Lignin as a High-Value Bioaditive in 3D-DLP Printable Acrylic Resins and Polyaniline Conductive Composite
Abstract
:1. Introduction
2. Experimental Section/Methods
2.1. Materials and Samples Preparation
2.2. Sample Characterization
3. Results
3.1. Prepolymerization Studies
3.1.1. Viscosity
3.1.2. UV-Visible and Real Time-FTIR Spectroscopy
3.1.3. Jacobs Working Curves
3.2. Composites Characterization
3.2.1. Structural Characterization: Morphology and ATR
3.2.2. Thermal and Mechanical Properties of Printed Composites
3.2.3. Contact Angles (CA)
3.3. Use of Lignin as Dispersant of Conductive Fillers
3.3.1. pTSA-PANI Characterization
3.3.2. Characterization of Printed pTSA-PANI Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fertier, L.; Koleilat, H.; Stemmelen, M.; Giani, O.; Joly-Duhamel, C.; Lapinte, V.; Robin, J.-J. The use of renewable feedstock in UV-curable materials—A new age for polymers and green chemistry. Prog. Polym. Sci. 2013, 38, 932–962. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Yao, Q.; Ji, C.; Liu, J.; Zhu, Q. 3D printing with cellulose materials. Cellulose 2018, 25, 4275–4301. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, P. 3D printing of photopolymers. Polym. Chem. 2018, 9, 1530–1540. [Google Scholar] [CrossRef]
- Mendes-Felipe, C.; Oliveira, J.; Etxebarria, I.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. State-of-the-Art and Future Challenges of UV Curable Polymer-Based Smart Materials for Printing Technologies. Adv. Mater. Technol. 2019, 4, 1–16. [Google Scholar] [CrossRef] [Green Version]
- El Magri, A.; Bencaid, S.E.; Vanaei, H.R.; Vaudreuil, S. Effects of Laser Power and Hatch Orientation on Final Properties of PA12 Parts Produced by Selective Laser Sintering. Polymers 2022, 14, 3674. [Google Scholar] [CrossRef]
- Bártolo, P.J. (Ed.) Stereolithography Materials, Processes and Applications; Springer: New York, NY, USA, 2011; ISBN 9780387929033. [Google Scholar]
- Ebers, L.S.; Arya, A.; Bowland, C.C.; Glasser, W.G.; Chmely, S.C.; Naskar, A.K.; Laborie, M.P. 3D printing of lignin: Challenges, opportunities and roads onward. Biopolymers 2021, 112, e23431. [Google Scholar] [CrossRef]
- Wang, F.; Ouyang, D.; Zhou, Z.; Page, S.J.; Liu, D.; Zhao, X. Lignocellulosic biomass as sustainable feedstock and materials for power generation and energy storage. J. Energy Chem. 2021, 57, 247–280. [Google Scholar] [CrossRef]
- Culebras, M.; Sanchis, M.J.; Beaucamp, A.; Carsí, M.; Kandola, B.K.; Horrocks, A.R.; Panzetti, G.; Birkinshaw, C.; Collins, M.N. Understanding the thermal and dielectric response of organosolv and modified kraft lignin as a carbon fibre precursor. Green Chem. 2018, 20, 4461–4472. [Google Scholar] [CrossRef]
- Collins, M.N.; Nechifor, M.; Tanasă, F.; Zănoagă, M.; McLoughlin, A.; Stróżyk, M.A.; Culebras, M.; Teacă, C.A. Valorization of lignin in polymer and composite systems for advanced engineering applications—A review. Int. J. Biol. Macromol. 2019, 131, 828–849. [Google Scholar] [CrossRef]
- Culebras, M.; Geaney, H.; Beaucamp, A.; Upadhyaya, P.; Dalton, E.; Ryan, K.M.; Collins, M.N. Bio-derived Carbon Nanofibres from Lignin as High-Performance Li-Ion Anode Materials. ChemSusChem 2019, 12, 4516–4521. [Google Scholar] [CrossRef]
- Lai, C.; Zhou, Z.; Zhang, L.; Wang, X.; Zhou, Q.; Zhao, Y.; Wang, Y.; Wu, X.F.; Zhu, Z.; Fong, H. Free-standing and mechanically flexible mats consisting of electrospun carbon nanofibers made from a natural product of alkali lignin as binder-free electrodes for high-performance supercapacitors. J. Power Sources 2014, 247, 134–141. [Google Scholar] [CrossRef]
- García-Negrón, V.; Chmely, S.C.; Ilavsky, J.; Keffer, D.J.; Harper, D.P. Development of Nanocrystalline Graphite from Lignin Sources. ACS Sustain. Chem. Eng. 2022, 10, 1786–1794. [Google Scholar] [CrossRef]
- Grossman, A.; Wilfred, V. Lignin-based polymers and nanomaterials. Curr. Opin. Biotechnol. 2019, 56, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in green polymer composites from lignin for multifunctional applications: A review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Wen, M.; Zhao, Y.; Li, Z.; Lai, S.; Zeng, Q.; Liu, C.; Liu, Y. Preparation of lignin-based carbon/polyaniline composites for advanced microwave absorber. Diam. Relat. Mater. 2021, 111, 108219. [Google Scholar] [CrossRef]
- Bozó, É.; Ervasti, H.; Halonen, N.; Shokouh, S.H.H.; Tolvanen, J.; Pitkänen, O.; Järvinen, T.; Pálvölgyi, P.S.; Szamosvölgyi, Á.; Sápi, A.; et al. Bioplastics and Carbon-Based Sustainable Materials, Components, and Devices: Toward Green Electronics. ACS Appl. Mater. Interfaces 2021, 13, 49301–49312. [Google Scholar] [CrossRef]
- Dianat, N.; Rahmanifar, M.S.; Noori, A.; El-Kady, M.F.; Chang, X.; Kaner, R.B.; Mousavi, M.F. Polyaniline-Lignin Interpenetrating Network for Supercapacitive Energy Storage. Nano Lett. 2021, 21, 9485–9493. [Google Scholar] [CrossRef]
- Tanguy, N.R.; Wu, H.; Nair, S.S.; Lian, K.; Yan, N. Lignin Cellulose Nanofibrils as an Electrochemically Functional Component for High-Performance and Flexible Supercapacitor Electrodes. ChemSusChem 2021, 14, 1057–1067. [Google Scholar] [CrossRef]
- Ye, W.; Li, X.; Luo, J.; Wang, X.; Sun, R. Lignin as a green reductant and morphology directing agent in the fabrication of 3D graphene-based composites for high-performance supercapacitors. Ind. Crops Prod. 2017, 109, 410–419. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Xu, H.; Wang, G. Construction of polyaniline/lignin composite with interpenetrating fibrous networks and its improved electrochemical capacitance performances. Synth. Met. 2019, 249, 40–46. [Google Scholar] [CrossRef]
- Razaq, A.; Asif, M.H.; Kalsoom, R.; Khan, A.F.; Awan, M.S.; Ishrat, S.; Ramay, S.M. Conductive and electroactive composite paper reinforced by coating of polyaniline on lignocelluloses fibers. J. Appl. Polym. Sci. 2015, 132, 3–7. [Google Scholar] [CrossRef]
- Arias-ferreiro, G.; Ares-pernas, A.; Lasagabáster-latorre, A.; Dopico-garcía, M.S.; Ligero, P.; Pereira, N.; Costa, P.; Lanceros-Mendez, S.; Abad, M. Photocurable Printed Piezocapacitive Pressure Sensor Based on an Acrylic Resin Modified with Polyaniline and Lignin. Adv. Mater. Technol 2022, 7, 2101503. [Google Scholar] [CrossRef]
- Yang, D.; Huang, W.; Qiu, X.; Lou, H.; Qian, Y. Modifying sulfomethylated alkali lignin by horseradish peroxidase to improve the dispersibility and conductivity of polyaniline. Appl. Surf. Sci. 2017, 426, 287–293. [Google Scholar] [CrossRef]
- Sutton, J.T.; Rajan, K.; Harper, D.P.; Chmely, S.C. Improving uv curing in organosolv lignin-containing photopolymers for stereolithography by reduction and acylation. Polymers 2021, 13, 3473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, M.; Hao, N.; Ragauskas, A.J. Stereolithography 3D Printing of Lignin-Reinforced Composites with Enhanced Mechanical Properties. ACS Omega 2019, 4, 20197–20204. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, F.; Mohan, D.; Sajab, M.S.; Bakarudin, S.B.; Kaco, H. Evaluation of the Compatibility of Organosolv Lignin-Graphene Nanoplatelets with Photo-Curable Polyurethane in Stereolithography 3D Printing. Polymers 2019, 11, 1544. [Google Scholar] [CrossRef] [Green Version]
- Sutton, J.T.; Rajan, K.; Harper, D.P.; Chmely, S.C. Lignin-Containing Photoactive Resins for 3D Printing by Stereolithography. ACS Appl. Mater. Interfaces 2018, 10, 36456–36463. [Google Scholar] [CrossRef]
- Dopico-garcía, M.S.; Ares, A.; Lasagabáster-latorre, A.; García, X. Extruded polyaniline/EVA blends: Enhancing electrical conductivity using gallate compatibilizers. Synth. Met. 2014, 189, 193–202. [Google Scholar] [CrossRef]
- Arias-Ferreiro, G.; Ares-Pernas, A.; Dopico-García, M.S.; Lasagabáster-Latorre, A.; Abad, M.-J. Photocured conductive PANI/acrylate composites for digital light processing. Influence of HDODA crosslinker in rheological and physicochemical properties. Eur. Polym. J. 2020, 136, 109887. [Google Scholar] [CrossRef]
- Arias-ferreiro, G.; Ares-pernas, A.; Lasagabáster-latorre, A.; Aranburu, N.; Guerrica-echevarria, G.; Dopico-garcía, M.S.; Abad, M.J. Printability study of a conductive polyaniline/acrylic formulation for 3d printing. Polymers 2021, 13, 2068. [Google Scholar] [CrossRef]
- Arias-Ferreiro, G.; Lasagabáster-Latorre, A.; Ares-Pernas, A.; Dopico-García, M.S.; Pereira, N.; Costa, P.; Lanceros-Mendez, S.; Abad, M.-J. Flexible 3D Printed Acrylic Composites based on Polyaniline/Multiwalled Carbon Nanotubes for Piezoresistive Pressure Sensors. Adv. Electron. Mater. 2022, 2200590. [Google Scholar] [CrossRef]
- UNE-EN ISO 868; Asociación Española de Normalización y Certificación. Determinación de la Dureza de Indentación por Medio de un Durómetro (Dureza Shore). AENOR: Madrid, Spain, 2003.
- Voet, V.S.D.D.; Strating, T.; Schnelting, G.H.M.M.; Dijkstra, P.; Tietema, M.; Xu, J.; Woortman, A.J.J.J.; Loos, K.; Jager, J.; Folkersma, R. Biobased Acrylate Photocurable Resin Formulation for Stereolithography 3D Printing. ACS Omega 2018, 3, 1403–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortés, A.; Cosola, A.; Sangermano, M.; Campo, M.; González Prolongo, S.; Pirri, C.F.; Jiménez-Suárez, A.; Chiappone, A. DLP 4D-Printing of Remotely, Modularly, and Selectively Controllable Shape Memory Polymer Nanocomposites Embedding Carbon Nanotubes. Adv. Funct. Mater. 2021, 31, 2106774. [Google Scholar] [CrossRef]
- Bennett, J. Measuring UV curing parameters of commercial photopolymers used in additive manufacturing. Addit. Manuf. 2017, 18, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Pezzana, L.; Malmström, E.; Johansson, M.; Sangermano, M. UV-curable bio-based polymers derived from industrial pulp and paper processes. Polymers 2021, 13, 1530. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhong, H.; Zhang, J.; Duan, Y.; Li, J.; Jiang, D. Fabrication of zirconia all-ceramic crown via DLP-based stereolithography. Int. J. Appl. Ceram. Technol. 2020, 17, 844–853. [Google Scholar] [CrossRef]
- Zhang, X.; Keck, S.; Qi, Y.; Baudis, S.; Zhao, Y. Study on Modified Dealkaline Lignin as Visible Light Macromolecular Photoinitiator for 3D Printing. ACS Sustain. Chem. Eng. 2020, 8, 10959–10970. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Johansson, M.; Sundell, P.E.; Claudino, M.; Pan, J.; Claesson, P.M. UV-curable acrylate-based nanocomposites: Effect of polyaniline additives on the curing performance. Polym. Adv. Technol. 2013, 24, 668–678. [Google Scholar] [CrossRef]
- Tomeckova, V.; Teyssandier, F.; Norton, S.J.; Love, B.J.; Halloran, J.W. Photopolymerization of acrylate suspensions. J. Photochem. Photobiol. A Chem. 2012, 247, 74–81. [Google Scholar] [CrossRef]
- Ganewatta, M.S.; Lokupitiya, H.N.; Tang, C. Lignin biopolymers in the age of controlled polymerization. Polymers 2019, 11, 1176. [Google Scholar] [CrossRef]
- Piccinino, D.; Capecchi, E.; Tomaino, E.; Gabellone, S.; Gigli, V.; Avitabile, D.; Saladino, R. Nano-structured lignin as green antioxidant and uv shielding ingredient for sunscreen applications. Antioxidants 2021, 10, 274. [Google Scholar] [CrossRef] [PubMed]
- Goliszek, M.; Podkościelna, B.; Klepka, T.; Sevastyanova, O. Preparation, Thermal, and Mechanical Characterization of UV-Cured Polymer Biocomposites with Lignin. Polymers 2020, 12, 1159. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, P.F. Rapid Prototyping & Manufacturing. Fundamentals of StereoLithography, 1st ed.; Society of Manufacturing Engineers: Dearborn, MI, USA, 1992; ISBN 0-87263-425-6. [Google Scholar]
- Gojzewski, H.; Guo, Z.; Grzelachowska, W.; Ridwan, M.G.; Hempenius, M.A.; Grijpma, D.W.; Vancso, G.J. Layer-by-Layer Printing of Photopolymers in 3D: How Weak is the Interface? ACS Appl. Mater. Interfaces 2020, 12, 8908–8914. [Google Scholar] [CrossRef] [Green Version]
- Rojo, E.; Peresin, M.S.; Sampson, W.W.; Hoeger, I.C.; Vartiainen, J.; Laine, J.; Rojas, O.J. Comprehensive elucidation of the effect of residual lignin on the physical, barrier, mechanical and surface properties of nanocellulose films. Green Chem. 2015, 17, 1853–1866. [Google Scholar] [CrossRef] [Green Version]
- Villaverde, J.J.; Li, J.; Ek, M.; Ligero, P.; De Vega, A. Native lignin structure of Miscanthus x giganteus and its changes during acetic and formic acid fractionation. J. Agric. Food Chem. 2009, 57, 6263–6270. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Choi, C.S.; Bae, J.H.; Jeong, H.; Lee, S.H.; Kim, Y.S. Preparation of a lignin/polyaniline composite and its application in Cr(VI) removal from aqueous solutions. BioResources 2019, 14, 9169–9182. [Google Scholar] [CrossRef]
- Fodil Cherif, M.; Trache, D.; Brosse, N.; Benaliouche, F.; Tarchoun, A.F. Comparison of the Physicochemical Properties and Thermal Stability of Organosolv and Kraft Lignins from Hardwood and Softwood Biomass for Their Potential Valorization. Waste Biomass Valoriz. 2020, 11, 6541–6553. [Google Scholar] [CrossRef]
- Tejado, A.; Peña, C.; Labidi, J.; Echeverria, J.M.; Mondragon, I. Physico-chemical characterization of lignins from different sources for use in phenol-formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef]
- Wen, J.L.; Xue, B.L.; Sun, S.L.; Sun, R.C. Quantitative structural characterization and thermal properties of birch lignins after auto-catalyzed organosolv pretreatment and enzymatic hydrolysis. J. Chem. Technol. Biotechnol. 2013, 88, 1663–1671. [Google Scholar] [CrossRef]
- Ke, J.; Singh, D.; Yang, X.; Chen, S. Thermal characterization of softwood lignin modification by termite Coptotermes formosanus (Shiraki). Biomass Bioenergy 2011, 35, 3617–3626. [Google Scholar] [CrossRef]
- Sahoo, S.; Seydibeyoĝlu, M.Ö.; Mohanty, A.K.; Misra, M. Characterization of industrial lignins for their utilization in future value added applications. Biomass Bioenergy 2011, 35, 4230–4237. [Google Scholar] [CrossRef]
- Cocca, M.; D’Arienzo, L.; D’Orazio, L.; Gentile, G.; Martuscelli, E. Polyacrylates for conservation: Chemico-physical properties and durability of different commercial products. Polym. Test. 2004, 23, 333–342. [Google Scholar] [CrossRef]
- Feng, X.; Yang, Z.; Chmely, S.; Wang, Q.; Wang, S.; Xie, Y. Lignin-coated cellulose nanocrystal filled methacrylate composites prepared via 3D stereolithography printing: Mechanical reinforcement and thermal stabilization. Carbohydr. Polym. 2017, 169, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Alsulami, Q.A.; Albukhari, S.M.; Hussein, M.A.; Tay, G.S.; Rozman, H.D. Biodegradable lignin as a reactive raw material in UV curable systems. Polym. Technol. Mater. 2020, 59, 1387–1406. [Google Scholar] [CrossRef]
- Neukäufer, J.; Seyfang, B.; Grützner, T. Investigation of Contact Angles and Surface Morphology of 3D-Printed Materials. Ind. Eng. Chem. Res. 2020, 59, 6761–6766. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, D.D.; Li, X.D.; Lu, Y.Y.; Qiu, X.H.; Zhang, J.S.; Kong, J. Topography, Wettability, and Electrostatic Charge Consist Mayor Surface Properties of Intraocular Lenses. Curr. Eye Res. 2017, 42, 201–210. [Google Scholar] [CrossRef]
- Scordo, G.; Bertana, V.; Scaltrito, L.; Ferrero, S.; Cocuzza, M.; Marasso, S.L.; Romano, S.; Sesana, R.; Catania, F.; Pirri, C.F. A novel highly electrically conductive composite resin for stereolithography. Mater. Today Commun. 2019, 19, 12–17. [Google Scholar] [CrossRef]
- Serna, D.L.; Martínez, P.E.; González, M.Á.R.; Cadena, A.A.Z.; Contreras, E.A.Z.; Anguiano, M.G.S. Synthesis and characterization of a lignin-styrene-butyl acrylate based composite. Polymers 2019, 11, 1080. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, A.A.; Collet, C.; Gaugler, M.; Lloyd-Jones, G. Integrating softwood biorefinery lignin into polyhydroxybutyrate composites and application in 3D printing. Mater. Today Commun. 2019, 19, 286–296. [Google Scholar] [CrossRef]
- Khalid, M.; Tumelero, M.A.; Brandt, I.S.; Zoldan, V.C.; Acuña, J.J.S.; Pasa, A.A. Electrical Conductivity Studies of Polyaniline Nanotubes Doped with Different Sulfonic Acids. Indian J. Mater. Sci. 2013, 2013, 718304. [Google Scholar] [CrossRef]
- Li, S.; Tao, Y.; Maryum, P.; Wang, Q.; Zhu, J.; Min, F.; Cheng, H.; Zhao, S.; Wang, C. Bifunctional polyaniline electroconductive hydrogels with applications in supercapacitor and wearable strain sensors. J. Biomater. Sci. Polym. Ed. 2020, 31, 938–953. [Google Scholar] [CrossRef] [PubMed]
- Usman, F.; Dennis, J.O.; Meriaudeau, F.; Ahmed, A.Y.; Seong, K.C.; Fen, Y.W.; Sadrolhosseini, A.R.; Abdulkadir, B.A.; Ayinla, R.T.; Daniyal, W.M.E.M.M.; et al. Dependence of the optical constant parameters of p-toluene sulfonic acid-doped polyaniline and its composites on dispersion solvents. Molecules 2020, 25, 4414. [Google Scholar] [CrossRef] [PubMed]
- Beygisangchin, M.; Rashid, S.A.; Shafie, S.; Sadrolhosseini, A.R. Polyaniline synthesized by different dopants for fluorene detection via photoluminescence spectroscopy. Materials 2021, 14, 7382. [Google Scholar] [CrossRef]
- Usman, F.; Dennis, J.O.; Ahmed, A.Y.; Seong, K.C.; Fen, Y.W.; Sadrolhosseini, A.R.; Meriaudeau, F.; Kumar, P.; Ayodele, O.B. Structural characterization and optical constants of p-toluene sulfonic acid doped polyaniline and its composites of chitosan and reduced graphene-oxide. J. Mater. Res. Technol. 2020, 9, 1468–1476. [Google Scholar] [CrossRef]
- Abdiryim, T.; Xiao-Gang, Z.; Jamal, R. Comparative studies of solid-state synthesized polyaniline doped with inorganic acids. Mater. Chem. Phys. 2005, 90, 367–372. [Google Scholar] [CrossRef]
- Horta-Romarís, L.; Abad, M.-J.; González-Rodríguez, M.V.; Lasagabáster, A.; Costa, P.; Lanceros-Méndez, S. Cyclic temperature dependence of electrical conductivity in polyanilines as a function of the dopant and synthesis method. Mater. Des. 2017, 114, 288–296. [Google Scholar] [CrossRef]
- Horta Romarís, L.; González Rodríguez, M.V.; Huang, B.; Costa, P.; Lasagabáster Latorre, A.; Lanceros-Mendez, S.; Abad López, M.J. Multifunctional electromechanical and thermoelectric polyaniline–poly(vinyl acetate) latex composites for wearable devices. J. Mater. Chem. C 2018, 6, 8502–8512. [Google Scholar] [CrossRef]
- Kulkarni, M.V.; Viswanath, A.K.; Aiyer, R.C.; Khanna, P.K. Synthesis, characterization, and morphology of p-toluene sulfonic acid-doped polyaniline: A material for humidity sensing application. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 2161–2169. [Google Scholar] [CrossRef]
- Makeiff, D.A.; Huber, T. Microwave absorption by polyaniline-carbon nanotube composites. Synth. Met. 2006, 156, 497–505. [Google Scholar] [CrossRef]
- Surajit, S.; Sambhu, B.; Khastgir, D. Effect of Dopant Type on the Properties of Polyaniline Surajit. J. Appl. Polym. Sci. 2009, 112, 3135–3140. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, Z.; Wan, M. Nanostructures of Polyaniline Doped with Inorganic Acids. Macromolecules 2002, 35, 5937–5942. [Google Scholar] [CrossRef]
- Reig, M.; Farrokhzad, H.; Van der Bruggen, B.; Gibert, O.; Cortina, J.L. Synthesis of a monovalent selective cation exchange membrane to concentrate reverse osmosis brines by electrodialysis. Desalination 2015, 375, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Khandelwal, V.; Sahoo, S.K.; Kumar, A.; Manik, G. Electrically conductive green composites based on epoxidized linseed oil and polyaniline: An insight into electrical, thermal and mechanical properties. Compos. Part B Eng. 2018, 136, 149–157. [Google Scholar] [CrossRef]
- Khandelwal, V.; Sahoo, S.K.; Kumar, A.; Manik, G. Study on the effect of carbon nanotube on the properties of electrically conductive epoxy/polyaniline adhesives. J. Mater. Sci. Mater. Electron. 2017, 28, 14240–14251. [Google Scholar] [CrossRef]
- Han, H.; Cho, S. Fabrication of Conducting Polyacrylate Resin Solution with Polyaniline Nanofiber and Graphene for Conductive 3D Printing Application. Polymers 2018, 10, 1003. [Google Scholar] [CrossRef] [Green Version]
- Joo, H.; Cho, S. Comparative studies on polyurethane composites filled with polyaniline and graphene for DLP-type 3D printing. Polymers 2020, 12, 67. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, G.; Chiappone, A.; Roppolo, I.; Fantino, E.; Bertana, V.; Perrucci, F.; Scaltrito, L.; Pirri, F.; Sangermano, M. Development of 3D printable formulations containing CNT with enhanced electrical properties. Polymer 2017, 109, 246–253. [Google Scholar] [CrossRef]
- Mu, Q.; Wang, L.; Dunn, C.K.; Kuang, X.; Duan, F.; Zhang, Z.; Qi, H.J.; Wang, T. Digital light processing 3D printing of conductive complex structures. Addit. Manuf. 2017, 18, 74–83. [Google Scholar] [CrossRef]
- Mendes-Felipe, C.; Oliveira, J.; Costa, P.; Ruiz-Rubio, L.; Iregui, A.; González, A.; Vilas, J.L.; Lanceros-Mendez, S. Stimuli responsive UV cured polyurethane acrylated/carbon nanotube composites for piezoresistive sensing. Eur. Polym. J. 2019, 120, 109226. [Google Scholar] [CrossRef]
- Fantino, E.; Chiappone, A.; Roppolo, I.; Manfredi, D.; Bongiovanni, R.; Pirri, C.F.; Calignano, F. 3D Printing of Conductive Complex Structures with in Situ Generation of Silver Nanoparticles. Adv. Mater. 2016, 28, 3712–3717. [Google Scholar] [CrossRef]
- Sciancalepore, C.; Moroni, F.; Messori, M.; Bondioli, F. Acrylate-based silver nanocomposite by simultaneous polymerization–reduction approach via 3D stereolithography. Compos. Commun. 2017, 6, 11–16. [Google Scholar] [CrossRef]
- Qian, C.; Xiao, T.; Chen, Y.; Wang, N.; Li, B.; Gao, Y. 3D Printed Reduced Graphene Oxide/Elastomer Resin Composite with Structural Modulated Sensitivity for Flexible Strain Sensor. Adv. Eng. Mater. 2022, 24, 2101068. [Google Scholar] [CrossRef]
- Mendes-Felipe, C.; Costa, P.; Roppolo, I.; Sangermano, M.; Lanceros-Mendez, S. Bio-based Piezo- and Thermo-Resistive Photo-Curable Sensing Materials from Acrylated Epoxidized Soybean Oil. Macromol. Mater. Eng. 2022, 307, 2100934. [Google Scholar] [CrossRef]
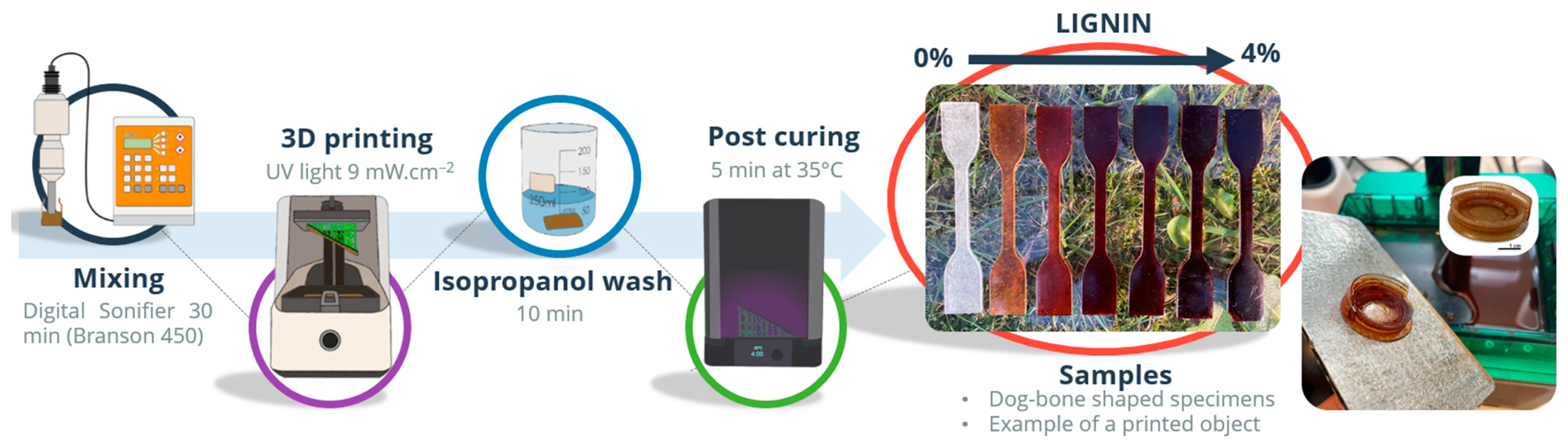



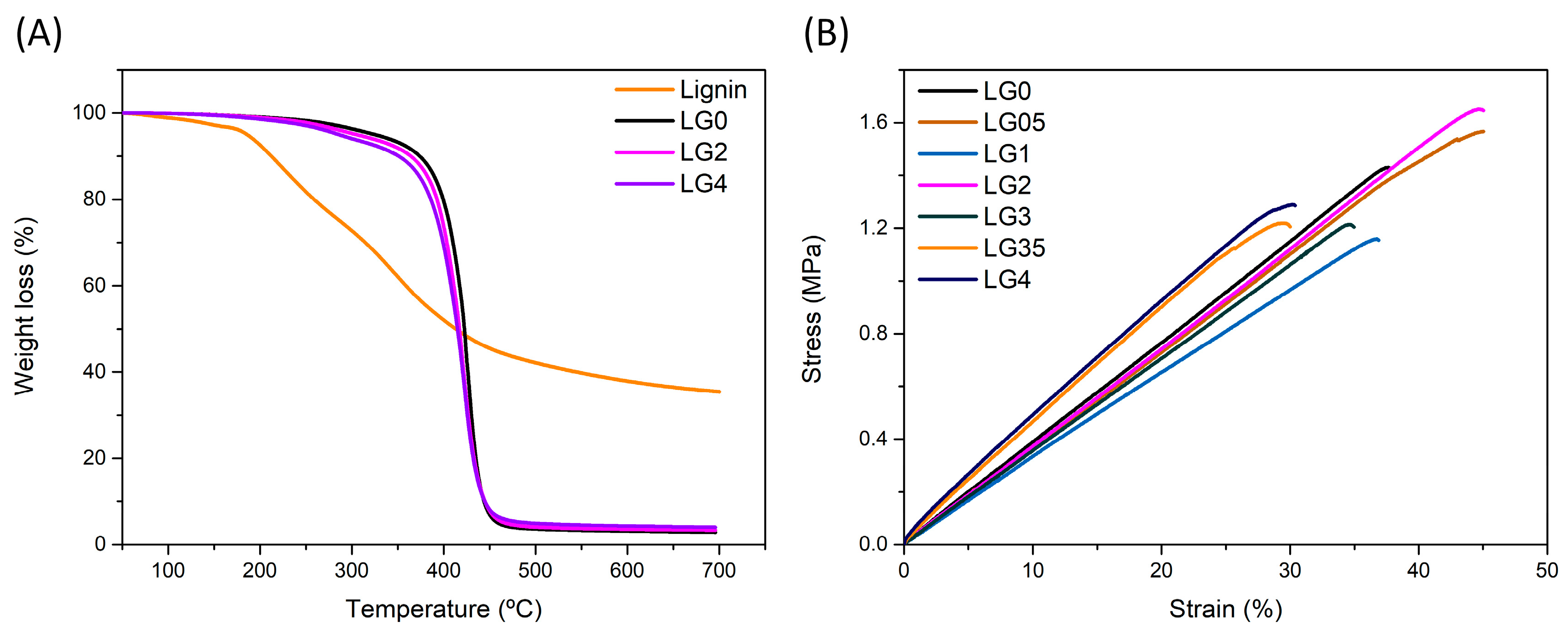


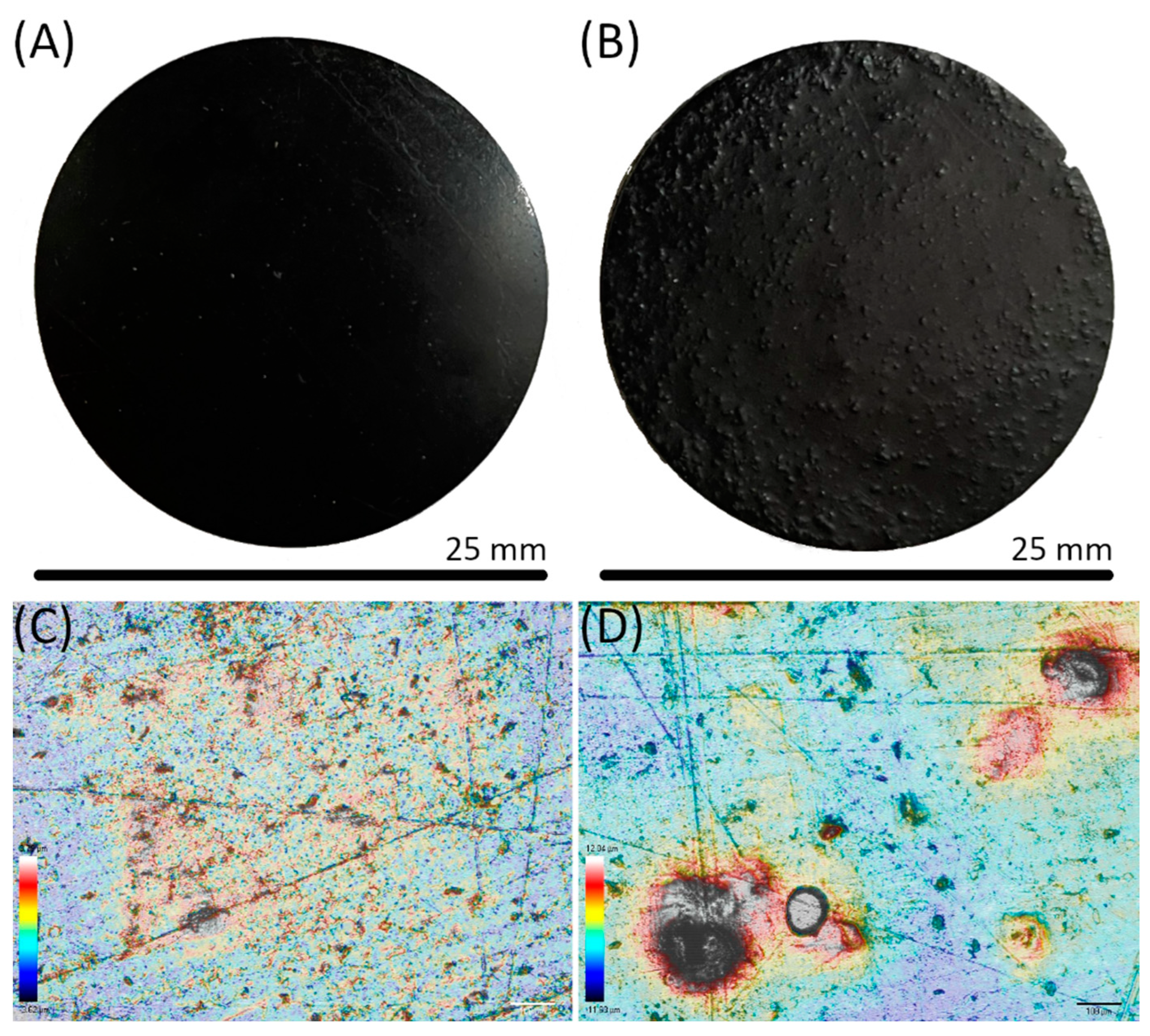
| Sample | Lignin (wt.%) | Exposure Time (s) | Bottom Exposure (s) | Viscosity (Pa.s) 1 s−1 | Induction Period (s) | Max. Rate of Polymerization (mol L−1 s−1) | Ultimate DBC∞ (%) |
|---|---|---|---|---|---|---|---|
| LG0 | 0 | 1 | 15 | 0.011 | <8 | 62.0 ± 0.6 | 98.0 ± 0.2 |
| LG05 | 0.5 | 2 | 15 | 0.019 | <8 | 17.9 ± 2.4 | 97.9 ± 1.6 |
| LG1 | 1 | 2.5 | 20 | 0.026 | 24 ± 7 | 5.8 ± 1.8 | 94.4 ± 0.1 |
| LG2 | 2 | 5 | 30 | 0.056 | 32 ± 13 | 1.9 ± 0.5 | 95.3 ± 0.5 |
| LG3 | 3 | 20 | 50 | 0.060 | 38 ± 4 | 1.9 ± 0.1 | 94.8 ± 2.0 |
| LG35 | 3.5 | 30 | 75 | 0.064 | - | - | - |
| LG4 | 4 | 35 | 90 | 0.072 | - * | 0.9 ± 0.2 | 79.0 ± 11.0 |
| Sample | T10 (°C) | Tonset (°C) | DTGmax (°C) | Residue (wt.%) |
|---|---|---|---|---|
| Lignin | 208.2 ± 3.7 | 176.7 ± 4.0 | 223 and 346 | 35.1 ± 0.2 |
| LG0 | 374.5 ± 0.6 | 400.0 ± 0.1 | 426.5 ± 3.5 | 2.9 ± 0.4 |
| LG05 | 369.2 ± 0.2 | 395.6 ± 1.6 | 420.5 ± 2.1 | 3.2 ± 0.2 |
| LG1 | 366.9 ± 1.6 | 395.5 ± 0.3 | 421.5 ± 0.7 | 3.4 ± 0.1 |
| LG2 | 363.6 ± 0.8 | 389.4 ± 3.8 | 419.5 ± 0.7 | 3.4 ± 0.1 |
| LG3 | 359.0 ± 0.2 | 386.5 ± 3.5 | 419.0 ± 1.4 | 3.7 ± 0.1 |
| LG35 | 356.9 ± 0.9 | 384.8 ± 1.9 | 420.0 ± 1.4 | 3.9 ± 0.1 |
| LG4 | 353.2 ± 3.0 | 383.8 ± 4.4 | 419.5 ± 0.7 | 4.1 ± 0.1 |
| Sample | Hardness Shore A (°Sh) | E (MPa) | σ (MPa) | ε (%) |
|---|---|---|---|---|
| LG0 | 78.3 ± 1.5 | 4.5 ± 0.3 | 1.58 ± 0.26 | 40.6 ± 5.1 |
| LG05 | 78.8 ± 0.6 | 4.2 ± 0.2 | 1.60 ± 0.10 | 43.9 ± 2.2 |
| LG1 | 78.0 ± 1.1 | 3.9 ± 0.3 | 1.30 ± 0.13 | 37.9 ± 2.1 |
| LG2 | 77.7 ± 1.6 | 4.3 ± 0.1 | 1.71 ± 0.11 | 44.13 ± 2.4 |
| LG3 | 80.9 ± 1.3 | 5.4 ± 0.4 | 1.49 ± 0.20 | 36.1 ± 5.8 |
| LG35 | 81.6 ± 0.8 | 5.77 ± 0.4 | 1.02 ± 0.30 | 29.2 ± 9.0 |
| LG4 | 82.2 ± 1.2 | 6.4 ± 0.6 | 1.29 ± 0.30 | 30.4 ± 8.0 |
| Sample | Ra | Rv | Rp |
|---|---|---|---|
| PANI5LG0 | 3.6 ± 0.5 | 80.1 ± 9.9 | 72.5 ± 16.3 |
| PANI5LG1 | 1.4 ± 0.2 | 32.2 ± 6.2 | 51.5 ± 26.5 |
| Printing Technique | Filler | Loading (wt.%) | Base Matrix | Conductivity (S cm−1) | Ref |
|---|---|---|---|---|---|
| DLP | graphene sheets/PANI | 1.2/5 | Polyacrylate resin | 4.0 × 10−9 | [78] |
| DLP | PANI | 5 | Polyacrylate resin | 1.0 × 10−10 | [78] |
| DLP | PANI | 6 | Polyurethane | 9.3 × 10−7 | [79] |
| DLP | PANI | 3 | Acrylic resin | 2.2 × 10−5 | [31] |
| DLP | PANI/MWCNT | 2 | Acrylic resin | 7.4 × 10−4 | [32] |
| DLP | pTSA-PANI | 5 | Acrylic resin | 1.7 × 10−7 | Present work |
| DLP | pTSA-PANI/LIGNIN | 5/1 | Acrylic resin | 1.6 × 10−6 | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias-Ferreiro, G.; Lasagabáster-Latorre, A.; Ares-Pernas, A.; Ligero, P.; García-Garabal, S.M.; Dopico-García, M.S.; Abad, M.-J. Lignin as a High-Value Bioaditive in 3D-DLP Printable Acrylic Resins and Polyaniline Conductive Composite. Polymers 2022, 14, 4164. https://doi.org/10.3390/polym14194164
Arias-Ferreiro G, Lasagabáster-Latorre A, Ares-Pernas A, Ligero P, García-Garabal SM, Dopico-García MS, Abad M-J. Lignin as a High-Value Bioaditive in 3D-DLP Printable Acrylic Resins and Polyaniline Conductive Composite. Polymers. 2022; 14(19):4164. https://doi.org/10.3390/polym14194164
Chicago/Turabian StyleArias-Ferreiro, Goretti, Aurora Lasagabáster-Latorre, Ana Ares-Pernas, Pablo Ligero, Sandra María García-Garabal, María Sonia Dopico-García, and María-José Abad. 2022. "Lignin as a High-Value Bioaditive in 3D-DLP Printable Acrylic Resins and Polyaniline Conductive Composite" Polymers 14, no. 19: 4164. https://doi.org/10.3390/polym14194164






