Cellulose Fibre Degradation in Cellulose/Steel Hybrid Geotextiles under Outdoor Weathering Conditions
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Chemicals
2.2. Production of Geo Textile Prototypes/Wet-Laying Process
2.3. Biodegradation Tests
2.4. Determination of Moisture Content and Carboxyl Group Content
2.5. Determination of Viscosity-Average Degree of Polymerisation
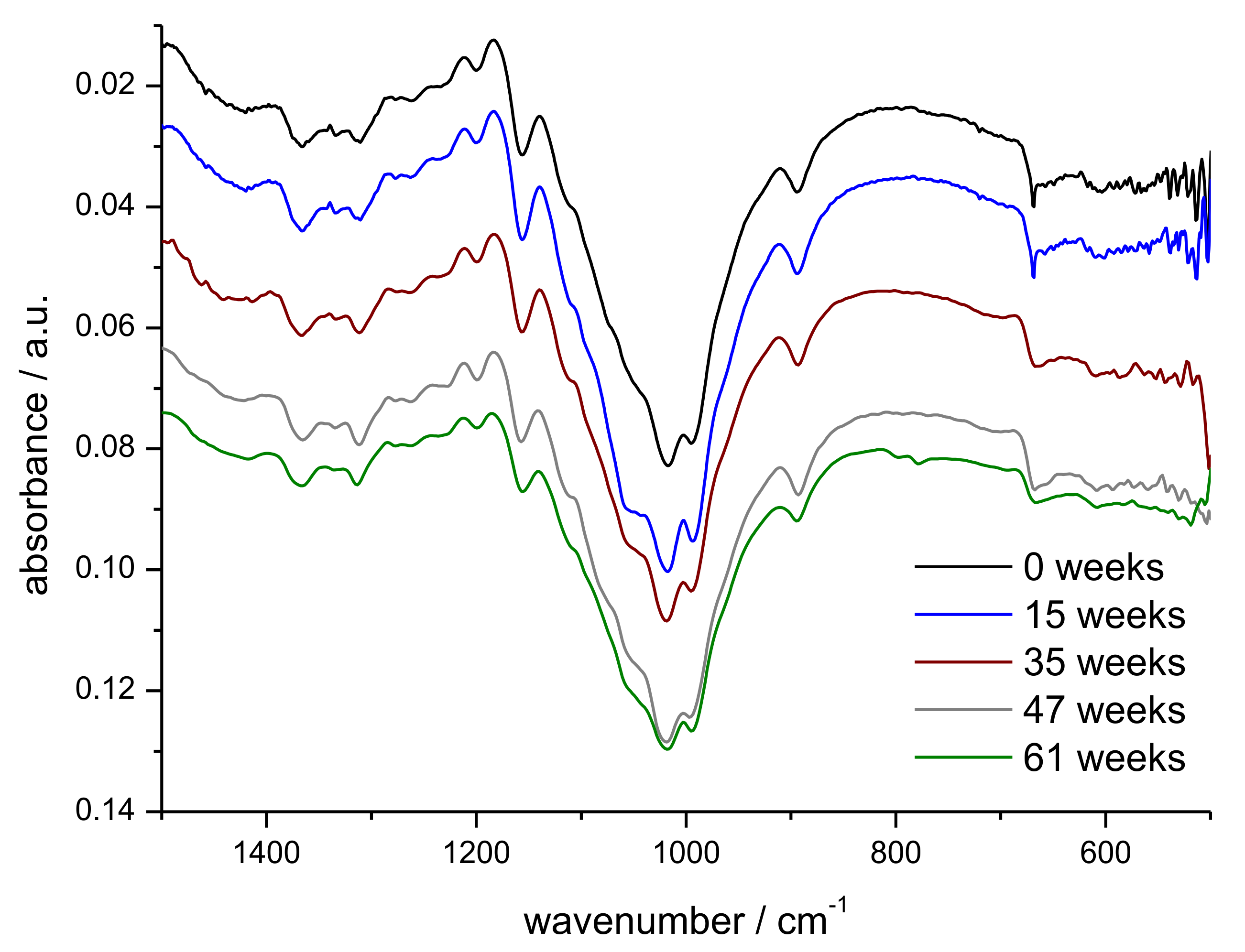
2.6. Confocal 3D Laser-Scanning Microscopy and FTIR Spectroscopy
3. Results and Discussion
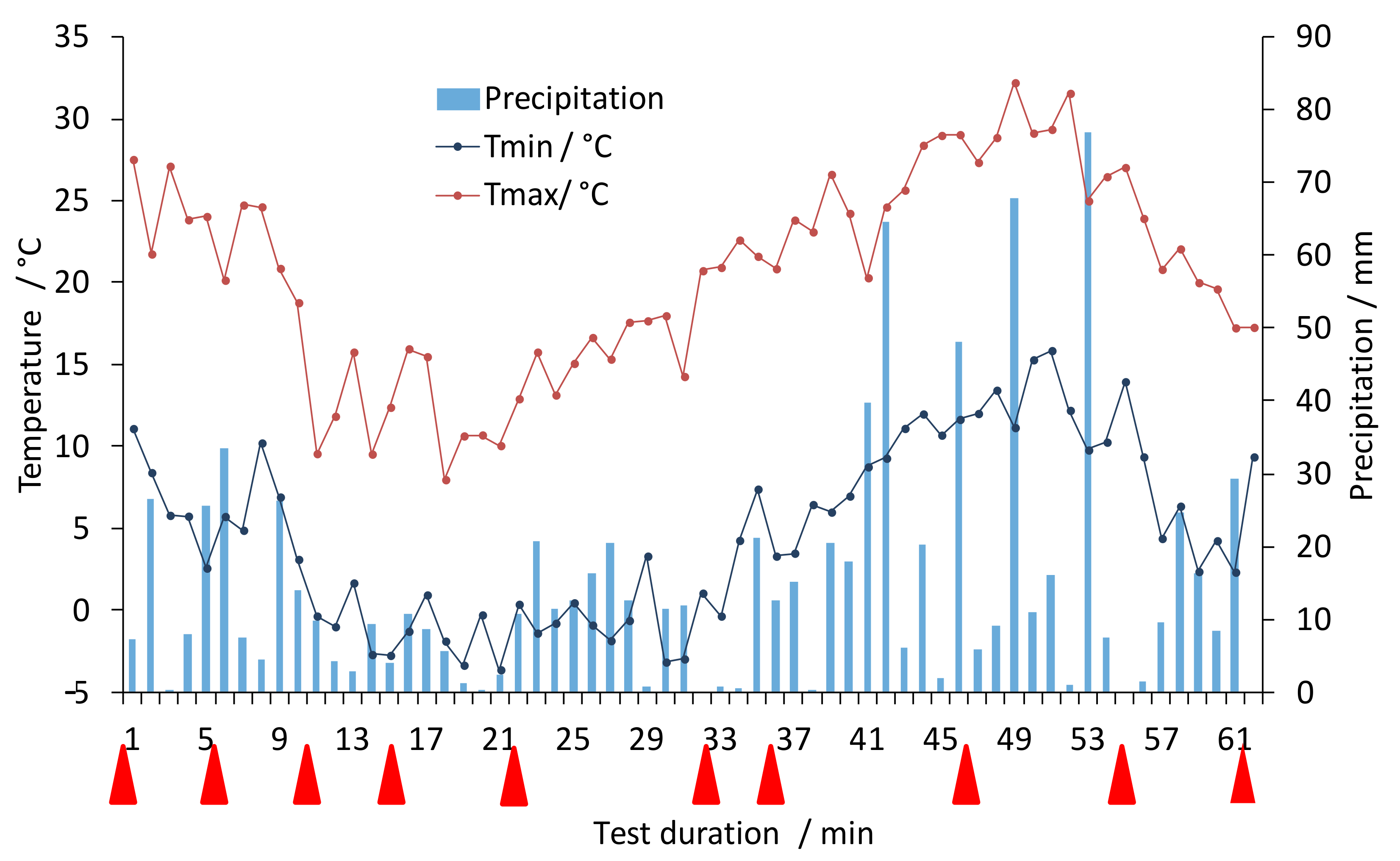
3.1. Exposure to Weathering and Visual Inspection
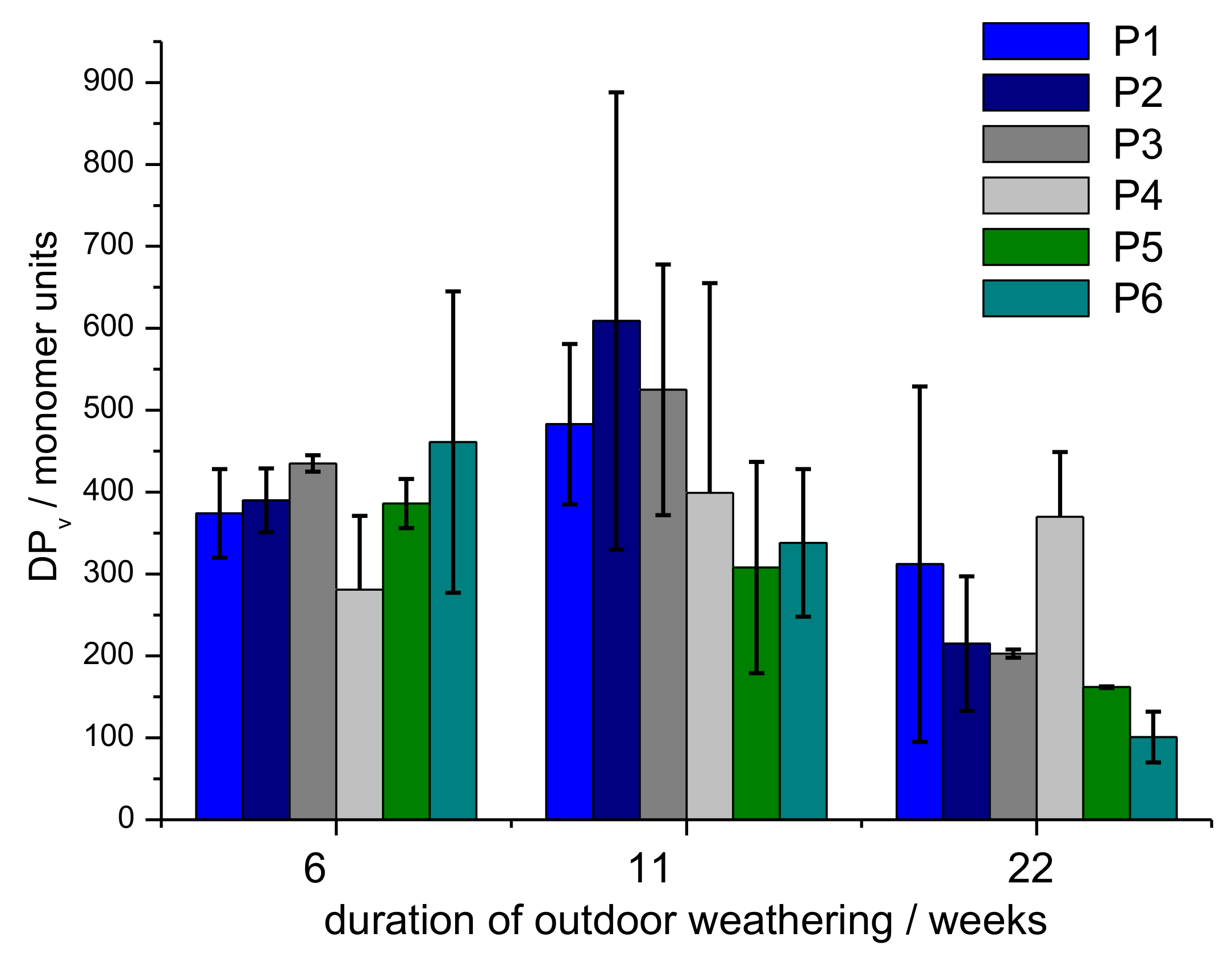
3.2. Monitoring of Fibre Degradation
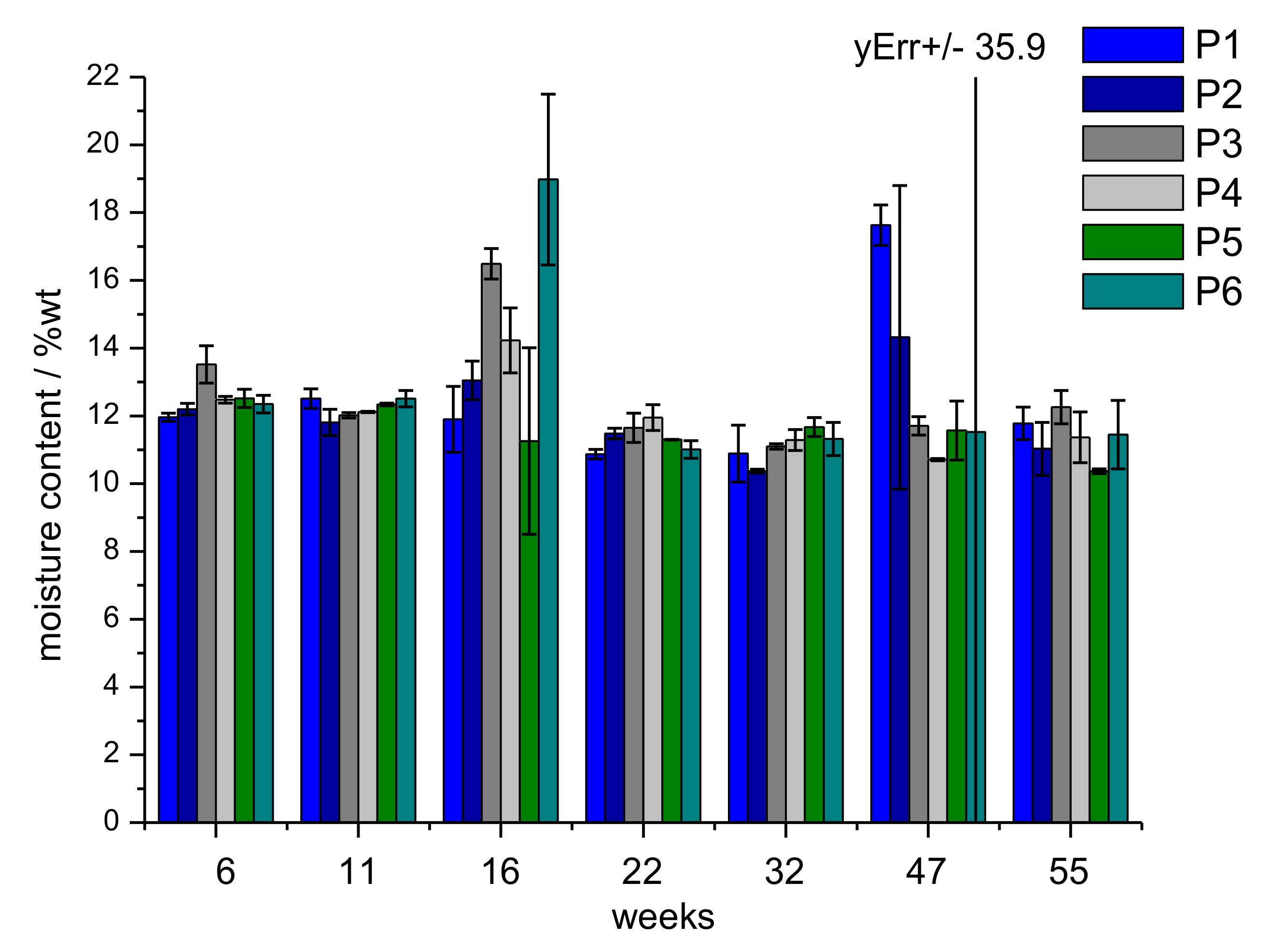
3.3. Determination of Moisture Content and Carboxyl Group Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eriksson, K.-E.L.; Blanchette, R.A.; Ander, P. Biodegradation of Cellulose. In Microbial and Enzymatic Degradation of Wood and Wood Components; Springer: Berlin/Heidelberg, Germany, 1990; pp. 89–180. [Google Scholar] [CrossRef]
- Potthast, A.; Ahn, K.; Becker, M.; Eichinger, T.; Kostic, M.; Böhmdorfer, S.; Jeong, M.J.; Rosenau, T. Acetylation of Cellulose—Another Pathway of Natural Cellulose Aging during Library Storage of Books and Papers. Carbohydr. Polym. 2022, 287, 119323. [Google Scholar] [CrossRef]
- Mayr, G.; Zeppetzauer, F.; Zweckmair, T.; Bauer, D.; Hild, S.; Potthast, A.; Rosenau, T.; Roeder, T. The Reactions of Cellulose and Hemicellulose Degradation Products in the Viscose Fibre Spin Bath. Lenzing. Ber. 2015, 92, 53–58. [Google Scholar]
- Roeder, T.; Kraft, G.; Borgards, A.; Zuckerstaetter, G.; Rosenau, T. Cellulose Degradation during Viscose Processing. In Abstracts of Papers, 241st ACS National Meeting & Exposition, Anaheim, CA, USA, 27–31 March 2011; American Chemical Society: Washington, DC, USA, 2011; p. CELL CO-69NZUR. [Google Scholar]
- Roeder, T.; Kliba, G.; Milacher, W.; Kraft, G.; Potthast, A.; Rosenau, T. Cellulose Degradation during Deformation Processing and Analytics. In Abstracts of Papers, 249th ACS National Meeting & Exposition, Denver, CO, USA, 22–26 March 2015; American Chemical Society: Washington, DC, USA, 2015; p. CELL CO-69TQWW. [Google Scholar]
- Bisaria, V.S.; Ghose, T.K. Biodegradation of Cellulosic Materials: Substrates, Microorganisms, Enzymes and Products. Enzyme Microb. Technol. 1981, 3, 90–104. [Google Scholar] [CrossRef]
- Yu, Z.; Ji, Y.; Bourg, V.; Bilgen, M.; Meredith, J.C. Chitin- and Cellulose-Based Sustainable Barrier Materials: A Review. Emergent Mater. 2020, 3, 919–936. [Google Scholar] [CrossRef]
- Beguin, P.; Aubert, J.-P. The Biological Degradation of Cellulose. FEMS Microbiol. Rev. 1994, 13, 25–58. [Google Scholar] [CrossRef] [PubMed]
- Polman, E.M.N.; Gruter, G.J.M.; Parsons, J.R.; Tietema, A. Comparison of the Aerobic Biodegradation of Biopolymers and the Corresponding Bioplastics: A Review. Sci. Total Environ. 2021, 753, 141953. [Google Scholar] [CrossRef] [PubMed]
- Berdugo-Clavijo, C.; Scheffer, G.; Sen, A.; Gieg, L.M. Biodegradation of Polymers Used in Oil and Gas Operations: Towards Enzyme Biotechnology Development and Field Application. Polymers 2022, 14, 1871. [Google Scholar] [CrossRef]
- Tsavkelova, E.A.; Netrusov, A.I. Biogas Production from Cellulose-Containing Substrates: A Review. Appl. Biochem. Microbiol. 2012, 48, 421–433. [Google Scholar] [CrossRef]
- Raschle, P. Microbial Influence on Cellulosic Textiles and Microbiological Testing. Int. Biodeterior. 1989, 25, 237–244. [Google Scholar] [CrossRef]
- Sewalt, V.J.H.; Glasser, W.G.; Beauchemin, K.A. Lignin Impact on Fiber Degradation. 3. Reversal of Inhibition of Enzymatic Hydrolysis by Chemical Modification of Lignin and by Additives. J. Agric. Food Chem. 1997, 45, 1823–1828. [Google Scholar] [CrossRef]
- Lykaki, M.; Zhang, Y.Q.; Markiewicz, M.; Brandt, S.; Kolbe, S.; Schrick, J.; Rabe, M.; Stolte, S. The Influence of Textile Finishing Agents on the Biodegradability of Shed Fibres. Green Chem. 2021, 23, 5212–5221. [Google Scholar] [CrossRef]
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of Recent Advances in the Biodegradability of Polyhydroxyalkanoate (PHA) Bioplastics and Their Composites. Green Chem. 2020, 22, 5519–5558. [Google Scholar] [CrossRef]
- Kim, S.; Cho, Y.; Park, C.H. Effect of Cotton Fabric Properties on Fiber Release and Marine Biodegradation. Text. Res. J. 2022, 92, 2121–2137. [Google Scholar] [CrossRef]
- Arshad, K.; Mujahid, M. Biodegradation of Textile Materials. Master’s Thesis, University of Boras, Boras, Sweden, 2011. [Google Scholar]
- Hayakawa, C.; Funakawa, S.; Fujii, K.; Kadono, A.; Kosaki, T. Effects of Climatic and Soil Properties on Cellulose Decomposition Rates in Temperate and Tropical Forests. Biol. Fertil. Soils 2014, 50, 633–643. [Google Scholar] [CrossRef]
- Warnock, M.; Davis, K.; Wolf, D.; Gbur, E. Biodegradation of Three Cellulosic Fabrics in Soil. In Summaries of Arkansas Cotton Research 2009; Oosterhuis, D.M., Ed.; Arkansas Agricultural Experiment Station, University of Arkansas: Favetteville, NC, USA, 2010. [Google Scholar]
- Sülar, V.; Devrim, G. Biodegradation Behaviour of Different Textile Fibres: Visual, Morphological, Structural Properties and Soil Analyses. Fibres Text. East. Eur. 2019, 27, 100–111. [Google Scholar] [CrossRef]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chemie—Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Lv, C. Durability of Cellulosic-Fiber-Reinforced Geopolymers: A Review. Molecules 2022, 27, 796. [Google Scholar] [CrossRef]
- Röder, T.; Kogler, M.; Innerlohinger, J.; Schuster, K.C. Regenerated Cellulose—Developments and Biodegradability. In Proceedings of the 6th EPNOE International Polysaccharide Conference, Aveiro, Portugal, 21–25 October 2019; p. 93. [Google Scholar]
- Jaturapiree, A.; Manian, A.P.; Bechtold, T. Sorption Studies on Regenerated Cellulosic Fibers in Salt-Alkali Mixtures. Cellulose 2006, 13, 647–654. [Google Scholar] [CrossRef]
- Philipp, B.; Rehder, W.; Lang, H. Determination of the Carboxyl Content of Dissolving Pulp. Papier 1965, 19, 1–9. [Google Scholar]
- Fitz-Binder, C.; Bechtold, T. One-Sided Surface Modification of Cellulose Fabric by Printing a Modified TEMPO-Mediated Oxidant. Carbohydr. Polym. 2014, 106, 142–147. [Google Scholar] [CrossRef]
- Klemm, D.; Philipp, B.; Heinze, T.; Heinze, U.; Wagenknecht, W. Comprehensive Cellulose Chemistry; Volume I: Fundamentals and Analytical Methods; Wiley-VCH Verlag: Weinheim, Germany, 1998. [Google Scholar]
- Fitz-Binder, C.; Bechtold, T. Ca2+ Sorption on Regenerated Cellulose Fibres. Carbohydr. Polym. Technol. Appl. 2012, 90, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Široký, J.; Blackburn, R.S.; Bechtold, T.; Taylor, J.; White, P. Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy Analysis of Crystallinity Changes in Lyocell Following Continuous Treatment with Sodium Hydroxide. Cellulose 2010, 17, 103–115. [Google Scholar] [CrossRef]
- Reddy, K.O.; Maheswari, C.U.; Shukla, M. Physico-Chemical Characterization of Cellulose Extracted from Ficus Leaves. J. Biobased Mater. Bioenergy 2013, 7, 496–499. [Google Scholar] [CrossRef]
- Oh, S.Y.; Il Yoo, D.; Shin, Y.; Seo, G. FTIR Analysis of Cellulose Treated with Sodium Hydroxide and Carbon Dioxide. Carbohydr. Res. 2005, 340, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Makarov, I.S.; Golova, L.K.; Smyslov, A.G.; Vinogradov, M.I.; Palchikova, E.E.; Legkov, S.A. Flax Noils as a Source of Cellulose for the Production of Lyocell Fibers. Fibers 2022, 10, 45. [Google Scholar] [CrossRef]
- Munasinghe, P.; Druckman, A.; Dissanayake, D.G.K. A Systematic Review of the Life Cycle Inventory of Clothing. J. Clean. Prod. 2021, 320, 128852. [Google Scholar] [CrossRef]







| Prototype | Mass per Area Viscose Fibres g m−2 | Mass per Area Nonwoven Fabric g m−2 | Ratio of Binding Agent per Fibre, Binder g m−2 |
|---|---|---|---|
| P 1 | 200 | 200 | 0.25 S1 |
| P 2 | 200 | 200 | 0.25 S1 |
| P 3 | 200 | 200 | 0.85 S2 |
| P 4 | 200 | 200 | 0.25 S2 |
| P 5 | 200 | - | 0.25 S2 |
| P 6 | 130 | 200 | 0.25 S2 |
| Month | Weeks | Days | ||
|---|---|---|---|---|
| Start of weather trials | August | 0 | 1 | |
| 1 | sampling | October | 6 | 42 |
| 2 | sampling | November | 11 | 77 |
| 3 | sampling | December | 15 | 108 |
| 4 | sampling | January | 22 | 157 |
| 5 | sampling | April | 32 | 228 |
| 6 | sampling | May | 35 | 248 |
| 7 | sampling | July | 47 | 335 |
| 8 | sampling | September | 55 | 365 |
| 9 | End of weather trials | October | 61 | 427 |
| Wavenumber | Literature | Type of Vibration |
|---|---|---|
| cm−1 | cm−1 | |
| 3000–3500 | 3000–3500 | O-H hydrogen bonded stretching |
| 2926–2886 | 2892 | C-H stretching |
| 1417–1420 | 1430 | H-C-H and O-C-H in plane bending |
| 1365–1366 | 1375 | C-O-C, C-C-O, C-C-H deformation and stretching |
| 1310–1313 | 1312 | O-H bending |
| 1156–1157 | 1157 | C-O asym. valence |
| 1018 | 1026 | C-O-C pyranose ring skeletal |
| 893 | 892 | C-O-C valence |
| 666–668 | 668 | C-OH out of plane bending |
| 1636, 1641 | 1635, 1638 | Adsorbed water |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manian, A.P.; Paul, B.; Lanter, H.; Bechtold, T.; Pham, T. Cellulose Fibre Degradation in Cellulose/Steel Hybrid Geotextiles under Outdoor Weathering Conditions. Polymers 2022, 14, 4179. https://doi.org/10.3390/polym14194179
Manian AP, Paul B, Lanter H, Bechtold T, Pham T. Cellulose Fibre Degradation in Cellulose/Steel Hybrid Geotextiles under Outdoor Weathering Conditions. Polymers. 2022; 14(19):4179. https://doi.org/10.3390/polym14194179
Chicago/Turabian StyleManian, Avinash Pradip, Barbara Paul, Helene Lanter, Thomas Bechtold, and Tung Pham. 2022. "Cellulose Fibre Degradation in Cellulose/Steel Hybrid Geotextiles under Outdoor Weathering Conditions" Polymers 14, no. 19: 4179. https://doi.org/10.3390/polym14194179








