-dynamics study of a three-dimensional one-component model for distortive phase transitions. Phys. Rev. B 1978, 17, 1302–1322. [Google Scholar] [CrossRef]
- Grest, G.S.; Kremer, K. Molecular dynamics simulation for polymers in the presence of a heat bath. Phys. Rev. A 1986, 33, 3628–3631. [Google Scholar] [CrossRef]
- Pieranski, P. Two-dimensional interfacial colloidal crystals. Phys. Rev. Lett. 1980, 45, 569. [Google Scholar] [CrossRef]
- Joanny, J.F.; de Gennes, P.G. A model for contact angle hysteresis. J. Chem. Phys. 1984, 81, 552–562. [Google Scholar] [CrossRef]
- Bigioni, T.P.; Lin, X.M.; Nguyen, T.T.; Corwin, E.I.; Witten, T.A.; Jaeger, H.M. Kinetically driven self-assembly of highly ordered nanoparticle monolayers. Nat. Mater. 2006, 5, 265–270. [Google Scholar] [CrossRef]
- Kremer, K.; Grest, G.S. Dynamics of entangled linear polymer melts: A molecular-dynamics simulation. J. Chem. Phys. 1990, 92, 5057. [Google Scholar] [CrossRef]
- Everaers, R.; Ejtehadi, M.R. Interaction potentials for soft and hard ellipsoids. Phys. Rev. E 2003, 67, 041710. [Google Scholar] [CrossRef] [Green Version]
- In ’t Veld, P.J.; Plimpton, S.J.; Grest, G.S. Accurate and efficient methods for modeling colloidal mixtures in an explicit solvent using molecular dynamics. Comput. Phys. Commun. 2008, 179, 320–329. [Google Scholar] [CrossRef]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; in ’t Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS-a flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comp. Phys. Comm. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, S. Chain conformations and phase separation in polymer solutions with varying solvent quality. J. Polym. Sci. 2021, 59, 2819–2831. [Google Scholar] [CrossRef]
- Rubinstein, M.; Colby, R.H. Polymer Physics; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Zhou, J.; Jiang, Y.; Doi, M. Cross interaction drives stratification in drying film of binary colloidal mixtures. Phys. Rev. Lett. 2017, 118, 108002. [Google Scholar] [CrossRef]
- Makepeace, D.K.; Fortini, A.; Markov, A.; Locatelli, P.; Lindsay, C.; Moorhouse, S.; Lind, R.; Sear, R.P.; Keddie, J.L. Stratification in binary colloidal polymer films: Experiment and simulations. Soft Matter 2017, 13, 6969–6980. [Google Scholar] [CrossRef]
- Liu, W.; Midya, J.; Kappl, M.; Butt, H.J.; Nikoubashman, A. Segregation in drying binary colloidal droplets. ACS Nano 2019, 13, 4972–4979. [Google Scholar] [CrossRef]
- Xiao, M.; Hu, Z.; Gartner, T.E.; Yang, X.; Li, W.; Jayaraman, A.; Gianneschi, N.C.; Shawkey, M.D.; Dhinojwala, A. Experimental and theoretical evidence for molecular forces driving surface segregation in photonic colloidal assemblies. Sci. Adv. 2019, 5, eaax1254. [Google Scholar] [CrossRef]
- Gartner, T.E.; Heil, C.M.; Jayaraman, A. Surface composition and ordering of binary nanoparticle mixtures in spherical confinement. Mol. Syst. Des. Eng. 2020, 5, 864–875. [Google Scholar] [CrossRef]
- Howard, M.P.; Nikoubashman, A. Stratification of polymer mixtures in drying droplets: Hydrodynamics and diffusion. J. Chem. Phys. 2020, 153, 054901. [Google Scholar] [CrossRef]
- Lintingre, E.; Lequeux, F.; Talini, L.; Tsapis, N. Control of particle morphology in the spray drying of colloidal suspensions. Soft Matter 2016, 12, 7435–7444. [Google Scholar] [CrossRef] [Green Version]
- Lintingre, E.; Ducouret, G.; Lequeux, F.; Olanier, L.; Périé, T.; Talini, L. Controlling the buckling instability of drying droplets of suspensions through colloidal interactions. Soft Matter 2015, 11, 3660–3665. [Google Scholar] [CrossRef]
- Lishchuk, S.V.; Ettelaie, R. Detachment force of particles with pinning of contact line from fluid bubbles/droplets. Langmuir 2016, 32, 13040–13045. [Google Scholar] [CrossRef]
- Van der Kooij, H.M.; van de Kerkhof, G.T.; Sprakel, J. A mechanistic view of drying suspension droplets. Soft Matter 2016, 12, 2858–2867. [Google Scholar] [CrossRef]
- Grest, G.S.; Lacasse, M.; Kremer, K.; Gupta, A.M. Efficient continuum model for simulating polymer blends and copolymers. J. Chem. Phys. 1996, 105, 10583–10594. [Google Scholar] [CrossRef]
- Higuchi, T.; Tajima, A.; Yabu, H.; Shimomura, M. Spontaneous formation of polymer nanoparticles with inner micro-phase separation structures. Soft Matter 2008, 4, 1302. [Google Scholar] [CrossRef]
- Bates, F.S.; Fredrickson, G.H. Block copolymer thermodynamics: Theory and experiment. Ann. Rev. Phys. Chem. 1990, 41, 525–557. [Google Scholar] [CrossRef]
- Johnson, B.K.; Prud’homme, R.K. Chemical processing and micromixing in confined impinging jets. AIChE J. 2003, 49, 2264–2282. [Google Scholar] [CrossRef]
- Johnson, B.K.; Prud’homme, R.K. Mechanism for rapid self-assembly of block copolymer nanoparticles. Phys. Rev. Lett. 2003, 91, 118302. [Google Scholar] [CrossRef]
- Grundy, L.S.; Lee, V.E.; Li, N.; Sosa, C.; Mulhearn, W.D.; Liu, R.; Register, R.A.; Nikoubashman, A.; Prud’homme, R.K.; Panagiotopoulos, A.Z.; et al. Rapid production of internally structured colloids by flash nanoprecipitation of block copolymer blends. ACS Nano 2018, 12, 4660–4668. [Google Scholar] [CrossRef]
- Javaid, S.; Mahmood, A.; Nasir, H.; Iqbal, M.; Ahmed, N.; Ahmad, N.M. Layer-by-layer self-assembled dip coating for antifouling functionalized finishing of cotton textile. Polymers 2022, 14, 2540. [Google Scholar] [CrossRef]
- Rodrigues, S.; da Costa, A.M.R.; Flórez-Fernández, N.; Torres, M.D.; Faleiro, M.L.; Buttini, F.; Grenha, A. Inhalable spray-dried chondroitin sulphate microparticles: Effect of different solvents on particle properties and drug activity. Polymers 2020, 12, 425. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, T.; Khan, A.U.; Liu, G. Block copolymer-based porous carbon fibers. Sci. Adv. 2019, 5, eaau6852. [Google Scholar] [CrossRef]
- Hu, H.; Larson, R.G. Marangoni effect reverses coffee-ring depositions. J. Phys. Chem. B 2006, 110, 7090–7094. [Google Scholar] [CrossRef]
- Joshi, A.S.; Sun, Y. Wetting dynamics and particle deposition for an evaporating colloidal drop: A lattice Boltzmann study. Phys. Rev. E 2010, 82, 041401. [Google Scholar] [CrossRef] [Green Version]


| System | Pe | Pe | |
|---|---|---|---|
| MNP-1 | 1391 | 2098 | |
| MNP-2 | 348 | 525 | |
| SNP-1 | 198 | 2023 | |
| SNP-2 | 49 | 506 |
| 7.0 | 0.76 | |
| 29 | 3.2 | |
| 87 | 9.5 | |
| 870 | 95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; McLaughlan, J.E.; Grest, G.S.; Cheng, S. Modeling Solution Drying by Moving a Liquid-Vapor Interface: Method and Applications. Polymers 2022, 14, 3996. https://doi.org/10.3390/polym14193996
Tang Y, McLaughlan JE, Grest GS, Cheng S. Modeling Solution Drying by Moving a Liquid-Vapor Interface: Method and Applications. Polymers. 2022; 14(19):3996. https://doi.org/10.3390/polym14193996
Chicago/Turabian StyleTang, Yanfei, John E. McLaughlan, Gary S. Grest, and Shengfeng Cheng. 2022. "Modeling Solution Drying by Moving a Liquid-Vapor Interface: Method and Applications" Polymers 14, no. 19: 3996. https://doi.org/10.3390/polym14193996







