Shear Bond Strength and Mode of Failure of Polypropylene Fibers in Orthodontic Flash-Free Adhesive
Abstract
:1. Introduction
2. Materials and Methods
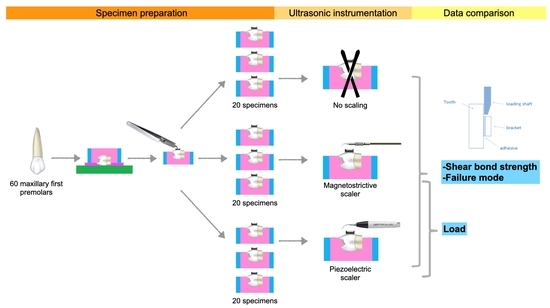
2.1. Specimen Preparation
2.2. Bonding Procedure
2.3. Ultrasonic Instrumentation
2.4. Data Collection
2.4.1. Shear Bond Strength
2.4.2. Mode of Failure

2.4.3. Load
2.5. Statistical Analysis
3. Results
3.1. Shear Bond Strength (SBS)
3.2. Mode of Failure

3.3. Load
4. Discussion
4.1. Shear Bond Strength
4.2. Mode of Failure
4.3. Load
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, J.M.; Choi, S.A.; Myung, J.Y.; Chun, Y.S.; Kim, M. Impact of Orthodontic Brackets on the Intraoral Scan Data Accuracy. Biomed. Res. Int. 2016, 2016, 5075182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paolone, M.G.; Kaitsas, R.; Paolone, G.; Kaitsas, V. Lingual orthodontics and forced eruption: A means for osseous and tissue regeneration. Prog. Orthod. 2008, 9, 46–57. [Google Scholar] [PubMed]
- Hennessy, J.; Al-Awadhi, E.A. Clear aligners generations and orthodontic tooth movement. J. Orthod. 2016, 43, 68–76. [Google Scholar] [CrossRef] [PubMed]
- González-Serrano, C.; Baena, E.; Fuentes, M.V.; Albaladejo, A.; Míguez-Contreras, M.; Lagravère, M.O.; Ceballos, L. Shear bond strength of a flash-free orthodontic adhesive system after thermal aging procedure. J. Clin. Exp. Dent. 2019, 11, e154–e161. [Google Scholar] [CrossRef]
- Al-Jewair, T.S.; Suri, S.; Tompson, B.D. Predictors of adolescent compliance with oral hygiene instructions during two-arch multibracket fixed orthodontic treatment. Angle Orthod. 2011, 81, 525–531. [Google Scholar] [CrossRef]
- Mazin, H.; Salman, S.; Salah, R.; Salah, B. The Effect of Fixed Orthodontic Appliances on Gingival Health. IOSR J. Dent. Med. Sci. 2016, 15, 82–88. [Google Scholar]
- Cardoso Mde, A.; Saraiva, P.P.; Maltagliati, L.; Rhoden, F.K.; Costa, C.C.; Normando, D.; Capelozza Filho, L. Alterations in plaque accumulation and gingival inflammation promoted by treatment with self-ligating and conventional orthodontic brackets. Dent. Press J. Orthod. 2015, 20, 35–41. [Google Scholar] [CrossRef]
- Kudirkaite, I.; Lopatiene, K.; Zubiene, J.; Saldunaite, K. Age and gender influence on oral hygiene among adolescents with fixed orthodontic appliances. Stomatologija 2016, 18, 61–65. [Google Scholar]
- Yousefimanesh, H.; Robati, M.; Kadkhodazadeh, M.; Molla, R. A comparison of magnetostrictive and piezoelectric ultrasonic scaling devices: An in vitro study. J. Periodontal. Implant. Sci. 2012, 42, 243–247. [Google Scholar] [CrossRef] [Green Version]
- Lea, S.C.; Felver, B.; Landini, G.; Walmsley, A.D. Three-dimensional analyses of ultrasonic scaler oscillations. J. Clin. Periodontol. 2009, 36, 44–50. [Google Scholar] [CrossRef]
- Alessandri Bonetti, G.; Incerti Parenti, S.; Ippolito, D.R.; Gatto, M.R.; Luigi, C. Effects of ultrasonic instrumentation with different scaler-tip angulations on the shear bond strength and bond failure mode of metallic orthodontic brackets. Korean J. Orthod. 2014, 44, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Scribante, A.; Sfondrini, M.F.; Collesano, V.; Tovt, G.; Bernardinelli, L.; Gandini, P. Dental Hygiene and Orthodontics: Effect of Ultrasonic Instrumentation on Bonding Efficacy of Different Lingual Orthodontic Brackets. Biomed. Res. Int. 2017, 2017, 3714651. [Google Scholar] [CrossRef]
- Hatipoglu, S.; Paksoy, T. Do conventional and new-generation multiple ultrasonic applications change the shear bond strength of metal brackets? J. Dent. Res. Rev. 2022, 9, 29–34. [Google Scholar] [CrossRef]
- ISO. Dental Materials-Testing of Adhesion to Tooth Structure; International Organisation for Standardization: Geneva, Switzerland, 2003. [Google Scholar]
- Bishara, S.E.; VonWald, L.; Laffoon, J.F.; Warren, J.J. Effect of a self-etch primer/adhesive on the shear bond strength of orthodontic brackets. Am. J. Orthod. Dentofac. Orthop. 2001, 119, 621–624. [Google Scholar] [CrossRef]
- Artun, J.; Bergland, S. Clinical trials with crystal growth conditioning as an alternative to acid-etch enamel pretreatment. Am. J. Orthod. 1984, 85, 333–340. [Google Scholar] [CrossRef]
- Chapman, J.A.; Roberts, W.E.; Eckert, G.J.; Kula, K.S.; González-Cabezas, C. Risk factors for incidence and severity of white spot lesions during treatment with fixed orthodontic appliances. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 188–194. [Google Scholar] [CrossRef]
- Sukontapatipark, W.; El-Agroudi, M.A.; Selliseth, N.J.; Thunold, K.; Selvig, K.A. Bacterial colonization associated with fixed orthodontic appliances. A scanning electron microscopy study. Eur. J. Orthod. 2001, 23, 475–484. [Google Scholar] [CrossRef]
- Guzman, U.A.; Jerrold, L.; Vig, P.S.; Abdelkarim, A. Comparison of shear bond strength and adhesive remnant index between precoated and conventionally bonded orthodontic brackets. Prog. Orthod. 2013, 14, 39. [Google Scholar] [CrossRef] [Green Version]
- Ozer, M.; Bayram, M.; Dincyurek, C.; Tokalak, F. Clinical bond failure rates of adhesive precoated self-ligating brackets using a self-etching primer. Angle Orthod. 2014, 84, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, A.; Pourkhameneh, S.; Sadrhaghighi, A.H. The effect of different force magnitudes for placement of orthodontic brackets on shear bond strength, in three adhesive systems. J. Clin. Exp. Dent. 2018, 10, e548–e554. [Google Scholar] [CrossRef]
- Lea, S.C.; Landini, G.; Walmsley, A.D. Thermal imaging of ultrasonic scaler tips during tooth instrumentation. J. Clin. Periodontol. 2004, 31, 370–375. [Google Scholar] [CrossRef]
- Sato, T.; Wakabayashi, M. Development of New Method to Reposition Orthodontic Brackets Without Debonding by Using an Ultrasonic Device: An In Vitro Study. J. Indian Orthod. Soc. 2020, 54, 030157422092138. [Google Scholar] [CrossRef]
- Oduncuoğlu, B.F.; Yamanel, K.; Koçak, Z. In Vitro Evaluation of Direct and Indirect Effects of Sonic and Ultrasonic Instrumentations on the Shear Bond Strength of Orthodontic Brackets. Turk. J. Orthod. 2020, 33, 37–42. [Google Scholar] [CrossRef]
- Bakhadher, W.; Halawany, H.; Talic, N.; Abraham, N.; Jacob, V. Factors Affecting the Shear Bond Strength of Orthodontic Brackets—A Review of In Vitro Studies. Acta Med. 2015, 58, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Kim, J.; Song, J.S.; Chung, S.H.; Hyun, H.K. Shear Bond Strength of Different MDP-Containing Adhesive Systems on Enamel and Dentin from Primary Teeth. J. Clin. Pediatr. Dent. 2021, 45, 186–192. [Google Scholar] [CrossRef]
- Sfondrini, M.F.; Cacciafesta, V.; Scribante, A.; De Angelis, M.; Klersy, C. Effect of blood contamination on shear bond strength of brackets bonded with conventional and self-etching primers. Am. J. Orthod. Dentofac. Orthop. 2004, 125, 357–360. [Google Scholar] [CrossRef]
- Scribante, A.; Gallo, S.; Pascadopoli, M.; Catalano, F.; Gandini, P.; Sfondrini, M.F. Effect of different enamel pretreating agents on bonding efficacy and survival rates of orthodontic brackets: In vitro study and split-mouth randomized clinical trial. Am. J. Orthod. Dentofac. Orthop. 2022, 162, 297–306. [Google Scholar] [CrossRef]
- Walmsley, A.D. Ultrasonics in Dentistry. Phys. Procedia 2015, 63, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Zappa, U.; Cadosch, J.; Simona, C.; Graf, H.; Case, D. In vivo scaling and root planing forces. J. Periodontol. 1991, 62, 335–340. [Google Scholar] [CrossRef]
- Busslinger, A.; Lampe, K.; Beuchat, M.; Lehmann, B. A comparative in vitro study of a magnetostrictive and a piezoelectric ultrasonic scaling instrument. J. Clin. Periodontol. 2001, 28, 642–649. [Google Scholar] [CrossRef]



| Group | N | Mean ± SD | Max | Min | Multiple Comparisons (Bonferroni Test) | |
|---|---|---|---|---|---|---|
| PE | MS | |||||
| Control | 20 | 9.65 ± 0.43 | 12.65 | 6.70 | p > 0.05 | p > 0.05 |
| PE | 20 | 9.45 ± 0.67 | 15.16 | 5.98 | ||
| MS | 20 | 9.54 ± 0.51 | 13.67 | 6.04 | ||
| Group | N | ARI Score 1 | Chi-Squared Test | |||
|---|---|---|---|---|---|---|
| 0 (%) | 1 (%) | 2 (%) | 3 (%) | |||
| Control | 20 | 0 (0%) | 2 (10%) | 18 (90%) | 0 (0%) | p = 0.21 |
| PE | 20 | 1 (5%) | 6 (30%) | 13 (65%) | 0 (0%) | |
| MS | 20 | 1 (5%) | 5 (25%) | 12 (60%) | 2 (10%) | |
| Group | N | Mean ± SD | Max | Min | Sig. (Unpaired t-Test) |
|---|---|---|---|---|---|
| PE | 20 | 0.24 ± 0.07 | 0.38 | 0.12 | p = 0.71 |
| MS | 20 | 0.23 ± 0.07 | 0.39 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaimaungchuen, K.; Riddhabhaya, A.; Niyomtham, N.; Sirisoontorn, I. Shear Bond Strength and Mode of Failure of Polypropylene Fibers in Orthodontic Flash-Free Adhesive. Polymers 2022, 14, 4167. https://doi.org/10.3390/polym14194167
Chaimaungchuen K, Riddhabhaya A, Niyomtham N, Sirisoontorn I. Shear Bond Strength and Mode of Failure of Polypropylene Fibers in Orthodontic Flash-Free Adhesive. Polymers. 2022; 14(19):4167. https://doi.org/10.3390/polym14194167
Chicago/Turabian StyleChaimaungchuen, Kitiporn, Apiwat Riddhabhaya, Nattisa Niyomtham, and Irin Sirisoontorn. 2022. "Shear Bond Strength and Mode of Failure of Polypropylene Fibers in Orthodontic Flash-Free Adhesive" Polymers 14, no. 19: 4167. https://doi.org/10.3390/polym14194167