Influence of Novel Experimental Light-Cured Resin Cement on Microtensile Bond Strength
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Used in the Study
2.2. Specimen Preparation
2.3. µTBS Test
2.4. Fracture Modes Analysis
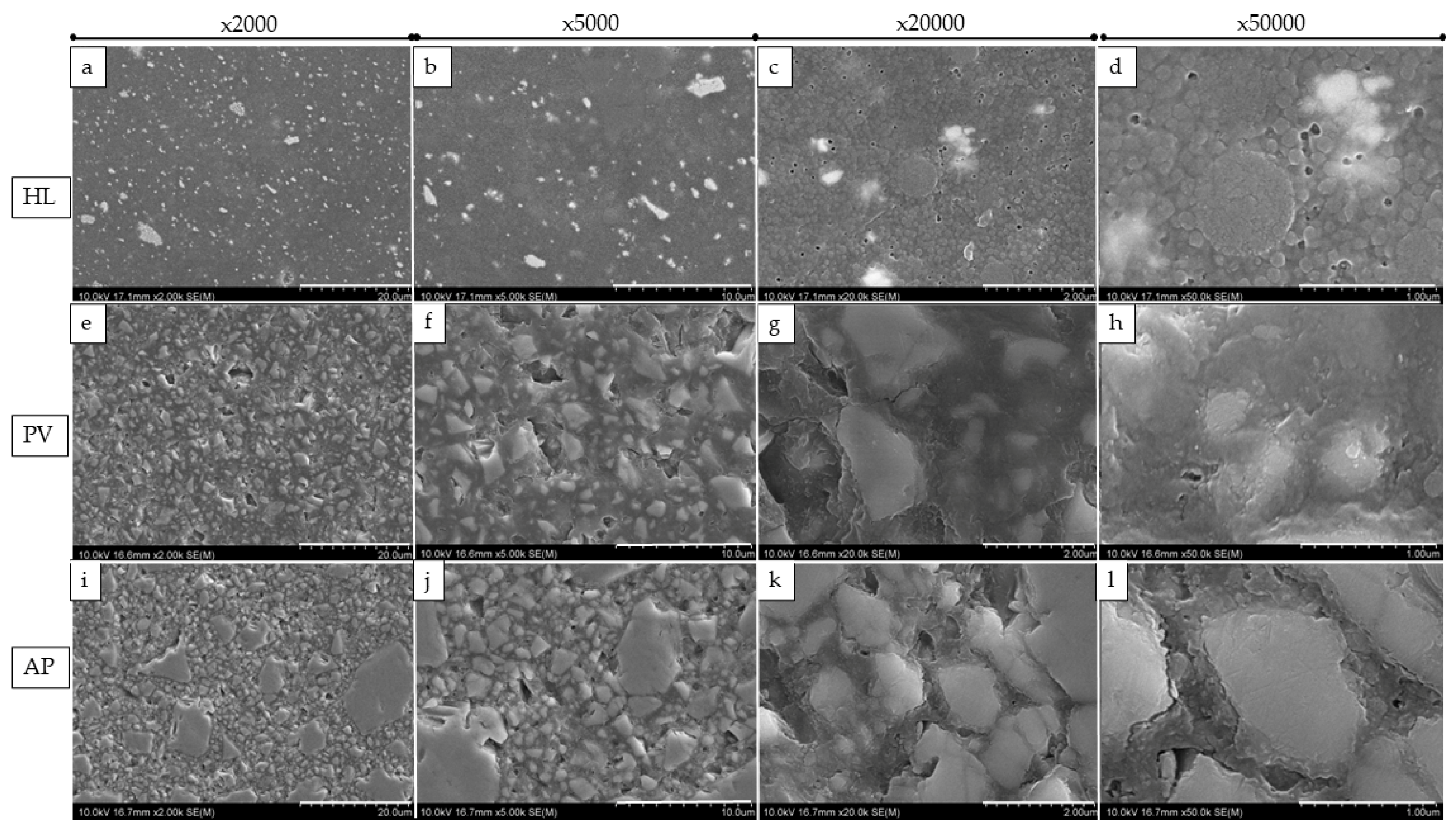
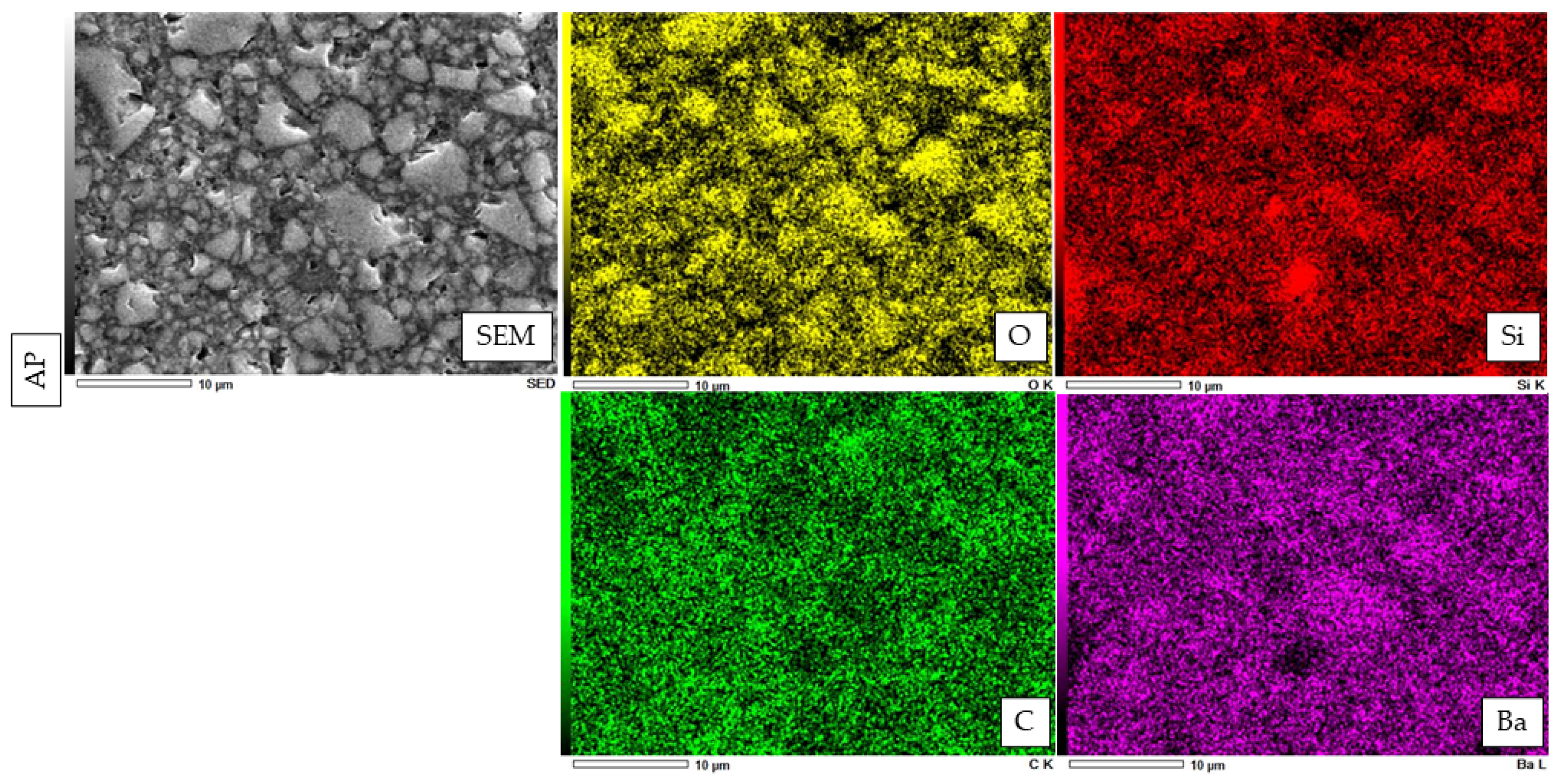
2.5. Filler Morphology Observation by Scanning Electron Microscope (SEM)
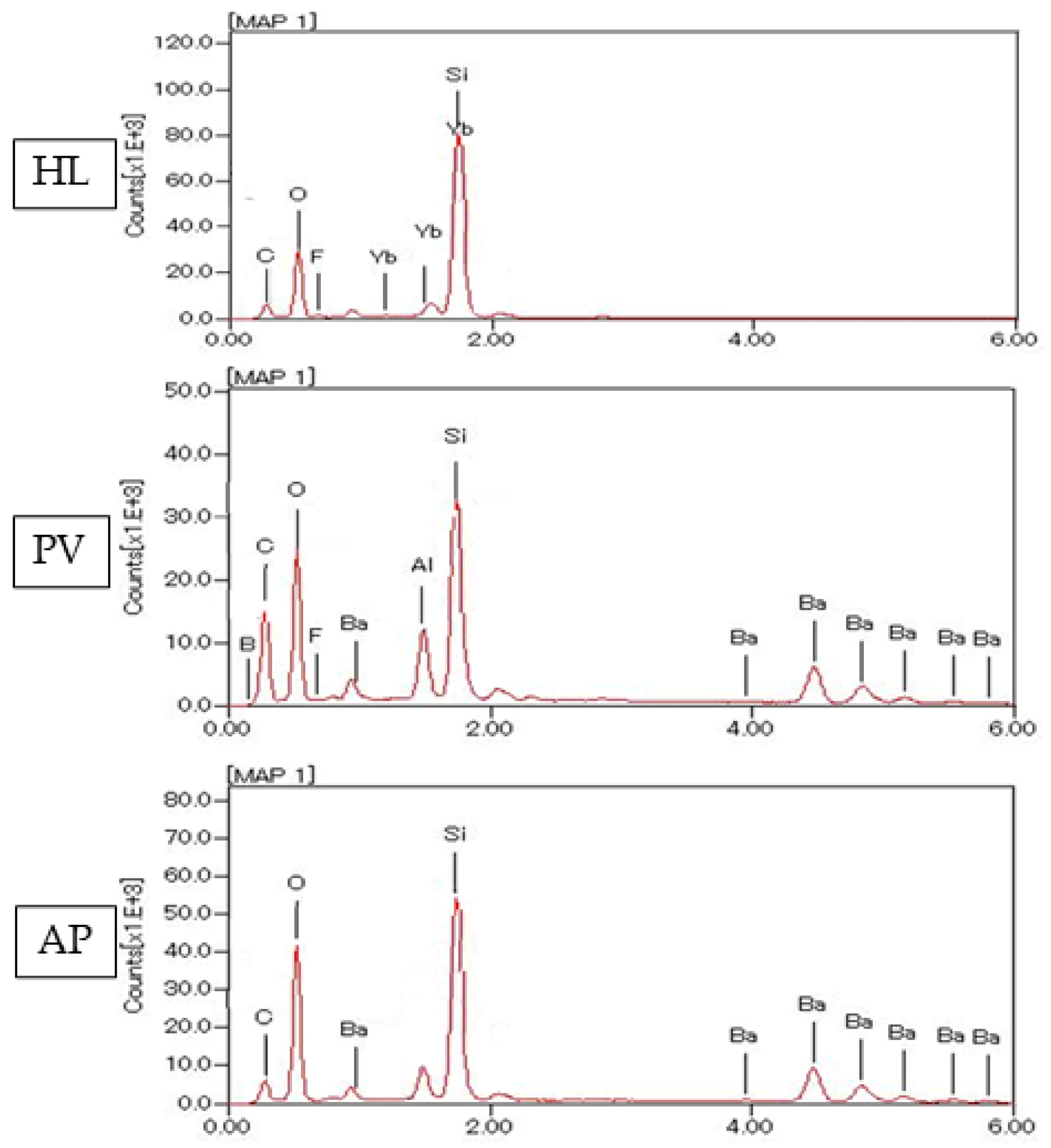
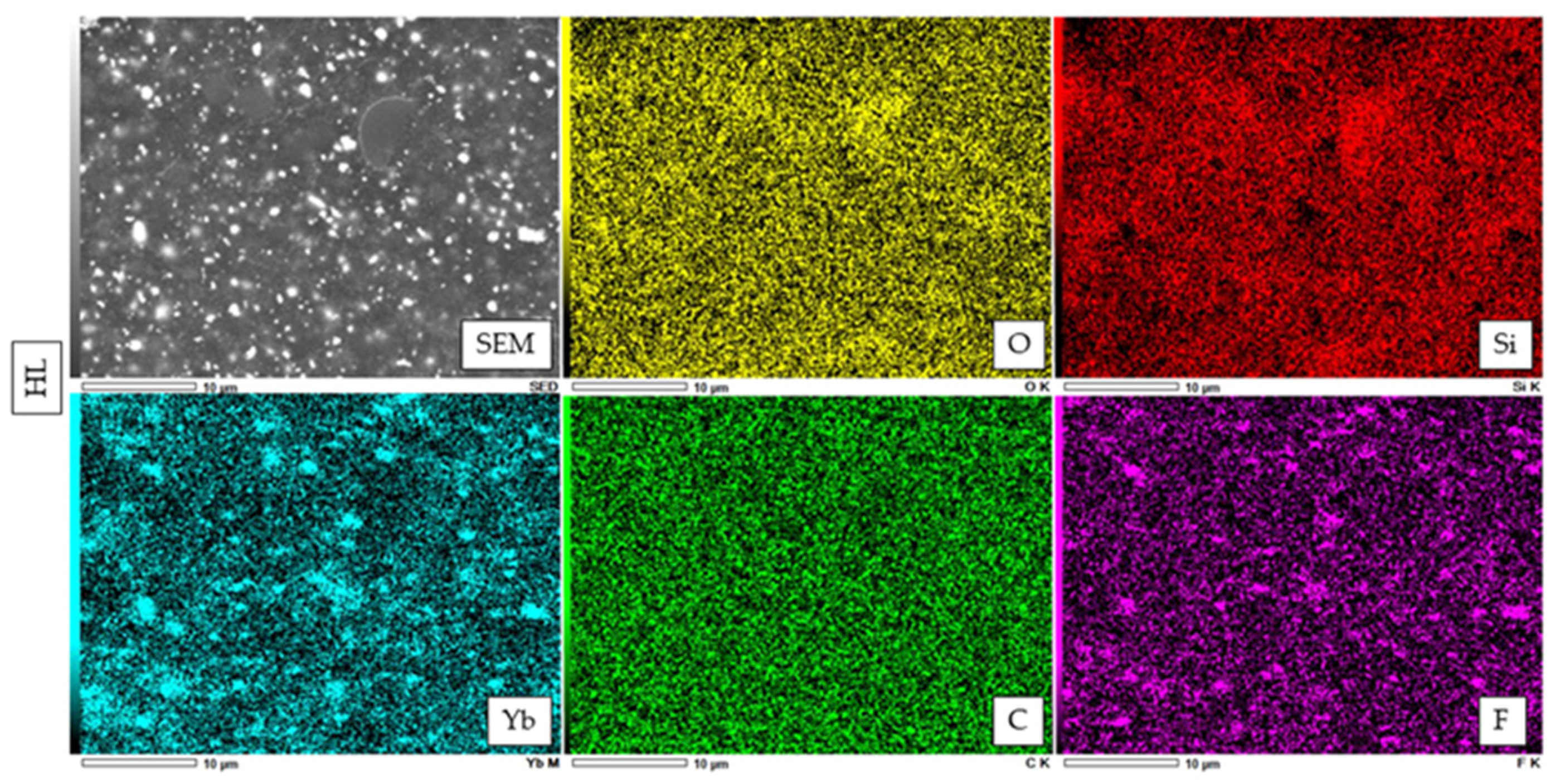
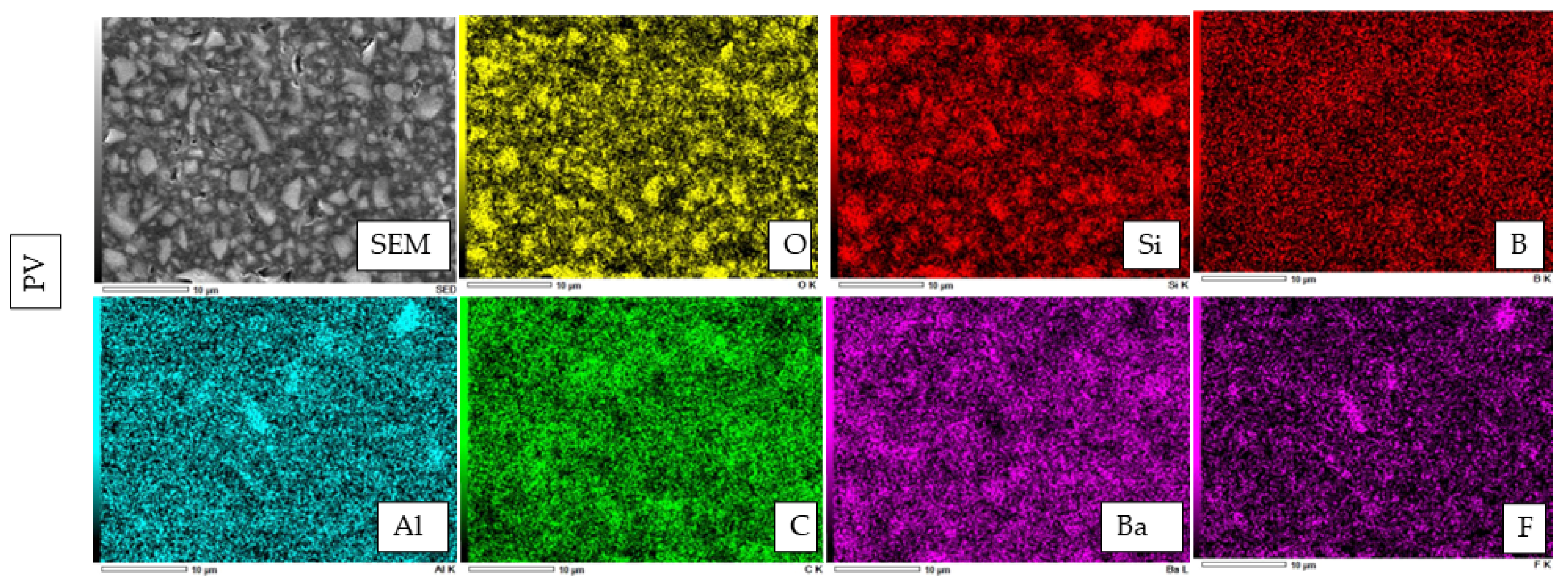
2.6. Atomic Elemental Analysis by Energy Dispersive X-ray (EDX)
2.7. Knoop Microhardness Test
2.8. Statistical Analysis
3. Results
3.1. µTBS
3.2. SEM Observation of the Failure Modes
3.3. SEM Observation of Fillers and Elemental Analysis of Cured Resin Cements and Composite
3.4. Microhardness Evaluation of Resin Materials
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Aker-Sagen, M.; Dahl, J.E.; Matinlinna, J.P.; Tibballs, J.E.; Rønold, H.J. The influence of the resin-based cement layer on ceramic dentin bond strength. Eur. J. Oral. Sci. 2021, 129, e12791. [Google Scholar] [CrossRef] [PubMed]
- Warreth, A.; Elkareimi, Y. All-ceramic restorations: A review of the literature. Saudi Dent. J. 2020, 32, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Araoka, D.; Hosaka, K.; Nakajima, M.; Foxton, R.; Thanatvarakorn, O.; Prasansuttiporn, T.; Chiba, A.; Sato, K.; Takahashi, M.; Otsuki, M.; et al. The strategies used for curing universal adhesives affect the micro-bond strength of resin cement used to lute indirect resin composites to human dentin. Dent. Mater. J. 2018, 37, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Baldissara, P.; Koci, B.; Monaco, C.; Scotti, N.; Breschi, L.; Ciocca, L. Fatigue failure and success rate of lithium disilicate table-tops as a function of cement thickness. J. Prosthodont. Res. 2021, 65, 528–534. [Google Scholar] [CrossRef]
- Johnson, A.C.; Versluis, A.; Tantbirojn, D.; Ahuja, S. Fracture strength of CAD/CAM composite and composite-ceramic occlusal veneers. J. Prosthodont. Res. 2014, 58, 107–114. [Google Scholar] [CrossRef]
- Chang, H.S.; Noh, Y.S.; Lee, Y.; Min, K.S.; Bae, J.M. Push-out bond strengths of fiber-reinforced composite posts with various resin cements according to the root level. J. Adv. Prosthodont. 2013, 5, 278–286. [Google Scholar] [CrossRef]
- Duarte, S., Jr.; Botta, A.C.; Meire, M.; Sadan, A. Microtensile bond strengths and scanning electron microscopic evaluation of self-adhesive and self-etch resin cements to intact and etched enamel. J. Prosthet. Dent. 2008, 100, 203–210. [Google Scholar] [CrossRef]
- Runnacles, P.; Correr, G.M.; Baratto Filho, F.; Gonzaga, C.C.; Furuse, A.Y. Degree of conversion of a resin cement light-cured through ceramic veneers of different thicknesses and types. Braz. Dent. J. 2014, 25, 38–42. [Google Scholar] [CrossRef]
- Hill, E.E.; Lott, J. A clinically focused discussion of luting materials. Aust. Dent. J. 2011, 56, 67–76. [Google Scholar] [CrossRef]
- Madrigal, E.L.; Tichy, A.; Hosaka, K.; Ikeda, M.; Nakajima, M.; Tagami, J. The effect of curing mode of dual-cure resin cements on bonding performance of universal adhesives to enamel, dentin and various restorative materials. Dent. Mater. J. 2021, 40, 446–454. [Google Scholar] [CrossRef]
- Aguiar, T.R.; Di-Francescantonio, M.; Arrais, C.A.; Ambrosano, G.M.; Davanzo, C.; Giannini, M. Influence of curing mode and time on degree of conversion of one conventional and two self-adhesive resin cements. Oper. Dent. 2010, 35, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.A.; Rohr, N.; Fischer, J. Evaluation of ISO 4049: Water sorption and water solubility of resin cements. Eur. J. Oral. Sci. 2017, 125, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Attar, N.; Tam, L.E.; McComb, D. Mechanical and physical properties of contemporary dental luting agents. J. Prosthet. Dent. 2003, 89, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, M.; Takahashi, H.; Asakawa, Y.; Iwasaki, N. Color stability of adhesive resin cements after immersion in coffee. Clin. Oral. Investig. 2015, 19, 309–317. [Google Scholar] [CrossRef]
- Guler, S.; Unal, M. The evaluation of color and surface roughness changes in resin based restorative materials with different contents after waiting in various liquids: An SEM and AFM study. Microsc. Res. Tech. 2018, 81, 1422–1433. [Google Scholar] [CrossRef]
- Santos, M.J.; Freitas, M.C.; Azevedo, L.M.; Santos, G.C., Jr.; Navarro, M.F.; Francischone, C.E.; Mondelli, R.F. Clinical evaluation of ceramic inlays and onlays fabricated with two systems: 12-year follow-up. Clin. Oral. Investig. 2016, 20, 1683–1690. [Google Scholar] [CrossRef]
- Gönülol, N.; Yilmaz, F. The effects of finishing and polishing techniques on surface roughness and color stability of nanocomposites. J. Dent. 2012, 40, 64–70. [Google Scholar] [CrossRef]
- Oei, J.D.; Mishriky, M.; Barghi, N.; Rawls, H.R.; Cardenas, H.L.; Aguirre, R.; Whang, K. Development of a low-color, color stable, dual cure dental resin. Dent. Mater. 2013, 29, 405–412. [Google Scholar] [CrossRef]
- Almeida, J.R.; Schmitt, G.U.; Kaizer, M.R.; Boscato, N.; Moraes, R.R. Resin-based luting agents and color stability of bonded ceramic veneers. J. Prosthet. Dent. 2015, 114, 272–277. [Google Scholar] [CrossRef]
- Itoh, S.; Nakajima, M.; Hosaka, K.; Okuma, M.; Takahashi, M.; Shinoda, Y.; Seki, N.; Ikeda, M.; Kishikawa, R.; Foxton, R.M.; et al. Dentin bond durability and water sorption/solubility of one-step self-etch adhesives. Dent. Mater. J. 2010, 29, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Kern, M. Resin bonding to oxide ceramics for dental restorations. J. Adhes. Sci. Technol. 2009, 23, 1097–1111. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, X.P.; Tan, R.X. Effect of Light-Cured Resin Cement Application on Translucency of Ceramic Veneers and Light Transmission of LED Polymerization Units. J. Prosthodont. 2019, 28, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Panavia Veneer LC. Challenging Task, Great Solution. Available online: https://www.kuraraynoritake.eu/en/panavia-veneer (accessed on 17 August 2022).
- Chowdhury, A.F.M.A.; Alam, A.; Yamauti, M.; Álvarez-Lloret, P.; Saikaew, P.; Carvalho, R.M.; Sano, H. Characterization of an Experimental Two-Step Self-Etch Adhesive’s Bonding Performance and Resin-Dentin Interfacial Properties. Polymers 2021, 13, 1009. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Hosaka, K.; Takahashi, M.; Ikeda, M.; Tian, F.; Komada, W.; Nakajima, M.; Foxton, R.; Nishitani, Y.; Pashley, D.H.; et al. Dentin Bonding Durability of Two-step Self-etch Adhesives with Improved of Degree of Conversion of Adhesive Resins. J. Adhes. Dent. 2017, 19, 31–37. [Google Scholar]
- Yoshida, Y.; Nagakane, K.; Fukuda, R.; Nakayama, Y.; Okazaki, M.; Shintani, H.; Inoue, S.; Tagawa, Y.; Suzuki, K.; De-Munck, J.; et al. Comparative study on adhesive performance of functional monomers. J. Dent. Res. 2004, 83, 454–458. [Google Scholar] [CrossRef]
- Politano, G.; Van-Meerbeek, B.; Peumans, M. Nonretentive Bonded Ceramic Partial Crowns: Concept and Simplified Protocol for Long-lasting Dental Restorations. J. Adhes. Dent. 2018, 20, 495–510. [Google Scholar]
- Aung, S.S.M.P.; Takagaki, T.; Ko, A.K.; Halabi, S.; Sato, T.; Ikeda, M.; Nikaido, T.; Burrow, M.F.; Tagami, J. Adhesion durability of dual-cure resin cements and acid-base resistant zone formation on human dentin. Dent. Mater. 2019, 35, 945–952. [Google Scholar] [CrossRef]
- Seki, N.; Nakajima, M.; Kishikawa, R.; Hosaka, K.; Foxton, R.M.; Tagami, J. The influence of light intensities irradiated directly and indirectly through resin composite to self-etch adhesives on dentin bonding. Dent. Mater. J. 2011, 30, 315–322. [Google Scholar] [CrossRef]
- Souza, E.M.; De-Munck, J.; Pongprueksa, P.; Van-Ende, A.; Van-Meerbeek, B. Correlative analysis of cement-dentin interfaces using an interfacial fracture toughness and micro-tensile bond strength approach. Dent. Mater. 2016, 32, 1575–1585. [Google Scholar] [CrossRef]
- Nawareg, M.M.; Zidan, A.Z.; Zhou, J.; Chiba, A.; Tagami, J.; Pashley, D.H. Adhesive sealing of dentin surfaces in vitro: A review. Am. J. Dent. 2015, 28, 321–332. [Google Scholar]
- Fronza, B.M.; Noronha, M.D.S.; Price, R.B.; Pecorari, V.G.A.; Giannini, M. Influence of Adhesion Promoter Primers on Polymerization Kinetics and Long-term Bond Strength of Composite Cements to Zirconia. J. Adhes. Dent. 2022, 24, 259–268. [Google Scholar] [PubMed]
- Sonmez, N.; Gultekin, P.; Turp, V.; Akgungor, G.; Sen, D.; Mijiritsky, E. Evaluation of five CAD/CAM materials by microstructural characterization and mechanical tests: A comparative in vitro study. BMC. Oral. Health 2018, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Jurišić, S.; Jurišić, G.; Zlatarić, D.K. In Vitro Evaluation and Comparison of the Translucency of Two Different All-Ceramic Systems. Acta. Stomatol. Croat. 2015, 49, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Um, C.M.; Lee, I.B. Rheological properties of resin composites according to variations in monomer and filler composition. Dent. Mater. 2006, 22, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Elfakhri, F.; Alkahtani, R.; Li, C.; Khaliq, J. Influence of filler characteristics on the performance of dental composites: A comprehensive review. Ceram. Int. 2022, 48, 27280–27294. [Google Scholar] [CrossRef]
- Makvandi, P.; Gu, J.T.; Zare, E.N.; Ashtari, B.; Moeini, A.; Tay, F.R.; Niu, L.-N. Polymeric and inorganic nanoscopical antimicrobial fillers in dentistry. Acta Biomater. 2020, 101, 69–101. [Google Scholar] [CrossRef]
- Gomes, G.M.; Rezende, E.C.; Gomes, O.M.; Gomes, J.C.; Loguercio, A.D.; Reis, A. Influence of the resin cement thickness on bond strength and gap formation of fiber posts bonded to root dentin. J. Adhes. Dent. 2014, 16, 71–78. [Google Scholar]
- Alhenaki, A.M.; Attar, E.A.; Alshahrani, A.; Farooq, I.; Vohra, F.; Abduljabbar, T. Dentin Bond Integrity of Filled and Unfilled Resin Adhesive Enhanced with Silica Nanoparticles—An SEM, EDX, Micro-Raman, FTIR and Micro-Tensile Bond Strength Study. Polymers 2021, 13, 1093. [Google Scholar] [CrossRef]
- Kim, J.S.; Cho, B.H.; Lee, I.B.; Um, C.M.; Lim, B.S.; Oh, M.H.; Chang, C.G.; Son, H.H. Effect of the hydrophilic nanofiller loading on the mechanical properties and the microtensile bond strength of an ethanol-based one-bottle dentin adhesive. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 72, 284–291. [Google Scholar] [CrossRef]
- Kasraei, S.H.; Atai, M.; Khamverdi, Z.; Nejad, S.K. Effect of Nanofiller Addition to an Experimental Dentin Adhesive on Microtensile Bond Strength to Human Dentin. Front. Dent. 2009, 6, 91–96. [Google Scholar]



| Materials | Compositions | Manufacturer | Lot No. |
|---|---|---|---|
| HL-100C | Paste: silanated spherical silica, UDMA, Ytterbium trifluoride, TEGDMA, hydrophilic aliphatic dimethacrylate, hydrophilic amide monomer, accelerators, dl-camphorquinone, pigments | Kuraray Noritake Dental, Tokyo, Japan | T200615-1 |
| Panavia V5 | Paste-A: Bis-GMA, TEGDMA, hydrophobic aromatic dimethacrylate, hydrophilic aliphatic dimethacrylate, initiators, accelerators, silanated barium glass filler, silanated fluoroalminosilicate glass filler, colloidal silica | Kuraray Noritake Dental, Tokyo, Japan | 8H0168 |
| Paste-B: Bis-GMA, hydrophobic aromatic dimethacrylate, hydrophilic aliphatic dimethacrylate, silanated barium glass filler, silanated aluminum oxide filler, accelerators, dl-camphorquinone, pigments | |||
| Clearfil AP-X | Paste: Bis-GMA, TEGDMA, silanated barium glass filler, silanated silica filler, silanated colloidal silica, dl-camphorquinone, catalysts, accelerators, pigments | Kuraray Noritake Dental, Tokyo, Japan | 850124 |
| Katana Avencia Block 2 | Mixed filler with colloidal silica and aluminum oxide, cured resins consisting of methacrylate monomer (copolymer of UDMA and other methacrylate monomers), pigments | Kuraray Noritake, Tokyo, Japan | 001122 |
| Clearfil Ceramic Primer Plus | Ceramic primer: 3-trimethoxysilylpropyl methacrylate, MDP, ethanol | Kuraray Noritake Dental, Tokyo, Japan | 2R0053 |
| Panavia V5 Tooth Primer | Tooth primer: MDP, HEMA, hydrophilic aliphatic dimethacrylate, accelerators, water | Kuraray Noritake Dental, Tokyo, Japan | AW0071 |
| Clearfil SE Bond 2 | Primer: MDP, HEMA, hydrophilic aliphatic dimethacrylate, dl-camphorquinone, hydrophobic aliphatic, water | Kuraray Noritake Dental, Tokyo, Japan | 4A0114 |
| Bond: MDP, Bis-GMA, HEMA, dl-camphorquinone, hydrophobic aliphatic dimethacrylate, initiators, accelerators, silanated colloidal silica | 4H0173 |
| Resin Cements | Mean ± SD (MPa) (24 h) (n = 15) | Fracture Mode (n) (24 h) IB/IB + C/IB + C + ID/CC/ID + C/ID | Mean ± SD (MPa) (7 Days) | Fracture Mode (n) (7 Days) IB/IB + C/IB + C + ID/CC/ID + C/ID |
|---|---|---|---|---|
| HL | 60.32 ± 11.28 a | 0/0/0/14/1/0 | 53.81 ± 9.56 B | 0/0/0/13/0/2 |
| PV | 37.24 ± 7.28 b | 0/0/0/15/0/0 | 40.23 ± 8.78 C | 0/0/0/14/0/1 |
| AP | 72.07 ± 21.10 a | 0/0/0/8/4/3 | 67.24 ± 13.82 A | 0/0/0/10/1/4 |
| Cements | KHN (24 h) | KHN (7 Days) |
|---|---|---|
| HL | 24.85 ± 3.39 a | 27.10 ± 4.90 a |
| PV | 11.01 ± 1.55 b | 10.87 ± 1.21 b |
| AP | 83.80 ± 14.43 c | 89.77 ± 14.08 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawamura, M.; Toida, Y.; Hoshika, S.; Islam, M.R.R.; Li, Y.; Yao, Y.; Liu, Y.; Islam, R.; Sato, T.; Shimada, Y.; et al. Influence of Novel Experimental Light-Cured Resin Cement on Microtensile Bond Strength. Polymers 2022, 14, 4075. https://doi.org/10.3390/polym14194075
Kawamura M, Toida Y, Hoshika S, Islam MRR, Li Y, Yao Y, Liu Y, Islam R, Sato T, Shimada Y, et al. Influence of Novel Experimental Light-Cured Resin Cement on Microtensile Bond Strength. Polymers. 2022; 14(19):4075. https://doi.org/10.3390/polym14194075
Chicago/Turabian StyleKawamura, Midori, Yu Toida, Shuhei Hoshika, Md Refat Readul Islam, Yitong Li, Ye Yao, Yunqing Liu, Rafiqul Islam, Takaaki Sato, Yasushi Shimada, and et al. 2022. "Influence of Novel Experimental Light-Cured Resin Cement on Microtensile Bond Strength" Polymers 14, no. 19: 4075. https://doi.org/10.3390/polym14194075
APA StyleKawamura, M., Toida, Y., Hoshika, S., Islam, M. R. R., Li, Y., Yao, Y., Liu, Y., Islam, R., Sato, T., Shimada, Y., & Sano, H. (2022). Influence of Novel Experimental Light-Cured Resin Cement on Microtensile Bond Strength. Polymers, 14(19), 4075. https://doi.org/10.3390/polym14194075








