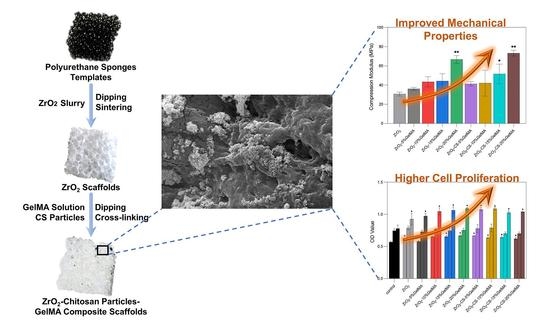
Preparation and Properties of Partial-Degradable ZrO2–Chitosan Particles–GelMA Composite Scaffolds
Abstract
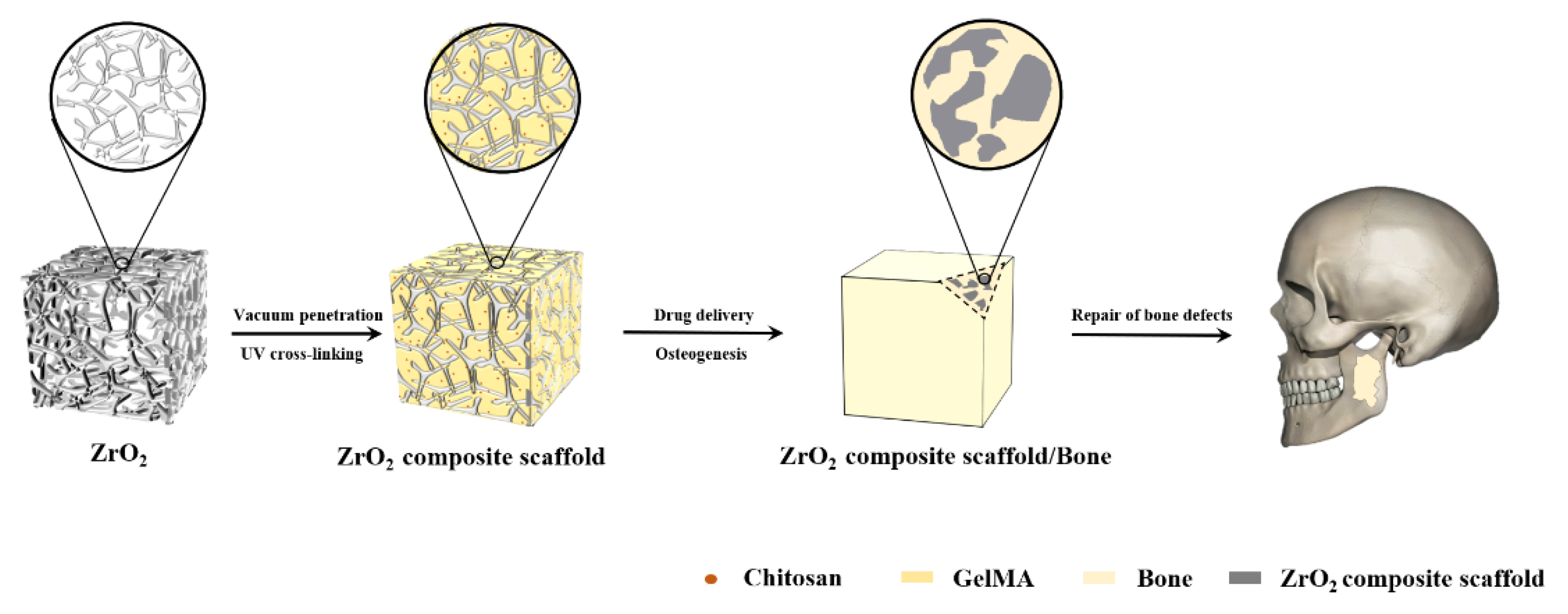
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Porous Zirconia Matrixes
2.2.2. Preparation of Chitosan Particles
2.2.3. Synthesis of GelMA Prepolymer
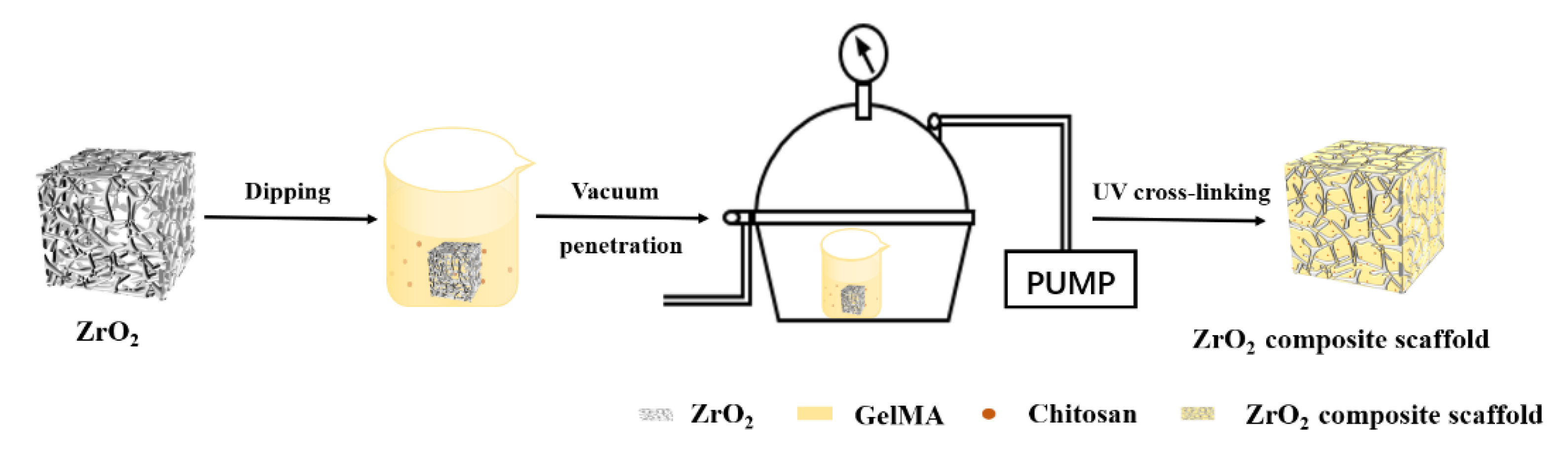
2.2.4. Preparation of Porous Zirconia–Chitosan Particles–Composite Scaffolds
2.2.5. Preparation of Porous Zirconia–Chitosan Particles–GelMA Hydrogel Composite Scaffolds
2.3. Characterizations
3. Results
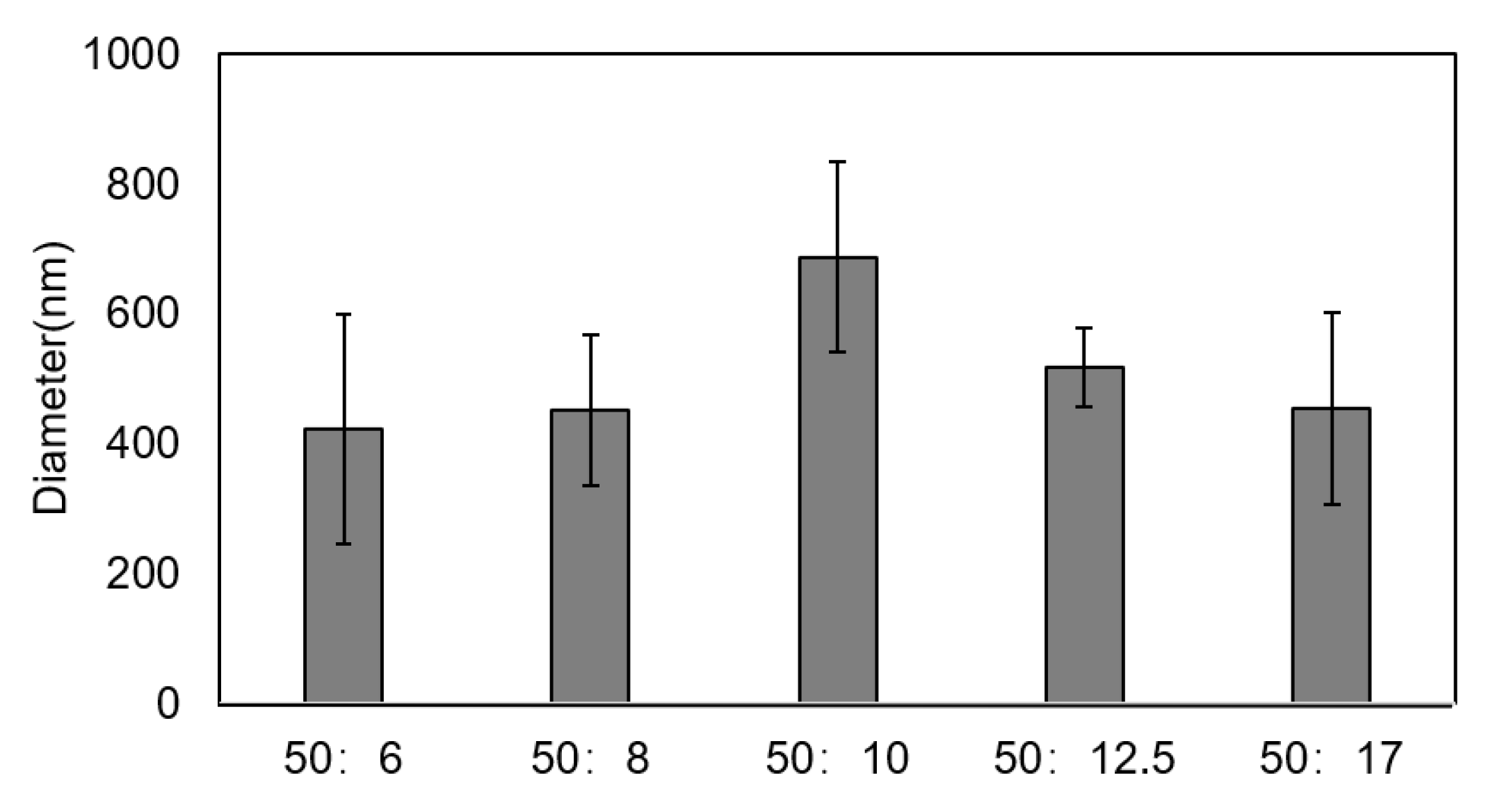
3.1. Size of Chitosan Particles
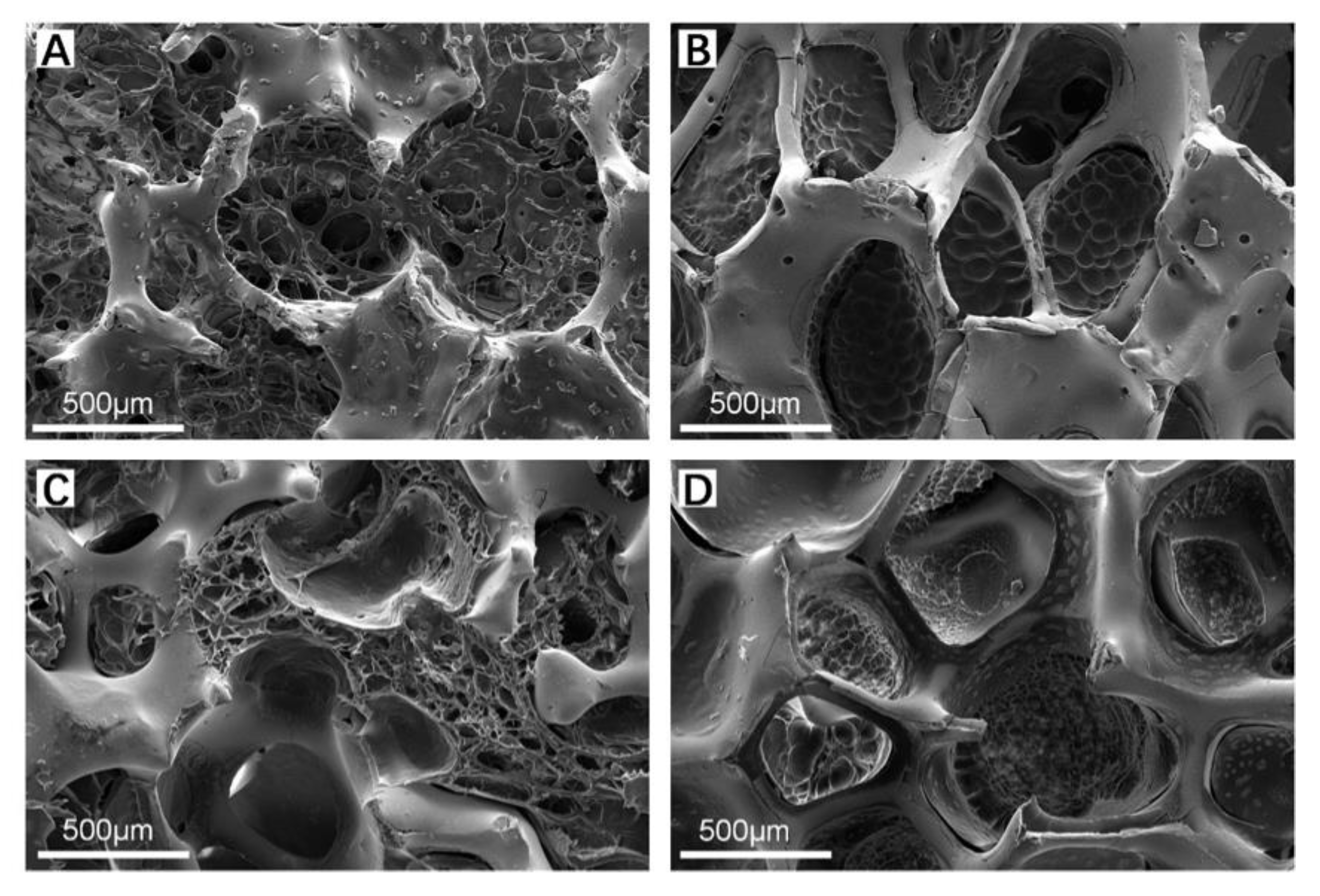
3.2. Morphologies and Structures of Zirconia-Based Composite Scaffolds
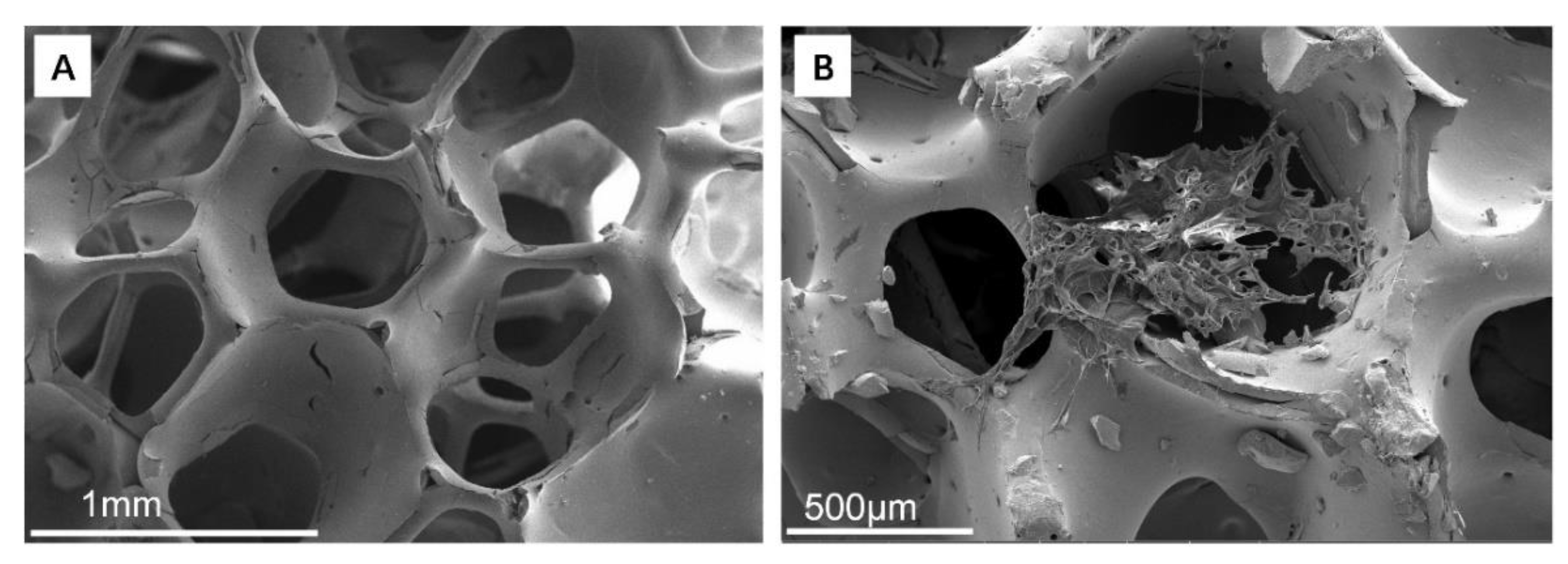
3.2.1. ZrO2 Matrix and ZrO2–Chitosan Composite Scaffold
3.2.2. ZrO2–GelMA Composite Scaffolds
3.2.3. ZrO2–Chitosan Particles–GelMA Composite Scaffolds
3.3. Swelling Properties of Composite Scaffolds
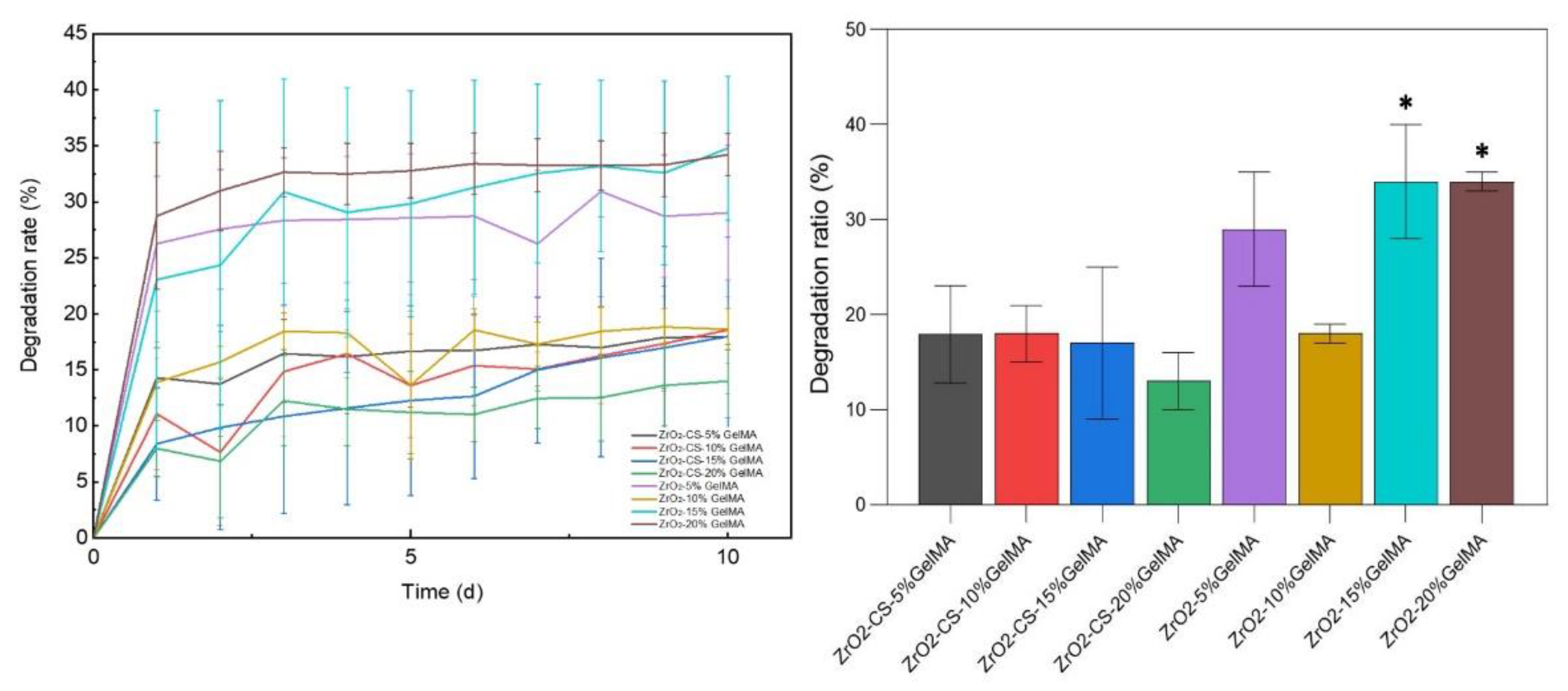
3.4. Degradation Properties of Zirconia Composite Scaffolds
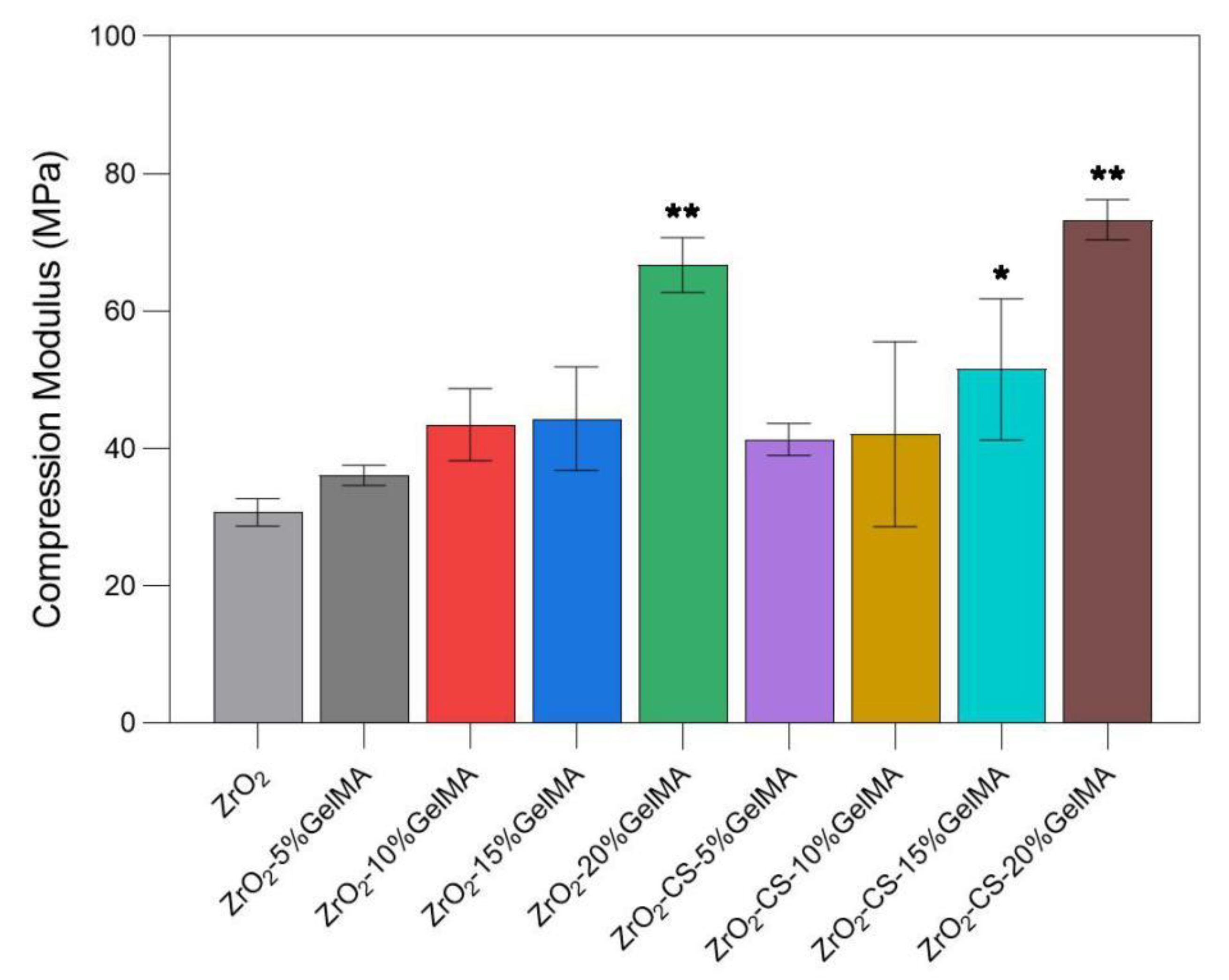
3.5. Mechanical Properties of Zirconia Composite Scaffolds
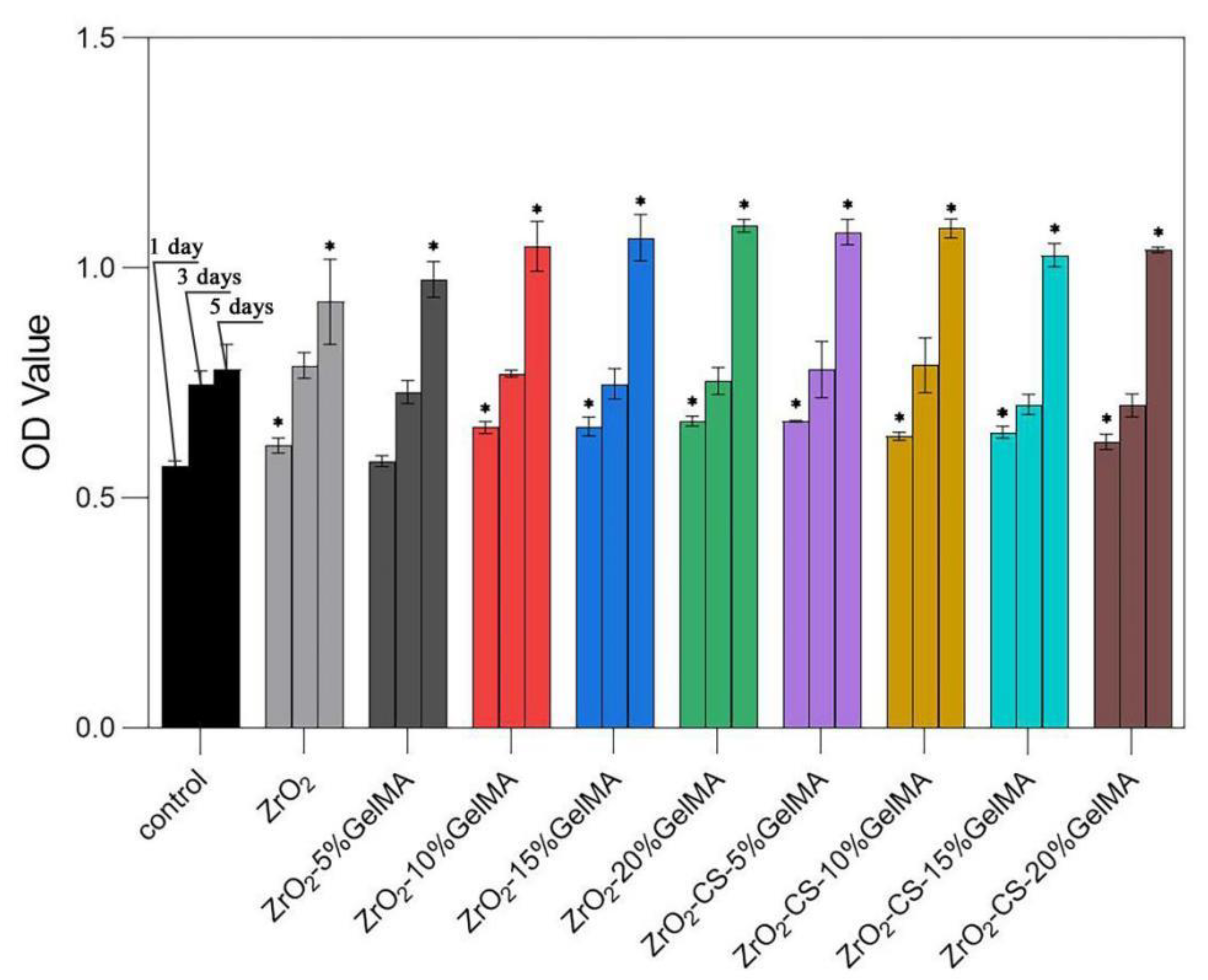
3.6. Cell Proliferation of Zirconia Composite Scaffolds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xue, N.; Ding, X.; Huang, R.; Jiang, R.; Huang, H.; Pan, X.; Min, W.; Chen, J.; Duan, J.-A.; Liu, P.; et al. Bone Tissue Engineering in the Treatment of Bone Defects. Pharmaceuticals 2022, 15, 879. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Fang, W.; Wu, G.; Li, Y.; Pathak, J.L.; Liu, Y. Low Dose BMP2-Doped Calcium Phosphate Graft Promotes Bone Defect Healing in a Large Animal Model. Front. Cell Dev. Biol. 2021, 8, 613891. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, S.-K.; Li, L.; Qin, L.; Wang, X.-L.; Lai, Y.-X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Transl. 2015, 3, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhu, T.; Li, J.; Cui, L.; Zhang, Z.; Zhuang, X.; Ding, J. Poly(lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact. Mater. 2021, 6, 346–360. [Google Scholar] [CrossRef]
- Ibrahim, M.Z.; Sarhan, A.A.D.; Yusuf, F.; Hamdi, M. Biomedical materials and techniques to improve the tribological, mechanical and biomedical properties of orthopedic implants—A review article. J. Alloys Compd. 2017, 714, 636–667. [Google Scholar] [CrossRef]
- Weng, W.; Wu, W.; Hou, M.; Liu, T.; Wang, T.; Yang, H. Review of zirconia-based biomimetic scaffolds for bone tissue engineering. J. Mater. Sci. 2021, 56, 8309–8333. [Google Scholar] [CrossRef]
- Yang, H.; Ji, Y. Low-temperature Degradation of Zirconia-based All-ceramic Crowns Materials: A Mini Review and Outlook. J. Mater. Sci. Technol. 2016, 32, 593–596. [Google Scholar] [CrossRef]
- Yoshimoto, H.; Shin, Y.M.; Terai, H.; Vacanti, J.P. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 2003, 24, 2077–2082. [Google Scholar] [CrossRef]
- Spasova, M.; Stoilova, O.; Manolova, N.; Rashkov, I. Electrospun PLLA/PEG scaffolds Materials resemble neural network. Mater. Today 2019, 28, 114–115. [Google Scholar] [CrossRef]
- Fiume, E.; Serino, G.; Bignardi, C.; Verne, E.; Baino, F. Bread-Derived Bioactive Porous Scaffolds: An Innovative and Sustainable Approach to Bone Tissue Engineering. Molecules 2019, 24, 2954. [Google Scholar] [CrossRef]
- Christ, B.; Glaubitt, W.; Berberich, K.; Weigel, T.; Probst, J.; Sextl, G.; Dembski, S. Sol-Gel-Derived Fibers Based on Amorphous alpha-Hydroxy-Carboxylate-Modified Titanium(IV) Oxide as a 3-Dimensional Scaffold. Materials 2022, 15, 2752. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-K.; Chen, P.-Y. Synthesis of silica-based scaffolds with high porosity and controllable microstructure by a sintering-free sol-gel/freeze-casting hybrid method under mild conditions. J. Mater. Res. Technol. 2020, 9, 16167–16178. [Google Scholar] [CrossRef]
- Kim, M.; Franco, R.A.; Lee, B.-T. Synthesis of functional gradient BCP/ZrO2 bone substitutes using ZrO2 and BCP nanopowders. J. Eur. Ceram. Soc. 2011, 31, 1541–1548. [Google Scholar] [CrossRef]
- Autissier, A.; Le Visage, C.; Pouzet, C.; Chaubet, F.; Letourneur, D. Fabrication of porous polysaccharide-based scaffolds using a combined freeze-drying/cross-linking process. Acta Biomater. 2010, 6, 3640–3648. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, S.; Rahul, S.H.; Balasubramanian, K. Inkjet printing yttria stabilized zirconia coatings on porous and nonporous substrates. Ceram. Int. 2020, 46, 3994–3999. [Google Scholar] [CrossRef]
- Zhang, B.; Cristescu, R.; Chrisey, D.B.; Narayan, R.J. Solvent-based Extrusion 3D Printing for the Fabrication of Tissue Engineering Scaffolds. Int. J. Bioprinting 2020, 6, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.J.; Choi, K.; Park, S.J.; Kim, Y.-J.; Chung, S.; Kim, C.-H. Suture Fiber Reinforcement of a 3D Printed Gelatin Scaffold for Its Potential Application in Soft Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 11600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, M.; Liu, T.; Hou, M.; Yang, H. Effect of Different Additives on the Mechanical Properties of Gelatin Methacryloyl Hydrogel: A Meta-analysis. Acs Omega 2021, 6, 9112–9128. [Google Scholar] [CrossRef]
- Jiang, T.; Zhao, J.; Yu, S.; Mao, Z.; Gao, C.; Zhu, Y.; Mao, C.; Zheng, L. Untangling the response of bone tumor cells and bone forming cells to matrix stiffness and adhesion ligand density by means of hydrogels. Biomaterials 2019, 188, 130–143. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Ying, G.-L.; Jiang, N.; Mahar, S.; Yin, Y.-X.; Chai, R.-R.; Cao, X.; Yang, J.-Z.; Miri, A.K.; Hassan, S.; Zhang, Y.S. Aqueous Two-Phase Emulsion Bioink-Enabled 3D Bioprinting of Porous Hydrogels. Adv. Mater. 2018, 30, 1805460. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef] [PubMed]
- Sharifianjazi, F.; Khaksar, S.; Esmaeilkhanian, A.; Bazli, L.; Eskandarinezhad, S.; Salahshour, P.; Sadeghi, F.; Rostamnia, S.; Vahdat, S.M. Advancements in Fabrication and Application of Chitosan Composites in Implants and Dentistry: A Review. Biomolecules 2022, 12, 155. [Google Scholar] [CrossRef]
- Castillo-Henriquez, L.; Sanabria-Espinoza, P.; Murillo-Castillo, B.; Montes de Oca-Vasquez, G.; Batista-Menezes, D.; Calvo-Guzman, B.; Ramirez-Arguedas, N.; Vega-Baudrit, J. Topical Chitosan-Based Thermo-Responsive Scaffold Provides Dexketoprofen Trometamol Controlled Release for 24 h Use. Pharmaceutics 2021, 13, 2100. [Google Scholar] [CrossRef]
- Cao, S.; Deng, Y.; Zhang, L.; Aleahmad, M. Chitosan nanoparticles, as biological macromolecule-based drug delivery systems to improve the healing potential of artificial neural guidance channels: A review. Int. J. Biol. Macromol. 2022, 201, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.R.; Chen, X.; Ismail, A.F.; Aziz, M.; Abdolahi, E.; Mahmoodiyan, F. Improved antibacterial properties of an Mg-Zn-Ca alloy coated with chitosan nanofibers incorporating silver sulfadiazine multiwall carbon nanotubes for bone implants. Polym. Adv. Technol. 2019, 30, 1333–1339. [Google Scholar] [CrossRef]
- Bohara, S.; Suthakorn, J. Surface coating of orthopedic implant to enhance the osseointegration and reduction of bacterial colonization: A review. Biomater. Res. 2022, 26, 26. [Google Scholar] [CrossRef]
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and Properties of Gelatin Methacryloyl (GelMA) Hydrogels and Their Recent Applications in Load-Bearing Tissue. Polymers 2018, 10, 1290. [Google Scholar] [CrossRef]
- Sun, M.; Sun, H.; Wang, Y.; Sanchez-Soto, M.; Schiraldi, D.A. The Relation between the Rheological Properties of Gels and the Mechanical Properties of Their Corresponding Aerogels. Gels 2018, 4, 33. [Google Scholar] [CrossRef]
- Modaresifar, K.; Hadjizadeh, A.; Niknejad, H. Design and fabrication of GelMA/chitosan nanoparticles composite hydrogel for angiogenic growth factor delivery. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1799–1808. [Google Scholar] [CrossRef]
- Suo, H.; Zhang, D.; Yin, J.; Qian, J.; Wu, Z.L.; Fu, J. Interpenetrating polymer network hydrogels composed of chitosan and photocrosslinkable gelatin with enhanced mechanical properties for tissue engineering. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018, 92, 612–620. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Hou, M.; Zhang, J.; Jin, M.; Wang, T.; Yang, H.; Zhang, X. Preparation and Properties of Partial-Degradable ZrO2–Chitosan Particles–GelMA Composite Scaffolds. Polymers 2022, 14, 4233. https://doi.org/10.3390/polym14194233
Ji Y, Hou M, Zhang J, Jin M, Wang T, Yang H, Zhang X. Preparation and Properties of Partial-Degradable ZrO2–Chitosan Particles–GelMA Composite Scaffolds. Polymers. 2022; 14(19):4233. https://doi.org/10.3390/polym14194233
Chicago/Turabian StyleJi, Yang, Mengdie Hou, Jin Zhang, Meiqi Jin, Tianlin Wang, Huazhe Yang, and Xiaodong Zhang. 2022. "Preparation and Properties of Partial-Degradable ZrO2–Chitosan Particles–GelMA Composite Scaffolds" Polymers 14, no. 19: 4233. https://doi.org/10.3390/polym14194233
APA StyleJi, Y., Hou, M., Zhang, J., Jin, M., Wang, T., Yang, H., & Zhang, X. (2022). Preparation and Properties of Partial-Degradable ZrO2–Chitosan Particles–GelMA Composite Scaffolds. Polymers, 14(19), 4233. https://doi.org/10.3390/polym14194233