Chitosan-Functionalized-Graphene Oxide (GO@CS) Beads as an Effective Adsorbent to Remove Cationic Dye from Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Characterization
2.3. Preparation of GO@CS Composite
2.4. Batch Adsorption
2.5. Kinetic Models
3. Results and Discussion
3.1. Characterization
3.1.1. SEM
3.1.2. FTIR
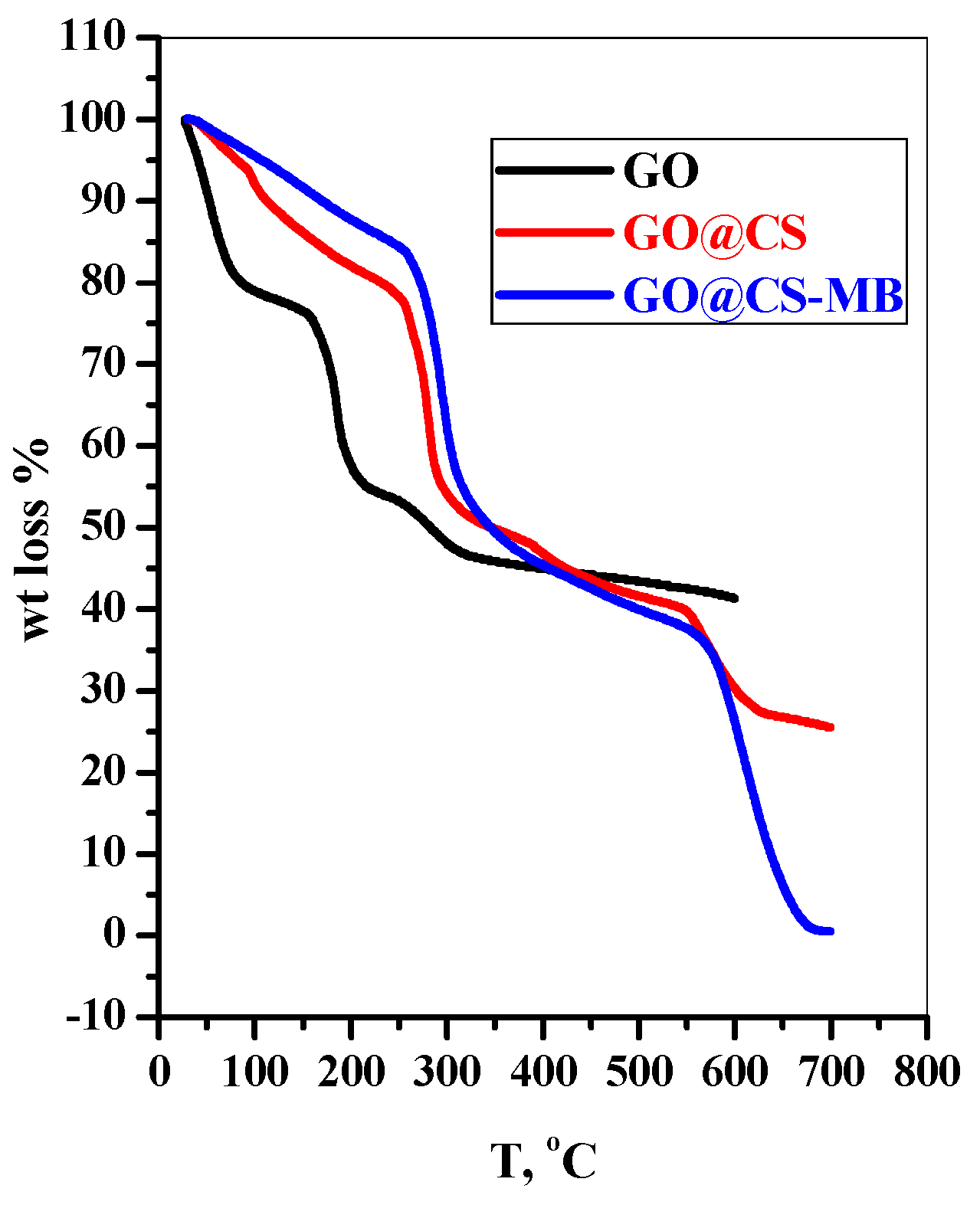
3.1.3. TGA
3.2. Adsorption Study
3.2.1. Effect of Contact Time
3.2.2. Effect of initial MB-Dye Concentrations
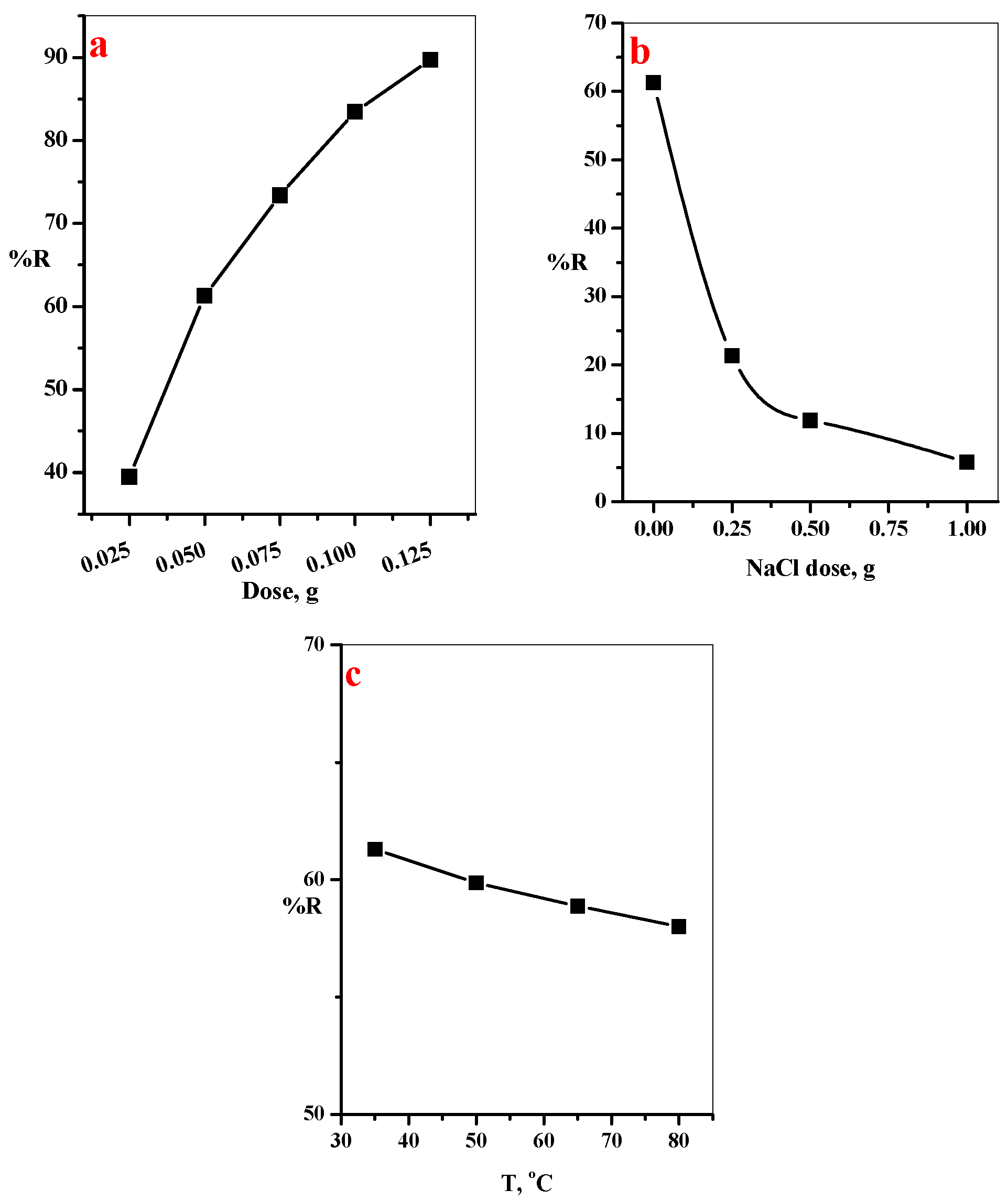
3.2.3. Effect of GO@CS Composite Beads Dose, NaCl Dose, and Temperature on %R of MB-Dye
3.2.4. Effect of pH on the Removal Percent of MB-Dye
3.3. Reusability Study
3.4. Application on the Real Dye Sample
3.5. Comparison Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khiam, G.K.; Karri, R.R.; Mubarak, N.M.; Khalid, M.; Walvekar, R.; Abdullah, E.C.; Rahman, M.E. Modelling and optimization for methylene blue adsorption using graphene oxide/chitosan composites via artificial neural network particle swarm optimization. Mater. Today Chem. 2022, 24, 100946. [Google Scholar] [CrossRef]
- Alharby, N.F.; Almutairi, R.S.; Mohamed, N.A. Adsorption Behavior of Methylene Blue Dye by Novel CrossLinked O-CMChitosan Hydrogel in Aqueous Solution: Kinetics, Isotherm and Thermodynamics. Polymers 2021, 13, 3659. [Google Scholar] [CrossRef]
- Sait, H.H.; Hussain, A.; Bassyouni, M.; Ali, I.; Kanthasamy, R.; Ayodele, B.V.; Elhenawy, Y. Anionic Dye Removal Using a Date Palm Seed-Derived Activated Carbon/Chitosan Polymer Microbead Biocomposite. Polymers 2022, 14, 2503. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.S.K.; Jiang, S.-J. Chitosan-functionalized graphene oxide: A novel adsorbent an efficient adsorption of arsenic from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 1698–1713. [Google Scholar] [CrossRef]
- Blachnio, M.; Budnyak, T.M.; Derylo-Marczewska, A.; Marczewski, A.W.; Tertykh, V.A. Chitosan-Silica Hybrid Composites for Removal of Sulfonated Azo Dyes from Aqueous Solutions. Langmuir 2018, 34, 2258–2273. [Google Scholar] [CrossRef]
- Sharma, J.; Kaith, B.S.; Sharma, A.K. Gum xanthan-psyllium-cl-poly (acrylic acid-co-itaconic acid) based adsorbent for e ff ective removal of cationic and anionic dyes: Adsorption isotherms, kinetics and thermodynamic studies. Ecotoxicol. Environ. Saf. 2018, 149, 150–158. [Google Scholar] [CrossRef]
- Ramakrishnan, R.K.; Padil, V.V.T.; Wacławek, S.; Černík, M.; Varma, R.S. Eco-Friendly and Economic, Adsorptive Removal of Cationic and Anionic Dyes by Bio-Based Karaya Gum—Chitosan Sponge. Polymers 2021, 13, 251. [Google Scholar] [CrossRef]
- Shen, X.; Huang, P.; Li, F.; Wang, X.; Yuan, T.; Sun, R. Compressive Alginate Sponge Derived from Seaweed Biomass Resources for Methylene Blue Removal from Wastewater. Polymers 2019, 11, 961. [Google Scholar] [CrossRef] [Green Version]
- Ghaedi, M.; Nazari, E.; Sahraie, R.; Purkait, M.K. Kinetic and isotherm study of Bromothymol Blue and Methylene blue removal using Au-NP loaded on activated carbon. Desalin. Water Treat. 2014, 52, 5504–5512. [Google Scholar] [CrossRef]
- Hoa, N.V.; Minh, N.C.; Cuong, H.N.; Dat, P.A.; Nam, P.V.; Viet, P.H.T.; Phuong, P.T.D.; Trung, T.S. Highly Porous Hydroxyapatite/Graphene Oxide/Chitosan Beads as an Efficient Adsorbent for Dyes and Heavy Metal Ions Removal. Molecules 2021, 26, 6127. [Google Scholar] [CrossRef]
- ALSamman, M.T.; Sánchez, J. Chitosan- and Alginate-Based Hydrogels for the Adsorption of Anionic and Cationic Dyes from Water. Polymers 2022, 14, 1498. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, X.; Zhu, J.; Bai, J.; Gao, L.; Liu, S.; Jiao, T. Facile preparation of self-assembled chitosan-based composite hydrogels with enhanced adsorption performances. Colloids Surf. A 2020, 598, 124860. [Google Scholar] [CrossRef]
- Biswas, S.; Rashid, T.U.; Debnath, T.; Haque, P.; Rahman, M.M. Application of Chitosan-Clay Biocomposite Beads for Removal of Heavy Metal and Dye from Industrial Effluent. J. Compos. Sci. 2020, 4, 16. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, G.; Li, Y. Preparation of Chitosan/Polyacrylamide/Graphene Oxide Composite Membranes and Study of Their Methylene Blue Adsorption Properties. Materials 2020, 13, 4407. [Google Scholar] [CrossRef]
- Marotta, A.; Luzzi, E.; Salzano de Luna, M.; Aprea, P.; Ambrogi, V.; Filippone, G. Chitosan/Zeolite Composite Aerogels for a Fast and Effective Removal of Both Anionic and Cationic Dyes from Water. Polymers 2021, 13, 1691. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, A.A.; Alorabi, A.Q.; Hassan, M.S.; Amna, T.; Azizi, M. Chitosan-Functionalized Hydroxyapatite-Cerium Oxide Heterostructure: An Efficient Adsorbent for Dyes Removal and Antimicrobial Agent. Nanomaterials 2022, 12, 2713. [Google Scholar] [CrossRef]
- Dindorkar, S.S.; Patel, R.V.; Yadav, A. Adsorptive removal of methylene blue dye from aqueous streams using photocatalytic CuBTC/ZnO chitosan composites. Water Sci. Technol. 2022, 85, 2748–2760. [Google Scholar] [CrossRef]
- Siregar, P.M.S.B.N.; Lesbani, A.; Mohadi, R. Mg/Al-chitosan as a Selective Adsorbent in The Removal of Methylene Blue from Aqueous Solutions. Sci. Technol. Indones. 2022, 7, 170–178. [Google Scholar] [CrossRef]
- Shi, Y.; Song, G.; Li, A.; Wang, J.; Wang, H.; Sun, Y.; Ding, G. Graphene oxide-chitosan composite aerogel for adsorption of methyl orange and methylene blue: Effect of pH in single and binary systems. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128595. [Google Scholar] [CrossRef]
- Sabzevari, M.; Cree, D.E.; Wilson, L.D. Graphene Oxide–Chitosan Composite Material for Treatment of a Model Dye Effluent. ACS Omega 2018, 3, 13045–13054. [Google Scholar] [CrossRef]
- Yan, M.; Huang, W.; Li, Z. Chitosan cross-linked graphene oxide/lignosulfonate composite aerogel for enhanced adsorption of methylene blue in water. Int. J. Biol. Macromol. 2019, 136, 927–935. [Google Scholar] [CrossRef]
- Abd-Elhamid, A.I.; Aly, H.F.; Soliman, H.A.M.; Shanshory, A.A. Graphene oxide: Follow the oxidation mechanism and its application in water treatment. J. Mol. Liq. 2018, 265, 226–237. [Google Scholar] [CrossRef]
- Fernandes Queiroz, M.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, K.D. Bio-adsorption of methylene blue dye using chitosan-extracted from Fenneropenaeus indicus shrimp shell waste. J. Aquac. Mar. Biol. 2021, 10, 146–150. [Google Scholar] [CrossRef]
- Nath, J.; Chowdhury, A.; Dolui, S.K. Chitosan/graphene oxide-based multifunctional pH-responsive hydrogel with significant mechanical strength, self-healing property, and shape memory effect. Adv. Polym. Technol. 2018, 37, 3665–3679. [Google Scholar] [CrossRef] [Green Version]
- Solano, M.A.; Galan, J.; Vallejo, W.; Arana, V.A.; Grande-Tovar, C.D. Chitosan Beads Incorporated with Graphene Oxide/Titanium Dioxide Nanoparticles for Removing an Anionic Dye. Appl. Sci. 2021, 11, 9439. [Google Scholar] [CrossRef]
- Guo, X.; Qu, L.; Tian, M.; Zhu, S.; Zhang, X.; Tang, X.; Sun, K. Chitosan/graphene oxide composite as an effective adsorbent for reactive red dye removal. Water Environ. Res. 2016, 88, 579–588. [Google Scholar] [CrossRef]
- Martis, L.J.; Parushuram, N.; Sangappa, Y. Preparation, characterization, and methylene blue dye adsorption study of silk fibroin–graphene oxide nanocomposites. Environ. Sci. Adv. 2022, 1, 285. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Z.; Wang, M.; Liu, S.; Zhang, J.; Zhang, J.; Han, R.; Xu, Q. Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): Kinetics, isotherm, thermodynamics and mechanism analysis. Chem. Eng. J. 2015, 259, 53–61. [Google Scholar] [CrossRef]
- Munir, M.; Nazar, M.F.; Zafar, M.N.; Zubair, M.; Ashfaq, M.; Hosseini-Bandegharaei, A.; Khan, S.U.-D.; Ahmad, A. Effective Adsorptive Removal of Methylene Blue from Water by Didodecyldimethylammonium Bromide-Modified Brown Clay. ACS Omega 2020, 5, 16711–16721. [Google Scholar] [CrossRef]
- Abd-Elhamid, A.I.; Doma, A.S.; El-syed, A.M.; Kenawy, E.-R. Eco-friendly activation of charcoal for purification of water from colored organic pollutants. Res. J. Chem. Environ. 2019, 23, 83–95. [Google Scholar]
- Li, Y.; Du, Q.; Liu, T.; Peng, X.; Wang, J.; Sun, J.; Wang, Y.; Wu, S.; Wang, Z.; Xia, Y.; et al. Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide and carbon nanotubes. Chem. Eng. Res. Des. 2013, 91, 361–368. [Google Scholar] [CrossRef]
- Salisu, A.; Sanagi, M.M.; Naim, A.A.; Karim, K.J. Removal of methylene blue dye from aqueous solution using alginate grafted polyacrylonitrile beads. Der Pharma Chem. 2015, 7, 237–242. [Google Scholar]
- Mustafa, I. Methylene blue removal from water using H2SO4 cross-linked magnetic chitosan nanocomposite beads. Microchem. J. 2019, 144, 397–402. [Google Scholar] [CrossRef]
- Liu, C.; Omer, A.; Ouyang, X. Adsorptive removal of cationic methylene blue dye using carboxymethyl cellulose/k-carrageenan/activated montmorillonite composite beads: Isotherm and kinetic studies. Int. J. Biol. Macromol. 2018, 106, 823–833. [Google Scholar] [CrossRef]
- Ruthiraan, M.; Abdullah, E.C.; Mubarak, N.M.; Nizamuddin, S. Adsorptive Removal of Methylene Blue Using Magnetic Biochar Derived from Agricultural Waste Biomass: Equilibrium, Isotherm, Kinetic Study. Int. J. Nanosci. 2018, 17, 1850002. [Google Scholar] [CrossRef] [Green Version]
- Al-Fawwaz, A.T.; Abdullah, M. Decolorization of Methylene Blue and Malachite Green by Immobilized Desmodesmus sp. Isolated from North Jordan. Int. J. Environ. Sci. Dev. 2016, 7, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Salisu, A.; Sanagi, M.M.; Karim, K.J.A.; Pourmand, N.; Ibrahim, W.A.W. Adsorption of Methylene Blue on Alginate-Grafted-Poly (Methyl Methacrylate). J. Teknol. 2015, 76, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Yu, Q.; He, Y.; Bai, J.; Jiao, T.; Zhang, L.; Bai, Z.; Zhou, J.; Peng, Q. Selfassembled polyelectrolyte-based composite hydrogels with enhanced stretchable and adsorption performances. J. Mol. Liq. 2019, 294, 111576. [Google Scholar] [CrossRef]
- Liu, L.; Sun, R.; Feng, S.; Wang, D.; Liu, H. Rapid synthesis of a silsesquioxane based disulfide-linked polymer for selective removal of cationic dyes from aqueous solutions. Chem. Eng. J. 2019, 359, 436–445. [Google Scholar] [CrossRef]
- Md Nurus, S.; Nanami, H.; Makoto, T.; Shoeb, A. Preparation of chitosan/laterite/iron oxide-based biocomposite and its application as a potential adsorbent for the removal of methylene blue from aqueous solution. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100658. [Google Scholar] [CrossRef]
- Popoola, L.T. Characterization and adsorptive behaviour of snail shell-rice husk (SS-RH) calcined particles (CPs) towards cationic dye. Heliyon 2019, 5, e01153. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]









| Dye | qe exp (mg/g) | Pseudo-First-Order Kinetic Parameter | Pseudo-Second-Order Kinetic Parameter | ||||
|---|---|---|---|---|---|---|---|
| K1 (min−1) | qe cal (mg/g) | R2 | K2 (g mg−1 min−1) | qe cal (mg/g) | R2 | ||
| MB | 9.99 | −0.06 | 9.46 | 0.990 | 0.008 | 11.23 | 0.992 |
| Dye | Langmuir Isotherm Model | Freundlich Isotherm Model | |||||
|---|---|---|---|---|---|---|---|
| qe (mg/g) | b (L/mg) | RL | R2 | n | Kf (mg/g) | R2 | |
| MB | 23.26 | 0.14 | 0.42 | 0.950 | 2.06 | 4.45 | 0.868 |
| Adsorbent | Adsorption Capacity (mg/g) | Reference |
|---|---|---|
| GO-CS composite | 7.53 | [1] |
| CS-GO hydrogel | 14.31 | [12] |
| CS-CNT hydrogel | 21.74 | [12] |
| Chitosan-Clay Biocomposite Beads | 2.385 | [13] |
| alginate graft-polyacrylonitrile beads | 3.79 | [33] |
| H2SO4 crosslinked magnetic chitosan nanocomposite beads | 20.40 | [34] |
| (CMC)/k-carrageenan (kC)/activated montmorillonite (AMMT) beads | 12.5 | [35] |
| Magnetic Biochar | 22.88 | [36] |
| Desmodesmus sp. Immobilized Alginate beads | 20 | [37] |
| Alginate-grafted-poly (methyl Methacrylate) | 5.25 | [38] |
| P-N-LDHs hydrogel | 6.03 | [39] |
| Silsesquioxane-based disulfide-linked polymer (DLP) | 12.90 | [40] |
| Chitosan/laterite/iron oxide | 16 | [41] |
| GO@CS beads | 23.26 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nayl, A.A.; Abd-Elhamid, A.I.; Arafa, W.A.A.; Ahmed, I.M.; El-Shanshory, A.A.; Abu-Saied, M.A.; Soliman, H.M.A.; Abdelgawad, M.A.; Ali, H.M.; Bräse, S. Chitosan-Functionalized-Graphene Oxide (GO@CS) Beads as an Effective Adsorbent to Remove Cationic Dye from Wastewater. Polymers 2022, 14, 4236. https://doi.org/10.3390/polym14194236
Nayl AA, Abd-Elhamid AI, Arafa WAA, Ahmed IM, El-Shanshory AA, Abu-Saied MA, Soliman HMA, Abdelgawad MA, Ali HM, Bräse S. Chitosan-Functionalized-Graphene Oxide (GO@CS) Beads as an Effective Adsorbent to Remove Cationic Dye from Wastewater. Polymers. 2022; 14(19):4236. https://doi.org/10.3390/polym14194236
Chicago/Turabian StyleNayl, AbdElAziz A., Ahmed I. Abd-Elhamid, Wael A. A. Arafa, Ismail M. Ahmed, Ahmed A. El-Shanshory, Mohamed A. Abu-Saied, Hesham M. A. Soliman, Mohamed A. Abdelgawad, Hazim M. Ali, and Stefan Bräse. 2022. "Chitosan-Functionalized-Graphene Oxide (GO@CS) Beads as an Effective Adsorbent to Remove Cationic Dye from Wastewater" Polymers 14, no. 19: 4236. https://doi.org/10.3390/polym14194236








