Vardenafil-Loaded Bilosomal Mucoadhesive Sponge for Buccal Delivery: Optimization, Characterization, and In Vivo Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Preparation of Vardenafil-Loaded Bilosomes
2.4. Experimental Design
2.5. Characterization of VDF-Loaded Bilosomes
2.5.1. Transmission Electron Microscope (TEM)
2.5.2. Determination of Particle Size
2.5.3. Entrapment Efficiency (EE %)
2.5.4. Differential Scanning Calorimetry (DSC)
2.6. In Vitro Drug Release Study
2.7. Stability Studies for Vardenafil-Loaded Bilosomes
2.8. Preparation of Vardenafil-Loaded Bilosomal Sponge
2.9. Characterization of Vardenafil-Loaded Bilosomal Sponge
2.9.1. pH
2.9.2. Drug Content Determination
2.9.3. Ex Vivo Mucoadhesion Time
2.10. Ex Vivo Permeation Study
2.11. In Vivo Studies
2.11.1. Pharmacokinetic Study
2.11.2. Measurement of cGMP Level in Serum
2.12. Statistical Analysis
3. Results
3.1. Experimental Design and Optimization
3.2. Impact of Formulation Variables on Product Characteristics of VDF-Loaded Bilosomes
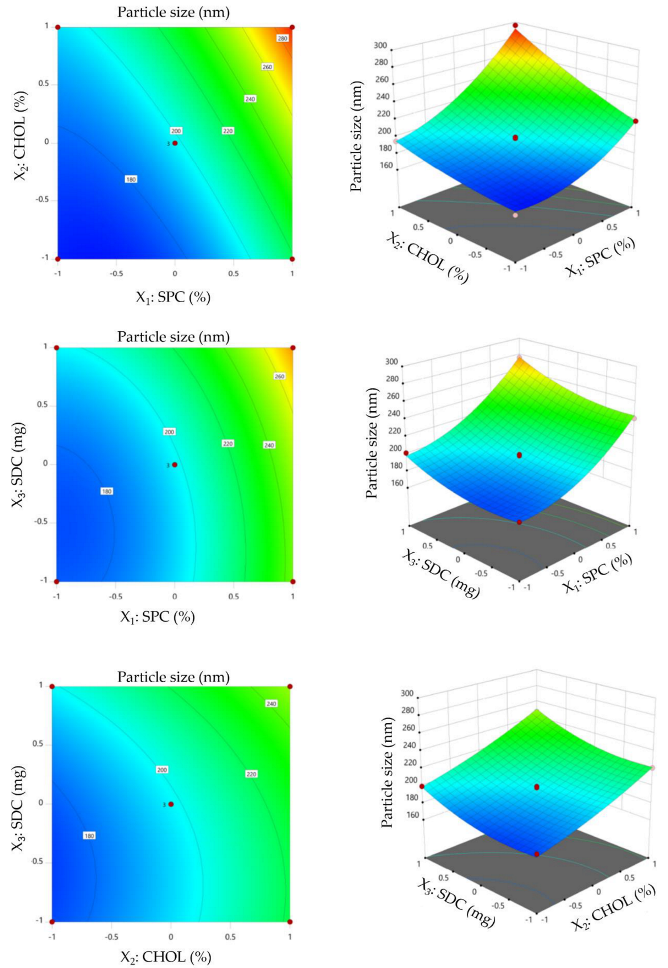
3.2.1. Effect of Formulation Variables on Particle Size
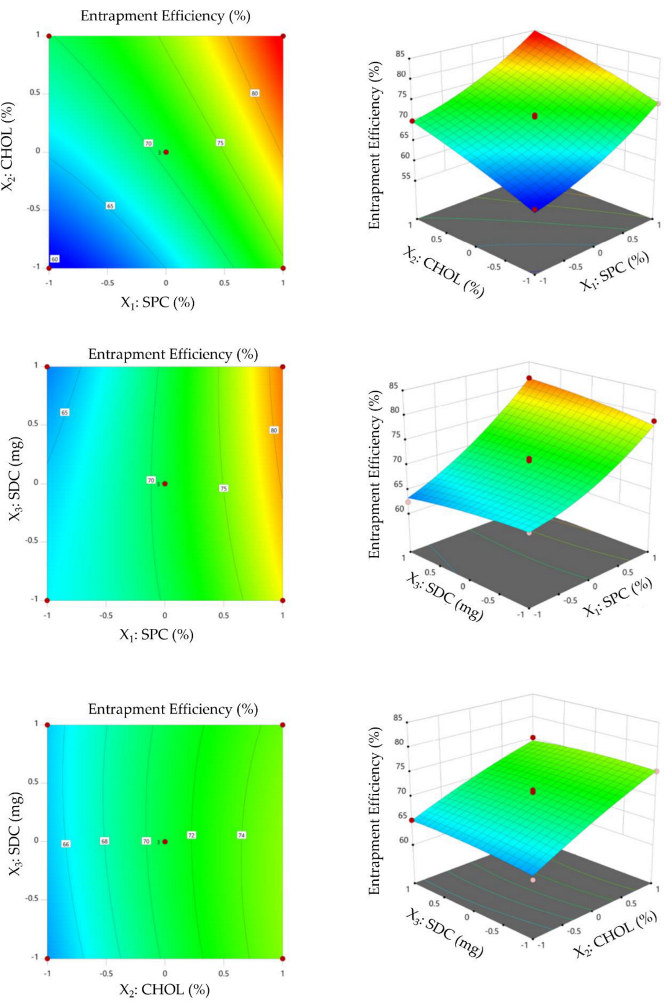
3.2.2. Impact of Formulation Variables on Entrapment Efficiency
3.2.3. Selection of Optimized VDF-Loaded Bilosomal Formulation
3.3. Characterization of Optimized VDF-Loaded Bilosomes
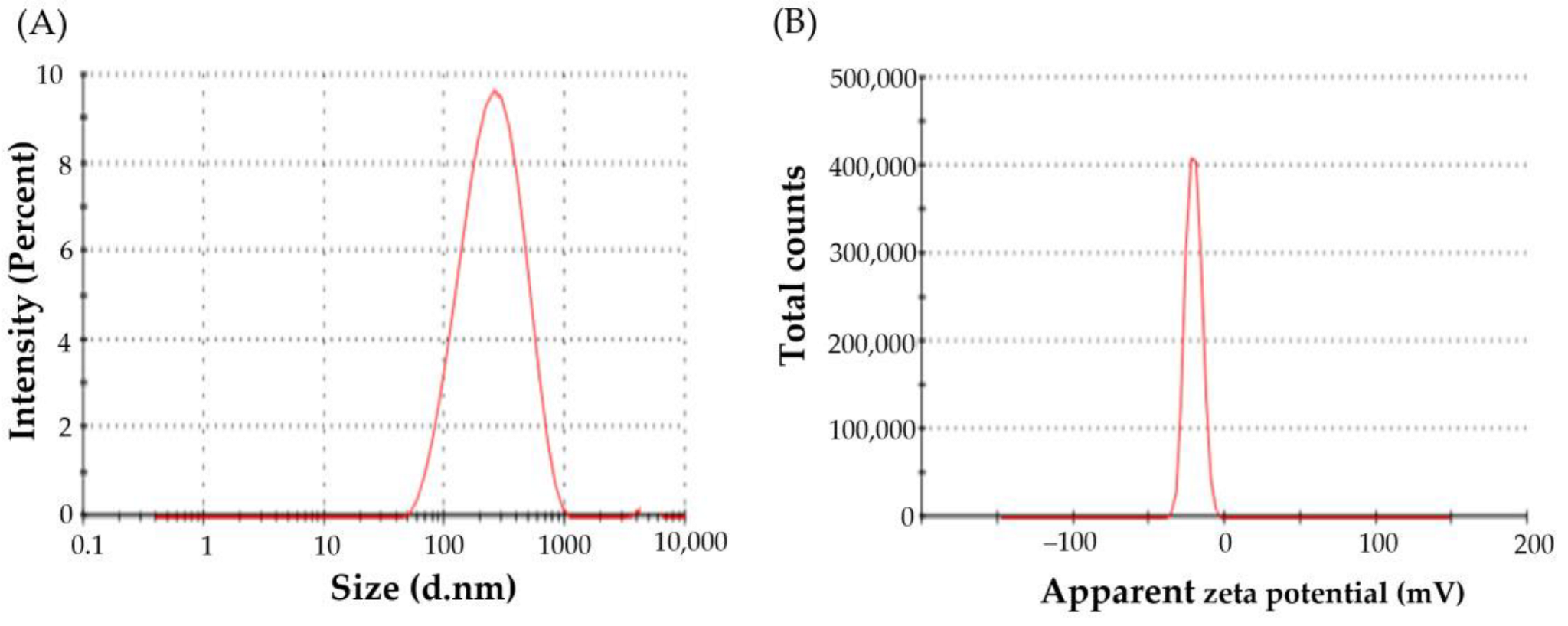
3.3.1. Particle Size, Zeta Potential, and Poly Dispersity Index
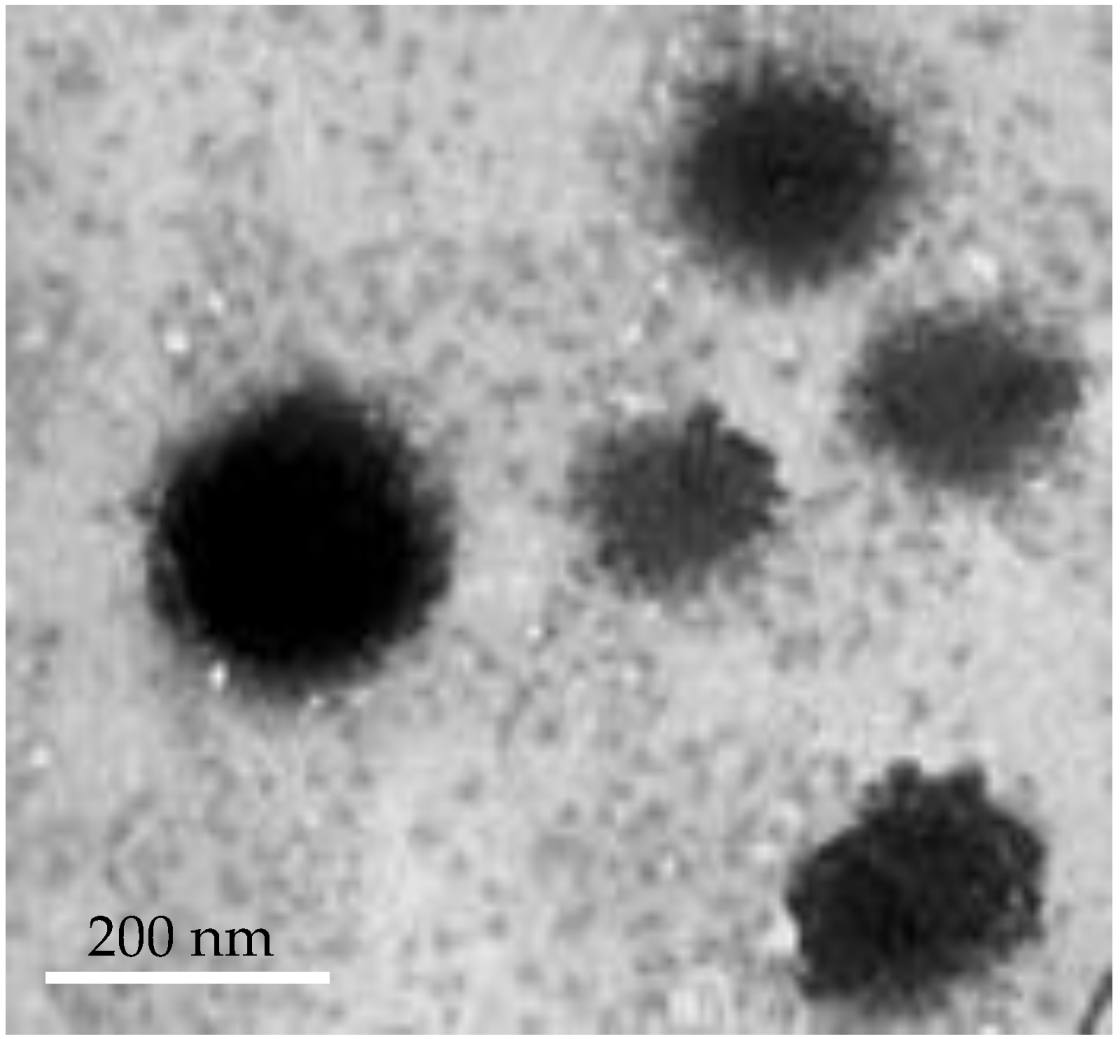
3.3.2. Transmission Electron Microscopy
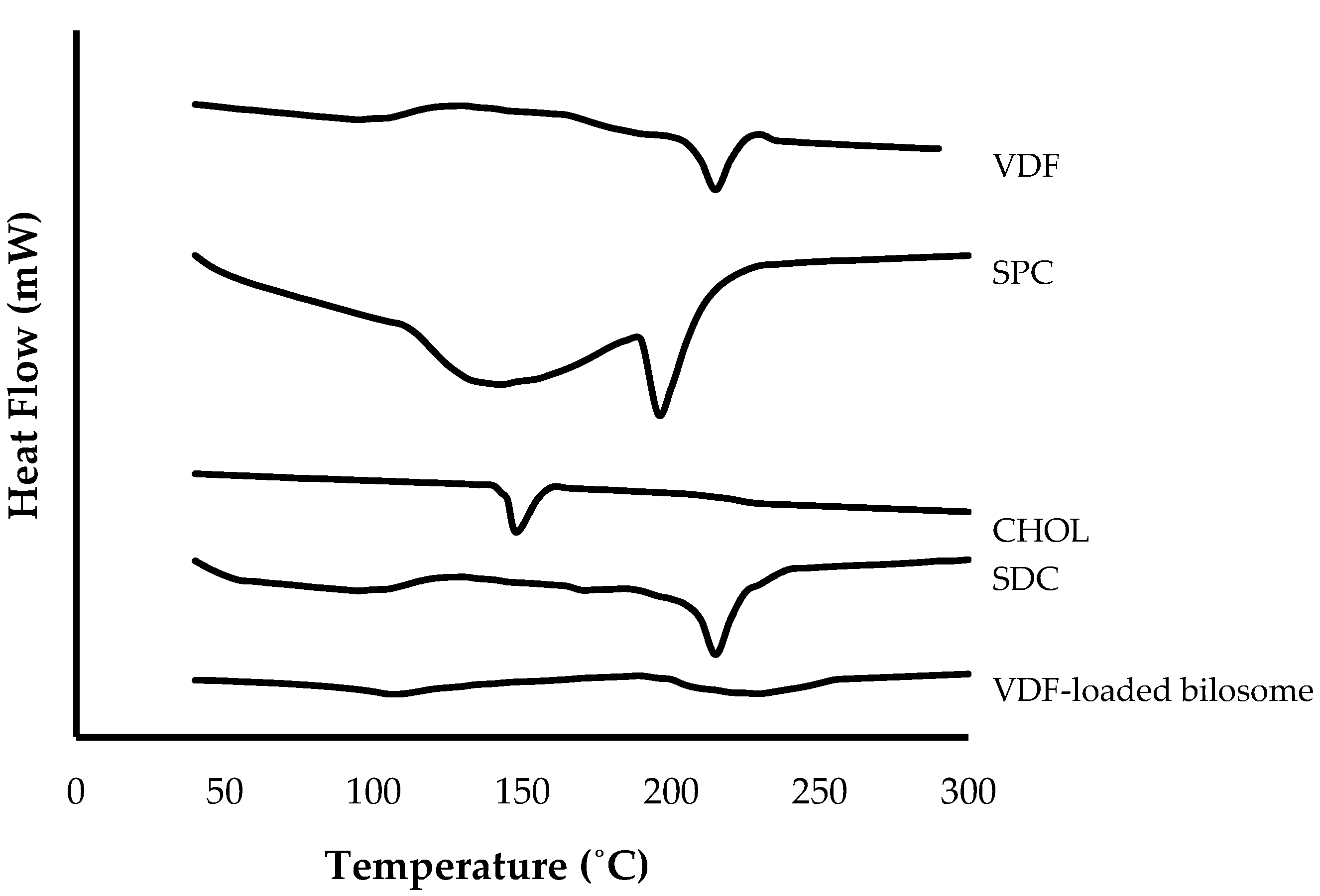
3.3.3. Differential Scanning Calorimetry (DSC)
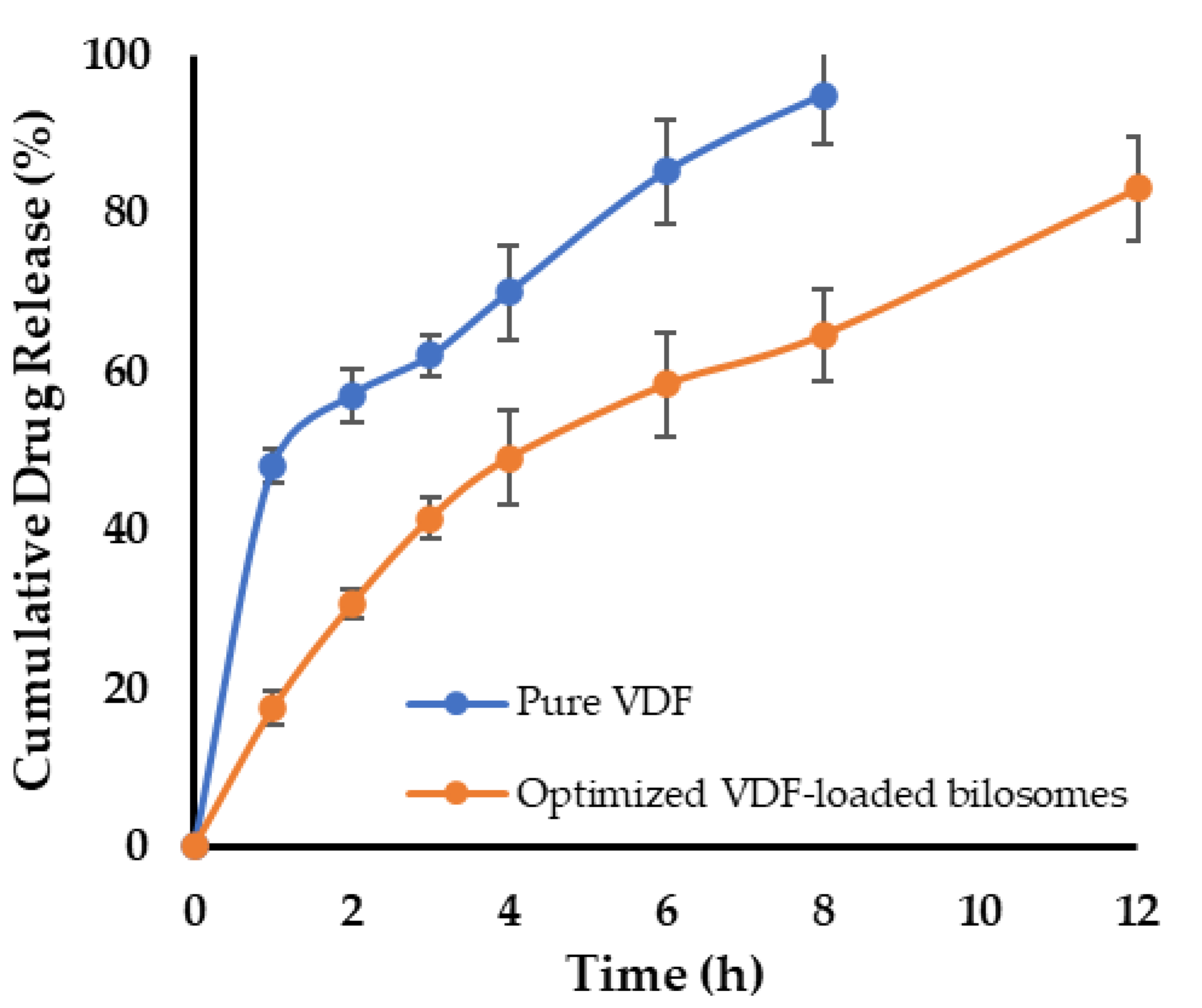
3.4. In Vitro Release Study
3.5. Stability Studies
3.6. Preparation of VDF-Loaded Bilosomal Mucoadhesive Sponge
3.7. Evaluation of the Developed VDF-Loaded Bilosomal Sponge
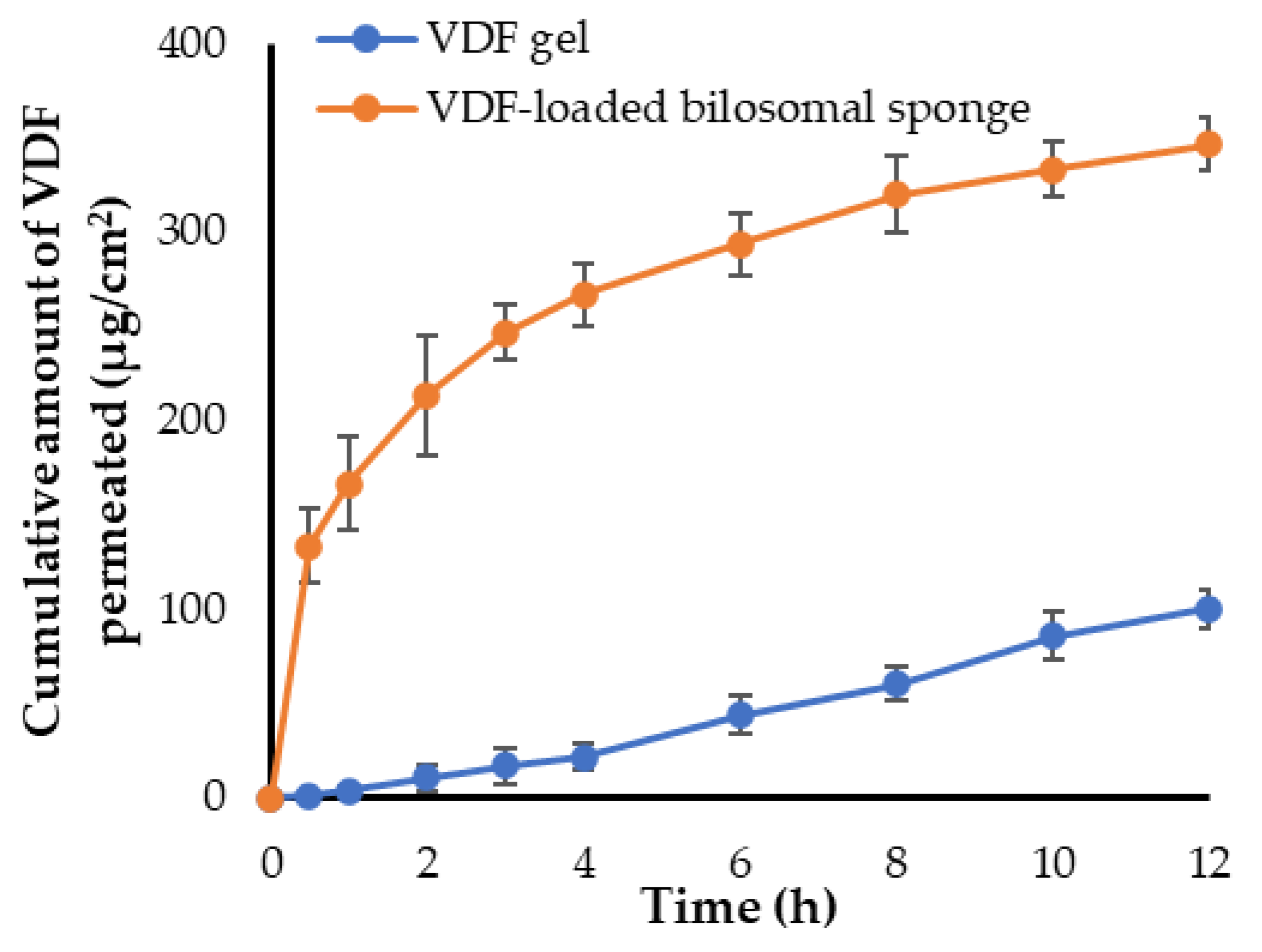
3.8. Ex Vivo Permeation Study through Sheep Buccal Mucosa
3.9. In Vivo Study
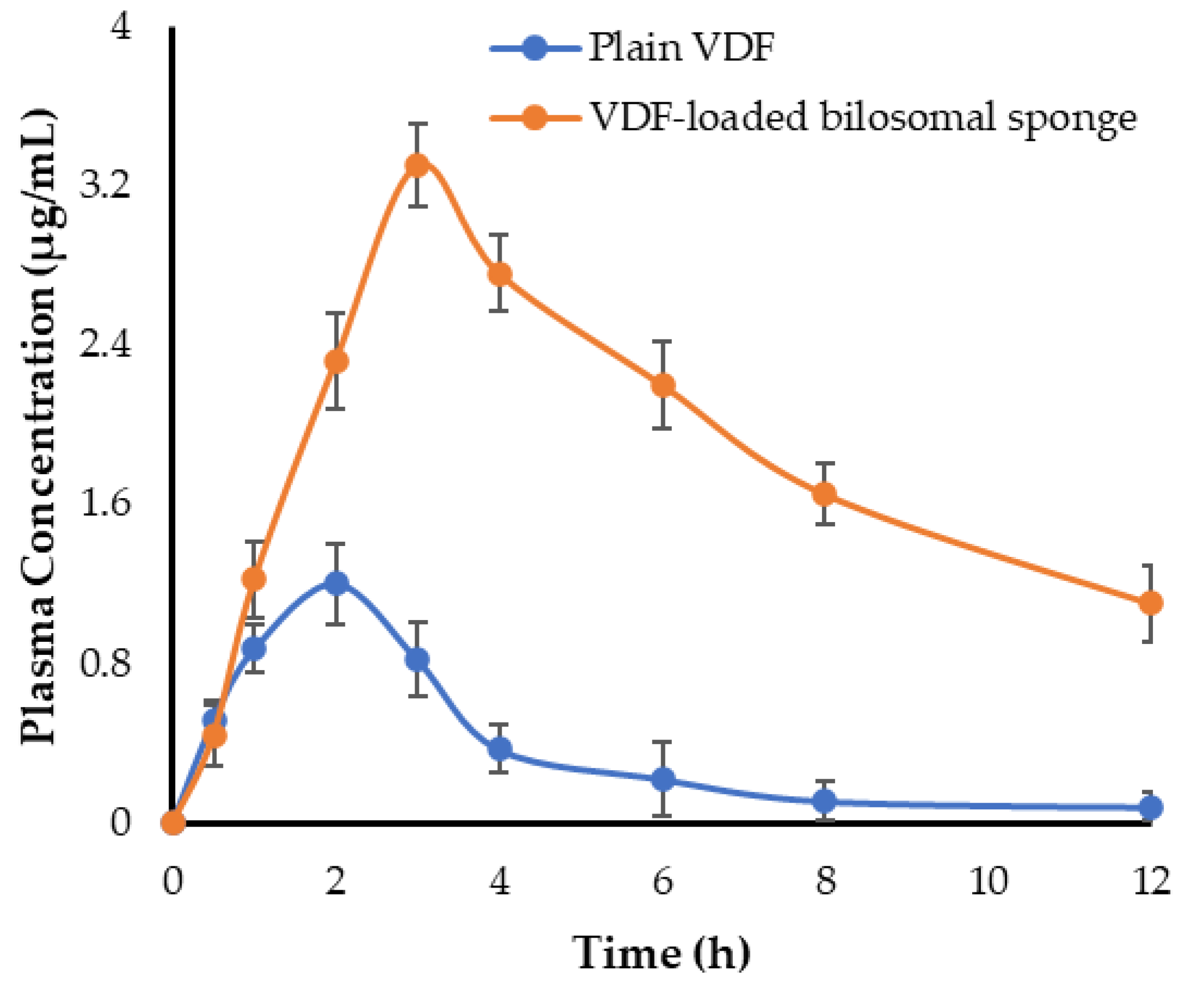
3.9.1. Bioavailability and Pharmacokinetic Study
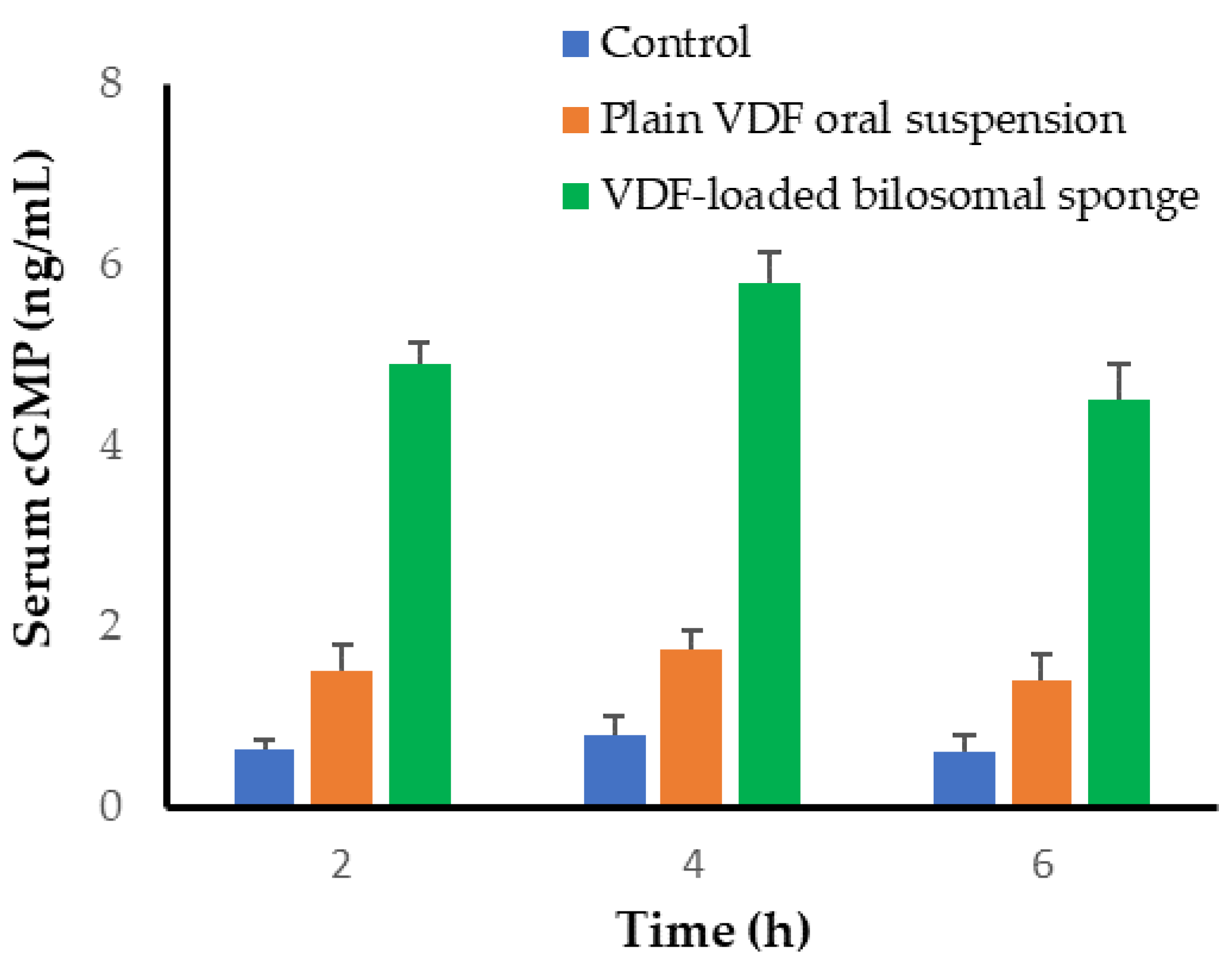
3.9.2. Serum Level of cGMP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kendirci, M.; Bivalacqua, T.J.; Hellstrom, W.J. Vardenafil: A novel type 5 phosphodiesterase inhibitor for the treatment of erectile dysfunction. Expert. Opin. Pharmacother. 2004, 5, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.A.; Lie, J.D. Phosphodiesterase-5 (PDE5) Inhibitors in the Management of Erectile Dysfunction. P T 2013, 38, 407–419. [Google Scholar] [PubMed]
- Abu Lila, A.S.; Gomaa, E.; Ghazy, F.E.S.; Hasan, A.A. Treatment of pulmonary arterial hypertension by vardenafil-solid dispersion lozenges as a potential alternative drug delivery system. J. Drug Deliv. Sci. Technol. 2020, 55, 101444. [Google Scholar] [CrossRef]
- Al-Gethmy, H.A.; Fahmy, U.A.; Alhakamy, N.A.; Ahmed, O.A.A.; El-Say, K.M. Optimization of the Factors Affecting the Absorption of Vardenafil from Oral Disintegrating Tablets: A Clinical Pharmacokinetic Investigation. Pharmaceutics 2019, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, S.; Zhang, M.; Sun, J. Nanocarriers for oral drug delivery. J. Drug Target. 2013, 21, 515–527. [Google Scholar] [CrossRef]
- Ahad, A.; Raish, M.; Ahmad, A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Eprosartan mesylate loaded bilosomes as potential nano-carriers against diabetic nephropathy in streptozotocin-induced diabetic rats. Eur. J. Pharm. Sci. 2018, 111, 409–417. [Google Scholar] [CrossRef]
- Khafagy, E.S.; Abu Lila, A.S.; Sallam, N.M.; Sanad, R.A.; Ahmed, M.M.; Ghorab, M.M.; Alotaibi, H.F.; Alalaiwe, A.; Aldawsari, M.F.; Alshahrani, S.M.; et al. Preparation and Characterization of a Novel Mucoadhesive Carvedilol Nanosponge: A Promising Platform for Buccal Anti-Hypertensive Delivery. Gels 2022, 8, 235. [Google Scholar] [CrossRef]
- Lila, A.S.; Kiwada, H.; Ishida, T. Selective delivery of oxaliplatin to tumor tissue by nanocarrier system enhances overall therapeutic efficacy of the encapsulated oxaliplatin. Biol. Pharm. Bull. 2014, 37, 206–211. [Google Scholar] [CrossRef] [Green Version]
- Kalepu, S.; Manthina, M.; Padavala, V. Oral lipid-based drug delivery systems—An overview. Acta Pharm. Sin. B 2013, 3, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Pouton, C.W.; Porter, C.J. Formulation of lipid-based delivery systems for oral administration: Materials, methods and strategies. Adv. Drug Deliv. Rev. 2008, 60, 625–637. [Google Scholar] [CrossRef]
- Sultan, A.A.; El-Gizawy, S.A.; Osman, M.A.; El Maghraby, G.M. Niosomes for oral delivery of nateglinide: In situ-in vivo correlation. J. Liposome. Res. 2018, 28, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Abd El Azim, H.; Nafee, N.; Ramadan, A.; Khalafallah, N. Liposomal buccal mucoadhesive film for improved delivery and permeation of water-soluble vitamins. Int. J. Pharm. 2015, 488, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Elkomy, M.H.; Alruwaili, N.K.; Elmowafy, M.; Shalaby, K.; Zafar, A.; Ahmad, N.; Alsalahat, I.; Ghoneim, M.M.; Eissa, E.M.; Eid, H.M. Surface-Modified Bilosomes Nanogel Bearing a Natural Plant Alkaloid for Safe Management of Rheumatoid Arthritis Inflammation. Pharmaceutics 2022, 14, 563. [Google Scholar] [CrossRef]
- Waglewska, E.; Pucek-Kaczmarek, A.; Bazylińska, U. Novel Surface-Modified Bilosomes as Functional and Biocompatible Nanocarriers of Hybrid Compounds. Nanomaterials 2020, 10, 2472. [Google Scholar] [CrossRef]
- Al-Mahallawi, A.M.; Abdelbary, A.A.; Aburahma, M.H. Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam. Int. J. Pharm. 2015, 485, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elbary, A.; Makky, A.M.; Tadros, M.I.; Alaa-Eldin, A.A. Laminated sponges as challenging solid hydrophilic matrices for the buccal delivery of carvedilol microemulsion systems: Development and proof of concept via mucoadhesion and pharmacokinetic assessments in healthy human volunteers. Eur. J. Pharm. Sci. 2016, 82, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Hoogstraate, J.A.J.; Wertz, P.W. Drug delivery via the buccal mucosa. Pharm. Sci. Technol. Today 1998, 1, 309–316. [Google Scholar] [CrossRef]
- Patel, V.F.; Liu, F.; Brown, M.B. Advances in oral transmucosal drug delivery. J. Control. Release 2011, 153, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Husseiny, R.A.; Abu Lila, A.S.; Abdallah, M.H.; El-ghamry, H.A. Fast disintegrating tablet of Valsartan for the treatment of pediatric hypertension: In vitro and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2018, 43, 194–200. [Google Scholar] [CrossRef]
- Kraisit, P.; Limmatvapirat, S.; Luangtana-Anan, M.; Sriamornsak, P. Buccal administration of mucoadhesive blend films saturated with propranolol loaded nanoparticles. Asian J. Pharm. Sci. 2018, 13, 34–43. [Google Scholar] [CrossRef]
- Portero, A.; Teijeiro-Osorio, D.; Alonso, M.J.; Remuñán-López, C. Development of chitosan sponges for buccal administration of insulin. Carbohydr. Polym. 2007, 68, 617–625. [Google Scholar] [CrossRef]
- Hazzah, H.A.; Farid, R.M.; Nasra, M.M.; El-Massik, M.A.; Abdallah, O.Y. Lyophilized sponges loaded with curcumin solid lipid nanoparticles for buccal delivery: Development and characterization. Int. J. Pharm. 2015, 492, 248–257. [Google Scholar] [CrossRef] [PubMed]
- El-Nabarawi, M.A.; Shamma, R.N.; Farouk, F.; Nasralla, S.M. Bilosomes as a novel carrier for the cutaneous delivery for dapsone as a potential treatment of acne: Preparation, characterization and in vivo skin deposition assay. J. Liposome. Res. 2020, 30, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vishwa, B.; Moin, A.; Gowda, D.V.; Rizvi, S.M.D.; Hegazy, W.A.H.; Abu Lila, A.S.; Khafagy, E.S.; Allam, A.N. Pulmonary Targeting of Inhalable Moxifloxacin Microspheres for Effective Management of Tuberculosis. Pharmaceutics 2021, 13, 79. [Google Scholar] [CrossRef]
- Abdallah, M.H.; Abu Lila, A.S.; Unissa, R.; Elsewedy, H.S.; Elghamry, H.A.; Soliman, M.S. Preparation, characterization and evaluation of anti-inflammatory and anti-nociceptive effects of brucine-loaded nanoemulgel. Colloids Surf. B Biointerfaces 2021, 205, 111868. [Google Scholar] [CrossRef]
- Kassem, M.A.; ElMeshad, A.N.; Fares, A.R. Lyophilized sustained release mucoadhesive chitosan sponges for buccal buspirone hydrochloride delivery: Formulation and in vitro evaluation. AAPS PharmSciTech 2015, 16, 537–547. [Google Scholar] [CrossRef]
- Ahmed, S.; Kassem, M.A.; Sayed, S. Bilosomes as Promising Nanovesicular Carriers for Improved Transdermal Delivery: Construction, in vitro Optimization, ex vivo Permeation and in vivo Evaluation. Int. J. Nanomed. 2020, 15, 9783–9798. [Google Scholar] [CrossRef]
- El Zaafarany, G.M.; Awad, G.A.; Holayel, S.M.; Mortada, N.D. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int. J. Pharm. 2010, 397, 164–172. [Google Scholar] [CrossRef]
- Abdelbary, A.A.; Abd-Elsalam, W.H.; Al-Mahallawi, A.M. Fabrication of novel ultradeformable bilosomes for enhanced ocular delivery of terconazole: In vitro characterization, ex vivo permeation and in vivo safety assessment. Int. J. Pharm. 2016, 513, 688–696. [Google Scholar] [CrossRef]
- Jain, S.; Harde, H.; Indulkar, A.; Agrawal, A.K. Improved stability and immunological potential of tetanus toxoid containing surface engineered bilosomes following oral administration. Nanomedicine 2014, 10, 431–440. [Google Scholar] [CrossRef]
- Qumbar, M.; Ameeduzzafar; Imam, S.S.; Ali, J.; Ahmad, J.; Ali, A. Formulation and optimization of lacidipine loaded niosomal gel for transdermal delivery: In-vitro characterization and in-vivo activity. Biomed. Pharmacother. 2017, 93, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Elkomy, M.H.; Eid, H.M.; Elmowafy, M.; Shalaby, K.; Zafar, A.; Abdelgawad, M.A.; Rateb, M.E.; Ali, M.R.A.; Alsalahat, I.; Abou-Taleb, H.A. Bilosomes as a promising nanoplatform for oral delivery of an alkaloid nutraceutical: Improved pharmacokinetic profile and snowballed hypoglycemic effect in diabetic rats. Drug Deliv. 2022, 29, 2694–2704. [Google Scholar] [CrossRef] [PubMed]
- Soliman, W.E.; Khan, S.; Rizvi, S.M.D.; Moin, A.; Elsewedy, H.S.; Abulila, A.S.; Shehata, T.M. Therapeutic Applications of Biostable Silver Nanoparticles Synthesized Using Peel Extract of Benincasa hispida: Antibacterial and Anticancer Activities. Nanomaterials 2020, 10, 1954. [Google Scholar] [CrossRef] [PubMed]
- Abou-Taleb, H.A.; Mustafa, W.W.; Makram, T.S.; Abdelaty, L.N.; Salem, H.; Abdelkader, H. Vardenafil Oral Dispersible Films (ODFs) with Advanced Dissolution, Palatability, and Bioavailability. Pharmaceutics 2022, 14, 517. [Google Scholar] [CrossRef]
- Aframian, D.J.; Davidowitz, T.; Benoliel, R. The distribution of oral mucosal pH values in healthy saliva secretors. Oral Dis. 2006, 12, 420–423. [Google Scholar] [CrossRef]
- Tayel, S.A.; El-Nabarawi, M.A.; Tadros, M.I.; Abd-Elsalam, W.H. Duodenum-triggered delivery of pravastatin sodium via enteric surface-coated nanovesicular spanlastic dispersions: Development, characterization and pharmacokinetic assessments. Int. J. Pharm. 2015, 483, 77–88. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Streisand, J.B. Oral mucosal drug delivery: Clinical pharmacokinetics and therapeutic applications. Clin. Pharm. 2002, 41, 661–680. [Google Scholar] [CrossRef]
- Friebe, A.; Sandner, P.; Schmidtko, A. cGMP: A unique 2nd messenger molecule—Recent developments in cGMP research and development. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 287–302. [Google Scholar] [CrossRef] [Green Version]
- Nakatsu, K.; Diamond, J. Role of cGMP in relaxation of vascular and other smooth muscle. Can. J. Physiol. Pharmacol. 1989, 67, 251–262. [Google Scholar] [CrossRef]
| Independent Variables | Level | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| X1: SPC molar concentration | 1 | 2 | 3 |
| X2: CHOL molar concentration | 0 | 10 | 30 |
| X3: SDC amount (mg) | 10 | 20 | 30 |
| Dependent variables | Desirability Constrains | ||
| Y1: Particle size (nm) | In range | ||
| Y2: Entrapment efficiency (%) | Maximize | ||
| Factors (Independent Variables) | Responses (Dependent Variables) | ||||
|---|---|---|---|---|---|
| X1: SPC (Molar Concentration) | X2: CHOL (Molar Concentration) | X3: SDC (mg) | Y1: Particle Size (nm) | Y2: Entrapment Efficiency (%) | |
| F1 | 2 | 30 | 10 | 221.1 ± 11.2 | 75.25 ± 6.9 |
| F2 | 3 | 10 | 10 | 241.3 ± 9.8 | 78.93 ± 5.1 |
| F3 | 2 | 10 | 20 | 198.1 ± 13.1 | 71.43 ± 5.8 |
| F4 | 1 | 30 | 20 | 194.6 ± 8.9 | 69.98 ± 4.7 |
| F5 | 1 | 0 | 20 | 165.8 ± 9.3 | 60.31 ± 4.1 |
| F6 | 3 | 0 | 20 | 218.5 ± 7.6 | 74.21 ± 6.8 |
| F7 | 2 | 0 | 30 | 199.6 ± 10.2 | 65.21 ± 5.9 |
| F8 | 2 | 10 | 20 | 195.6 ± 11.7 | 70.13 ± 6.3 |
| F9 | 3 | 30 | 20 | 296.1 ± 10.8 | 84.14 ± 7.5 |
| F10 | 2 | 0 | 10 | 178.5 ± 9.5 | 63.20 ± 4.2 |
| F11 | 2 | 10 | 20 | 199.5 ± 8.4 | 70.98 ± 6.3 |
| F12 | 1 | 10 | 10 | 178.4 ± 7.9 | 66.13 ± 4.9 |
| F13 | 2 | 30 | 30 | 247.6 ± 7.1 | 75.41 ± 6.4 |
| F14 | 3 | 10 | 30 | 276.2 + 11.2 | 81.51 ± 6.8 |
| F15 | 1 | 10 | 30 | 201.4 ± 8.3 | 62.45 ± 3.9 |
| Permeation Parameters | VDF Gel | VDF-Loaded Bilosomal Sponge |
|---|---|---|
| Total amount of drug permeated (μg/cm2) | 346.67 ± 14.2 | 100.13 ± 9.9 |
| Jmax (μg/cm2/h) | 28.88 ± 3.1 | 8.34 ± 1.1 |
| ER | 1 | 3.46 |
| Parameter | VDF Oral Suspension | VDF-Loaded Bilosomal Buccal Sponge |
|---|---|---|
| Cmax (μg/mL) | 1.2 ± 0.18 | 3.3 ± 0.21 |
| Tmax (h) | 2 ± 0.26 | 3 ± 0.31 |
| T1/2 (h) | 2.52 ± 0.37 | 5.98 ± 0.29 |
| Ke (h−1) | 0.275 ± 0.06 | 0.116 ± 0.04 |
| AUC0-t (μg.h/mL) | 4.41 ± 0.65 | 22.44 ± 1.35 |
| MRT (h) | 4.17 ± 0.51 | 10.03 ± 0.82 |
| Relative bioavailability (%) | - | 508.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldawsari, M.F.; Khafagy, E.-S.; Alotaibi, H.F.; Abu Lila, A.S. Vardenafil-Loaded Bilosomal Mucoadhesive Sponge for Buccal Delivery: Optimization, Characterization, and In Vivo Evaluation. Polymers 2022, 14, 4184. https://doi.org/10.3390/polym14194184
Aldawsari MF, Khafagy E-S, Alotaibi HF, Abu Lila AS. Vardenafil-Loaded Bilosomal Mucoadhesive Sponge for Buccal Delivery: Optimization, Characterization, and In Vivo Evaluation. Polymers. 2022; 14(19):4184. https://doi.org/10.3390/polym14194184
Chicago/Turabian StyleAldawsari, Mohammed F., El-Sayed Khafagy, Hadil Faris Alotaibi, and Amr Selim Abu Lila. 2022. "Vardenafil-Loaded Bilosomal Mucoadhesive Sponge for Buccal Delivery: Optimization, Characterization, and In Vivo Evaluation" Polymers 14, no. 19: 4184. https://doi.org/10.3390/polym14194184
APA StyleAldawsari, M. F., Khafagy, E.-S., Alotaibi, H. F., & Abu Lila, A. S. (2022). Vardenafil-Loaded Bilosomal Mucoadhesive Sponge for Buccal Delivery: Optimization, Characterization, and In Vivo Evaluation. Polymers, 14(19), 4184. https://doi.org/10.3390/polym14194184








