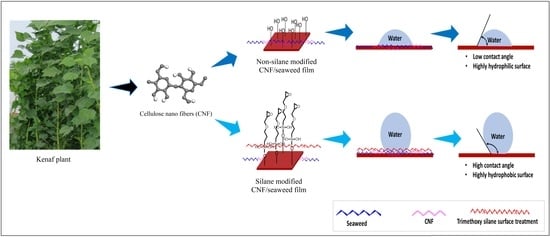
Hydrophobicity and Biodegradability of Silane-Treated Nanocellulose in Biopolymer for High-Grade Packaging Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation and Characterization of CNF via Supercritical CO2
2.3. Preparation of Macroalgae/CNF Biopolymer Film
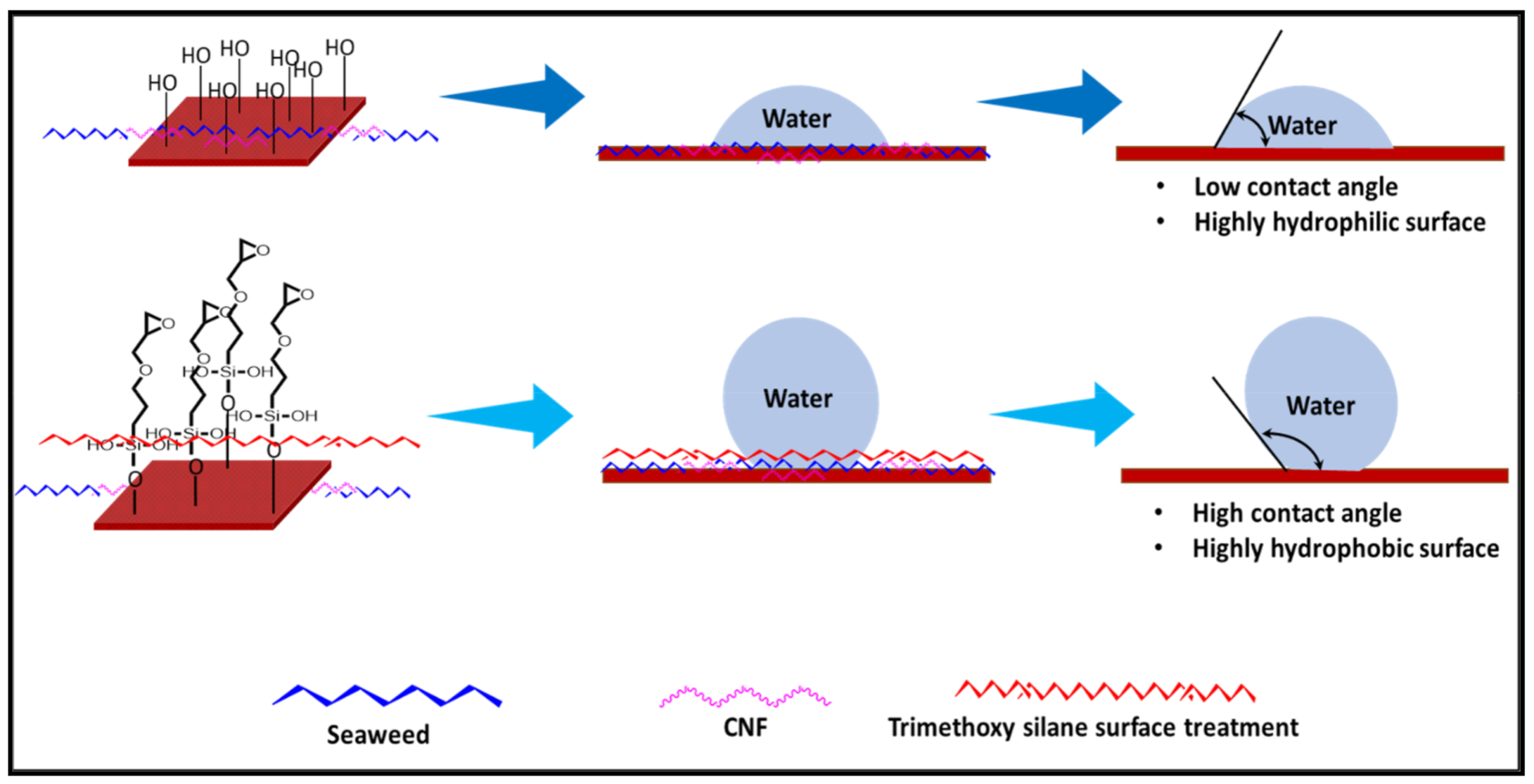
2.4. Surface Treatment of Macroalgae Film Using Silane
2.5. Characterization of Seaweed/CNF Biopolymer Film
2.5.1. Particle Size and Zeta Potential
2.5.2. FT-IR Characterization
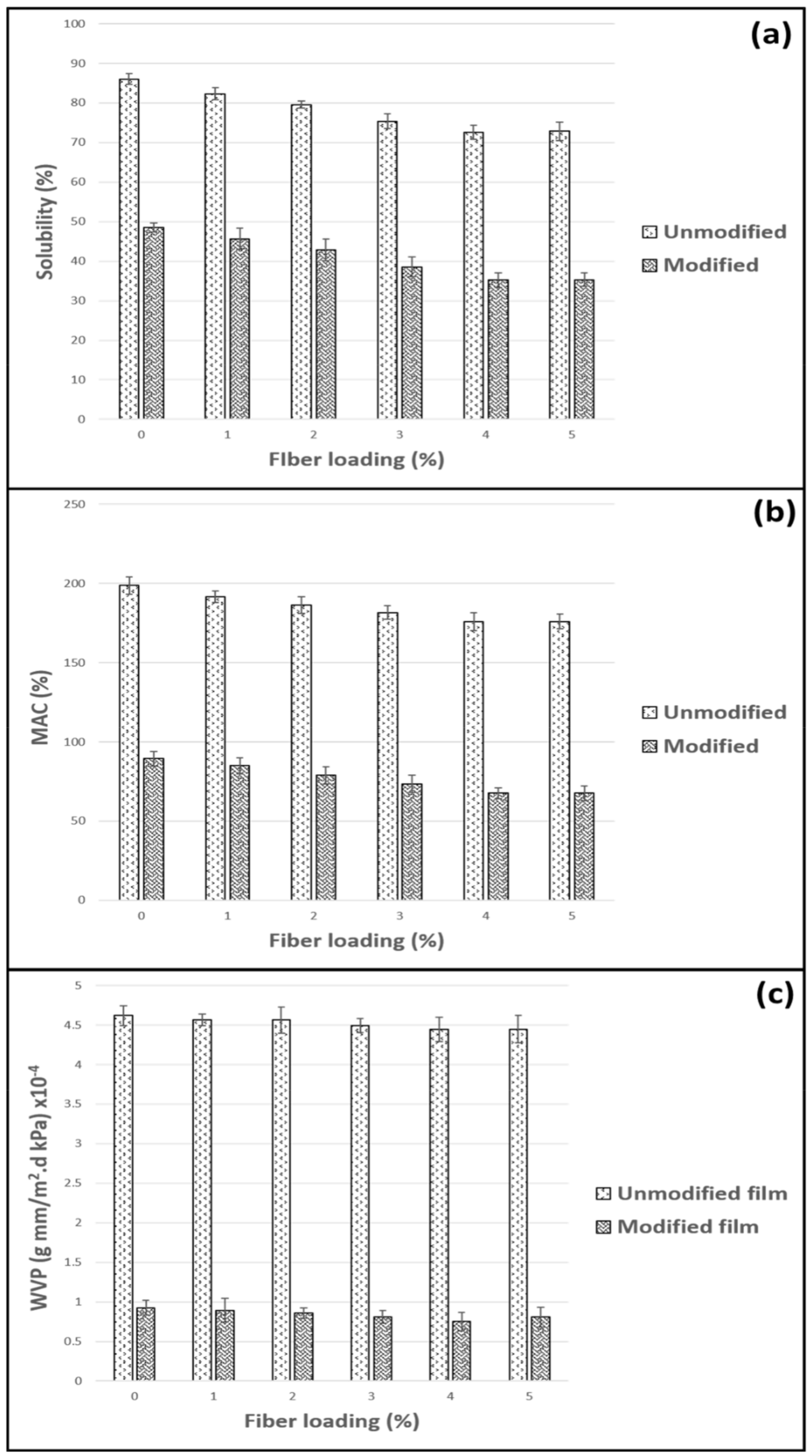
2.5.3. Water Solubility (WS)
2.5.4. Moisture Absorption Capacity (MAC)
2.5.5. Water Vapor Permeability (WVP)
2.5.6. Contact Angle (CA)
2.5.7. Soil Burial Testing
3. Results and Discussion
3.1. Zeta Potential and Particle Size of Kenaf CNF
3.2. FT-IR Characterization of Modified Seaweed Film
3.3. Physical Properties
3.4. Contact Angle (CA)
3.5. Soil Burial Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hermawan, D.; Lai, T.K.; Jafarzadeh, S.; Gopakumar, D.A.; Hasan, M.; Owolabi, F.T.; Aprilia, N.S.; Rizal, S.; Khalil, H.A. Development of seaweed-based bamboo microcrystalline cellulose films intended for sustainable food packaging applications. BioResources 2019, 14, 3389–3410. [Google Scholar] [CrossRef]
- Sheldon, R.A. The road to biorenewables: Carbohydrates to commodity chemicals. ACS Sustain. Chem. Eng. 2018, 6, 4464–4480. [Google Scholar] [CrossRef] [Green Version]
- Abdul Khalil, H.P.S.; Yahya, E.B.; Jummaat, F.; Adnan, A.; Olaiya, N.; Rizal, S.; Abdullah, C.; Pasquini, D.; Thomas, S. Biopolymers based Aerogels: A review on revolutionary solutions for smart therapeutics delivery. Prog. Mater. Sci. 2022, 131, 101014. [Google Scholar] [CrossRef]
- Iskandar, M.; Yahya, E.B.; Abdul Khalil, H.P.S.; Rahman, A.; Ismail, M. Recent progress in modification strategies of nanocellulose-based aerogels for oil absorption application. Polymers 2022, 14, 849. [Google Scholar] [CrossRef]
- Khalil, H.A.; Mohamad, H.C.C.; Khairunnisa, A.; Owolabi, F.; Asniza, M.; Rizal, S.; Fazita, M.N.; Paridah, M. Development and characterization of bamboo fiber reinforced biopolymer films. Mater. Res. Express 2018, 5, 085309. [Google Scholar] [CrossRef]
- Abdul Khalil, H.; Lai, T.K.; Tye, Y.Y.; Paridah, M.; Fazita, M.; Azniwati, A.; Dungani, R.; Rizal, S. Preparation and characterization of microcrystalline cellulose from sacred bali bamboo as reinforcing filler in seaweed-based composite film. Fibers Polym. 2018, 19, 423–434. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A review on synthesis, properties and applications of natural polymer based carrageenan blends and composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef]
- Khalil, H.; Tye, Y.; Saurabh, C.; Leh, C.; Lai, T.; Chong, E.; Fazita, M.; Hafiidz, J.M.; Banerjee, A.; Syakir, M. Biodegradable polymer films from seaweed polysaccharides: A review on cellulose as a reinforcement material. Express Polym. Lett. 2017, 11, 244–265. [Google Scholar] [CrossRef]
- Ogur, S. The physicochemical properties of edible protein films. Ital. J. Food Sci. 2015, 27, 64–74. [Google Scholar]
- Kumar, L.; Ramakanth, D.; Akhila, K.; Gaikwad, K.K. Edible films and coatings for food packaging applications: A review. Environ. Chem. Lett. 2021, 20, 875–900. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Gupta, R.K. Graft copolymers from natural polymers using free radical polymerization. Int. J. Polym. Anal. Charact. 2013, 18, 495–503. [Google Scholar] [CrossRef]
- Thakur, M.K.; Gupta, R.K.; Thakur, V.K. Surface modification of cellulose using silane coupling agent. Carbohydr. Polym. 2014, 111, 849–855. [Google Scholar] [CrossRef] [Green Version]
- Yahya, E.B.; Amirul, A.; Abdul Khalil, H.P.S.; Olaiya, N.G.; Iqbal, M.O.; Jummaat, F.; AK, A.S.; Adnan, A. Insights into the role of biopolymer aerogel scaffolds in tissue engineering and regenerative medicine. Polymers 2021, 13, 1612. [Google Scholar] [CrossRef]
- Arkles, B. Hydrophobicity, hydrophilicity and silane surface modification. Gelest Inc. Morrisville 2011, 215, 547–1015. [Google Scholar]
- Kargarzadeh, H.; Mariano, M.; Huang, J.; Lin, N.; Ahmad, I.; Dufresne, A.; Thomas, S. Recent developments on nanocellulose reinforced polymer nanocomposites: A review. Polymer 2017, 132, 368–393. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites–A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Hu, D.; Wang, H.; Wang, L. Physical properties and antibacterial activity of quaternized chitosan/carboxymethyl cellulose blend films. LWT Food Sci. Technol. 2016, 65, 398–405. [Google Scholar] [CrossRef]
- Haerunnisa, A.; Ramadhan, D.; Putra, H.; Afiifah, N.; Devita, R.; Rahayu, S.; Nandiyanto, A. Synthesis of crystalline nanocellulose by various methods. Arab J. Chem. Environ. Res. 2020, 7, 94–125. [Google Scholar]
- Abraham, E.; Deepa, B.; Pothen, L.; Cintil, J.; Thomas, S.; John, M.J.; Anandjiwala, R.; Narine, S. Environmental friendly method for the extraction of coir fibre and isolation of nanofibre. Carbohydr. Polym. 2013, 92, 1477–1483. [Google Scholar] [CrossRef]
- Ioelovich, M. Characterization of various kinds of nanocellulose. Handb. Nanocellulose Cellul. Nanocomposites 2017, 1, 51–100. [Google Scholar]
- Zhang, H.; Wu, S. Enhanced enzymatic cellulose hydrolysis by subcritical carbon dioxide pretreatment of sugarcane bagasse. Bioresour. Technol. 2014, 158, 161–165. [Google Scholar] [CrossRef]
- Gopakumar, D.A.; Pasquini, D.; Henrique, M.A.; de Morais, L.C.; Grohens, Y.; Thomas, S. Meldrum’s acid modified cellulose nanofiber-based polyvinylidene fluoride microfiltration membrane for dye water treatment and nanoparticle removal. ACS Sustain. Chem. Eng. 2017, 5, 2026–2033. [Google Scholar] [CrossRef]
- Alsharef, J.M.; Taha, M.R.; Khan, T.A. Physical dispersion of nanocarbons in composites–a review. J. Teknol. 2017, 79, 69–81. [Google Scholar]
- Gopakumar, D.A.; Arumughan, V.; Pottathara, Y.B.; KS, S.; Pasquini, D.; Bračič, M.; Seantier, B.; Nzihou, A.; Thomas, S.; Rizal, S. Robust superhydrophobic cellulose nanofiber aerogel for multifunctional environmental applications. Polymers 2019, 11, 495. [Google Scholar]
- Zhou, S.; Liu, P.; Wang, M.; Zhao, H.; Yang, J.; Xu, F. Sustainable, reusable, and superhydrophobic aerogels from microfibrillated cellulose for highly effective oil/water separation. ACS Sustain. Chem. Eng. 2016, 4, 6409–6416. [Google Scholar] [CrossRef]
- Pan, C.; Kou, K.; Jia, Q.; Zhang, Y.; Wu, G.; Ji, T. Improved thermal conductivity and dielectric properties of hBN/PTFE composites via surface treatment by silane coupling agent. Compos. Part B Eng. 2017, 111, 83–90. [Google Scholar] [CrossRef]
- Khan, R.A.; Salmieri, S.; Dussault, D.; Uribe-Calderon, J.; Kamal, M.R.; Safrany, A.; Lacroix, M. Production and properties of nanocellulose-reinforced methylcellulose-based biodegradable films. J. Agric. Food Chem. 2010, 58, 7878–7885. [Google Scholar] [CrossRef]
- Khalil, H.A.; Yap, S.W.; Tye, Y.Y.; Tahir, P.M.; Rizal, S.; Fazita, M.N. Effects of corn starch and Kappaphycus alvarezii seaweed blend concentration on the optical, mechanical, and water vapor barrier properties of composite films. BioResources 2018, 13, 1157–1173. [Google Scholar]
- Kanmani, P.; Rhim, J.-W. Development and characterization of carrageenan/grapefruit seed extract composite films for active packaging. Int. J. Biol. Macromol. 2014, 68, 258–266. [Google Scholar] [CrossRef]
- Nanaki, S.; Karavas, E.; Kalantzi, L.; Bikiaris, D. Miscibility study of carrageenan blends and evaluation of their effectiveness as sustained release carriers. Carbohydr. Polym. 2010, 79, 1157–1167. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Khezerlou, A.; Ehsani, A. Fabrication and characterization of the bionanocomposite film based on whey protein biopolymer loaded with TiO2 nanoparticles, cellulose nanofibers and rosemary essential oil. Ind. Crops Prod. 2018, 124, 300–315. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Cao, J.; Jiang, W. Improving the performance of edible food packaging films by using nanocellulose as an additive. Int. J. Biol. Macromol. 2021, 166, 288–296. [Google Scholar] [CrossRef]
- Wan, Y.; Luo, H.; He, F.; Liang, H.; Huang, Y.; Li, X. Mechanical, moisture absorption, and biodegradation behaviours of bacterial cellulose fibre-reinforced starch biocomposites. Compos. Sci. Technol. 2009, 69, 1212–1217. [Google Scholar] [CrossRef]
- Abdollahi, M.; Alboofetileh, M.; Behrooz, R.; Rezaei, M.; Miraki, R. Reducing water sensitivity of alginate bio-nanocomposite film using cellulose nanoparticles. Int. J. Biol. Macromol. 2013, 54, 166–173. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Ammayappan, L.; Huang, Q. Effect of Gum arabic on distribution behavior of nanocellulose fillers in starch film. Appl. Nanosci. 2011, 1, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Gomes, D.J.; de Souza, N.C.; Silva, J.R. Using a monocular optical microscope to assemble a wetting contact angle analyser. Measurement 2013, 46, 3623–3627. [Google Scholar] [CrossRef]
- Artus, G.R.; Jung, S.; Zimmermann, J.; Gautschi, H.P.; Marquardt, K.; Seeger, S. Silicone nanofilaments and their application as superhydrophobic coatings. Adv. Mater. 2006, 18, 2758–2762. [Google Scholar] [CrossRef]
- Rizal, S.; Yahya, E.B.; Abdul Khalil, H.P.S.; Abdullah, C.; Marwan, M.; Ikramullah, I.; Muksin, U. Preparation and characterization of nanocellulose/chitosan aerogel scaffolds using chemical-free approach. Gels 2021, 7, 246. [Google Scholar] [CrossRef]
- de Leon, A.C.C.; Pernites, R.B.; Advincula, R.C. Superhydrophobic colloidally textured polythiophene film as superior anticorrosion coating. ACS Appl. Mater. Interfaces 2012, 4, 3169–3176. [Google Scholar] [CrossRef]
- Tazi, M.; Erchiqui, F.; Godard, F.; Kaddami, H. Evaluation of mechanical properties and durability performance of HDPE-wood composites. J. Renew. Mater. 2014, 2, 258–263. [Google Scholar] [CrossRef] [Green Version]
- Deepa, B.; Abraham, E.; Pothan, L.A.; Cordeiro, N.; Faria, M.; Thomas, S. Biodegradable nanocomposite films based on sodium alginate and cellulose nanofibrils. Materials 2016, 9, 50. [Google Scholar] [CrossRef]
- Siddiquee, K.M.; Helali, M.M.; Gafur, M.A.; Chakraborty, S. Investigation of an optimum method of biodegradation process for jute polymer composites. Am. J. Eng. Res. 2014, 3, 200–206. [Google Scholar]



| Filler Loading (%) | Contact Angle (θ) | |
|---|---|---|
| Unmodified Film | Modified Film | |
| 0 |  | 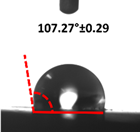 |
| 1 |  |  |
| 2 | 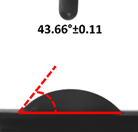 |  |
| 3 |  |  |
| 4 | 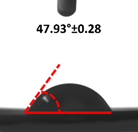 | 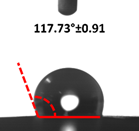 |
| 5 | 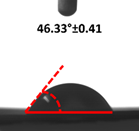 | 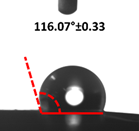 |
| Weeks (a) | Unmodified Seaweed/CNF Composite Film | |||||
|---|---|---|---|---|---|---|
| 0% | 1% | 2% | 3% | 4% | 5% | |
| 0 |  |  |  |  |  |  |
| 1 |  |  |  |  |  |  |
| 2 |  |  |  |  |  |  |
| 3 |  |  |  |  |  |  |
| 4 |  |  |  |  |  |  |
| Weeks (b) | Modified Seaweed/CNF Composite Film | |||||
| 0% | 1% | 2% | 3% | 4% | 5% | |
| 0 |  |  |  |  |  |  |
| 1 |  |  |  |  |  |  |
| 2 |  |  |  |  |  |  |
| 3 |  |  |  |  |  |  |
| 4 |  |  |  |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surya, I.; Hazwan, C.M.; Abdul Khalil, H.P.S.; Yahya, E.B.; Suriani, A.B.; Danish, M.; Mohamed, A. Hydrophobicity and Biodegradability of Silane-Treated Nanocellulose in Biopolymer for High-Grade Packaging Applications. Polymers 2022, 14, 4147. https://doi.org/10.3390/polym14194147
Surya I, Hazwan CM, Abdul Khalil HPS, Yahya EB, Suriani AB, Danish M, Mohamed A. Hydrophobicity and Biodegradability of Silane-Treated Nanocellulose in Biopolymer for High-Grade Packaging Applications. Polymers. 2022; 14(19):4147. https://doi.org/10.3390/polym14194147
Chicago/Turabian StyleSurya, Indra, C. M. Hazwan, H. P. S. Abdul Khalil, Esam Bashir Yahya, A. B. Suriani, Mohammed Danish, and Azmi Mohamed. 2022. "Hydrophobicity and Biodegradability of Silane-Treated Nanocellulose in Biopolymer for High-Grade Packaging Applications" Polymers 14, no. 19: 4147. https://doi.org/10.3390/polym14194147