Electro Fluid Dynamics: A Route to Design Polymers and Composites for Biomedical and Bio-Sustainable Applications
Abstract
1. Introduction
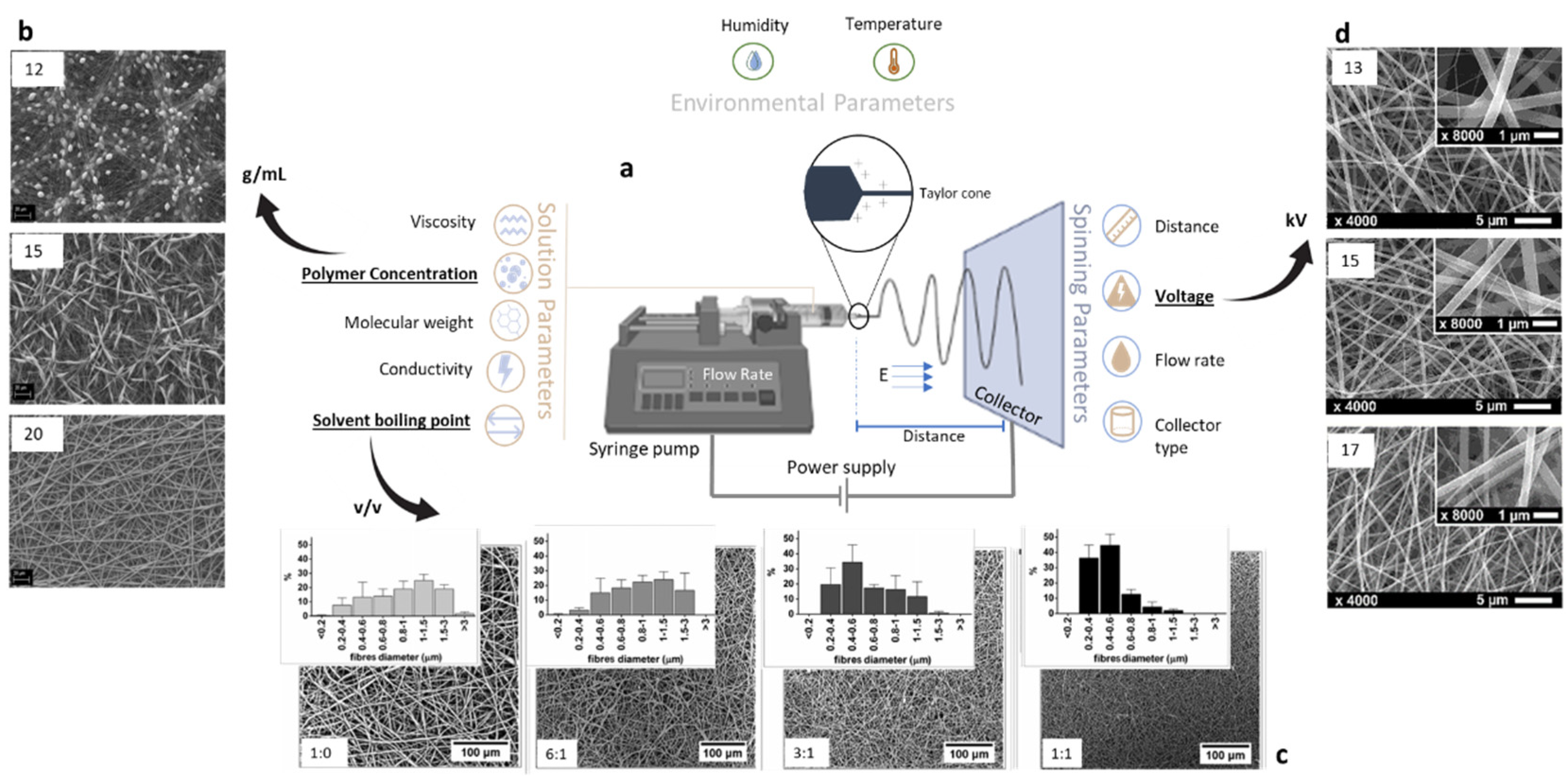
2. Electro Fluid Dynamics: Classification and Working Principles
3. Melt Polymers vs. Polymer Solution
3.1. Fibers from Melt Polymers
3.2. Fibers from Polymer Solution
4. Blended and Composite Fibers towards Bio-Sustainability
4.1. Biomedical Applications
4.2. Environmental Applications: Contaminants Adsorption in Water and Their Sensing
5. Monocomponent/Multicomponent Particles
5.1. Biomedical Applications
5.2. Other Green Applications
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, Z.; Huang, S. Functional Dynamics Inside Nano- or Microscale Bio-Hybrid Systems. Front. Chem. 2018, 6, 621. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Hosseinkhani, H.; Hosseinkhani, M.; Khademhosseini, A.; Yokoyama, Y.; Estrada, G.G.; Kobayashi, H. Quantitative analysis of cell adhesion on aligned micro- and nanofibers. J. Biomed. Mater. Res. A 2008, 84, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.; Hsiao, B.S.; Chu, B. Functional nanofibers for environmental applications. J. Mater. Chem. 2008, 18, 5326–5334. [Google Scholar] [CrossRef]
- Kim, H.N.; Jiao, A.; Hwang, N.S.; Kim, M.S.; Kim, D.H.; Suh, K.Y. Nanotopography-guided tissue engineering and regenerative medicine. Adv. Drug Deliv. Rev. 2013, 65, 536–558. [Google Scholar] [CrossRef] [PubMed]
- Alghoraibi, I.; Alomari, S. Different Methods for Nanofiber Design and Fabrication. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–46. [Google Scholar]
- Guarino, V.; Ambrosio, L. Electrofluidodynamics: Exploring a new toolbox to design biomaterials for tissue regeneration and degeneration. Nanomedicine 2016, 11, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Haider, S. Electrohydrodynamic Processes and Their Affecting Parameters; Haider, A., Ed.; IntechOpen: Rijeka, Croatia, 2019; Chapter 1. [Google Scholar]
- Guarino, V.; Cirillo, V.; Altobelli, R.; Ambrosio, L. Polymer-based platforms by electric field-assisted techniques for tissue engineering and cancer therapy. Expert Rev. Med. Devices 2015, 12, 113–129. [Google Scholar] [CrossRef]
- Aggarwal, A.; Sah, M.K. Chapter Three—Electrospun materials as scaffolds in tissue engineering and regenerative medicine. In Biomedical Applications of Electrospinning and Electrospraying; Woodhead Publishing Series in Biomaterials; Kasoju, N., Ye, H., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 83–121. [Google Scholar]
- Del Bakhshayesh, A.R.; Babaie, S.; Niknafs, B.; Abedelahi, A.; Mehdipour, A.; Ghahremani-Nasab, M. High efficiency biomimetic electrospun fibers for use in regenerative medicine and drug delivery: A review. Mater. Chem. Phys. 2022, 279, 125785. [Google Scholar] [CrossRef]
- Valachová, K.; el Meligy, M.A.; Šoltés, L. Hyaluronic acid and chitosan-based electrospun wound dressings: Problems and solutions. Int. J. Biol. Macromol. 2022, 206, 74–91. [Google Scholar] [CrossRef]
- Majumder, S.; Sagor, M.M.H.; Arafat, M.T. Functional electrospun polymeric materials for bioelectronic devices: A review. Mater. Adv. 2022, 3, 6753–6772. [Google Scholar] [CrossRef]
- Deng, Y.; Lu, T.; Cui, J.; Samal, S.K.; Xiong, R.; Huang, C. Bio-based electrospun nanofiber as building blocks for a novel eco-friendly air filtration membrane: A review. Sep. Purif. Technol. 2021, 277, 119623. [Google Scholar] [CrossRef]
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial Upscaling of Electrospinning and Applications of Polymer Nanofibers: A Review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- International Council for Harmonisation. Impurities: Guideline for Residual Solvents Q3C (R7); International Council for Harmonisation: Geneva, Switzerland, 2018. [Google Scholar]
- Onoe, H.; Takeuchi, S. Cell-laden microfibers for bottom-up tissue engineering. Drug Discov. Today 2015, 20, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Ambhorkar, P.; Rakin, R.H.; Wang, Z.; Kumar, H.; Kim, K. Biofabrication strategies for engineering heterogeneous artificial tissues. Addit. Manuf. 2020, 36, 101459. [Google Scholar] [CrossRef]
- Nie, M.; Takeuchi, S. Bottom-up biofabrication using microfluidic techniques. Biofabrication 2018, 10, 44103. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S.L.; Bates, W.D.; Frisch, H.L.; Wnek, G.E. Role of chain entanglements on fiber formation during electrospinning of polymer solutions: Good solvent, non-specific polymer–polymer interaction limit. Polymer 2005, 46, 3372–3384. [Google Scholar] [CrossRef]
- Hao, M.F.; Liu, Y.; He, X.T.; Ding, Y.M.; Yang, W.M. Factors Influencing Diameter of Polypropylene Fiber in Melt Electrospinning. Adv. Mater. Res. 2011, 221, 129–134. [Google Scholar] [CrossRef]
- Bambole, V.; Yakhmi, J.V. Chapter 14—Tissue engineering: Use of electrospinning technique for recreating physiological functions. In Nanobiomaterials in Soft Tissue Engineering; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 387–455. [Google Scholar]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Şener, A.G.; Altay, A.S.; Altay, F. Effect of voltage on morphology of electrospun nanofibers. In Proceedings of the 2011 7th International Conference on Electrical and Electronics Engineering (ELECO), Bursa, Turkey, 1–4 December 2011; pp. 324–328. [Google Scholar]
- Gade, H.; Nikam, S.; Chase, G.G.; Reneker, D.H. Effect of electrospinning conditions on β-phase and surface charge potential of PVDF fibers. Polymer 2021, 228, 123902. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Tan, N.C.B. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Bagbi, Y.; Pandey, A.; Solanki, P.R. Chapter 10—Electrospun Nanofibrous Filtration Membranes for Heavy Metals and Dye Removal. In Nanoscale Materials in Water Purification; Micro and Nano Technologies; Thomas, S., Pasquini, D., Leu, S.-Y., Gopakumar, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 275–288. [Google Scholar]
- Edikresnha, D.; Suciati, T.; Khairurrijal, K. Preliminary study of composite fibers polyvinylpyrrolidone/cellulose acetate loaded by garlic extract by means of electrospinning method. Mater. Today Proc. 2021, 44, A1–A4. [Google Scholar] [CrossRef]
- Unnithan, A.R.; Arathyram, R.S.; Kim, C.S. Chapter 3—Electrospinning of Polymers for Tissue Engineering. In Nanotechnology Applications for Tissue Engineering; Thomas, S., Grohens, Y., Ninan, N., Eds.; William Andrew Publishing: Oxford, UK, 2015; pp. 45–55. [Google Scholar]
- Liverani, L.; Boccaccini, A.R. Versatile Production of Poly(Epsilon-Caprolactone) Fibers by Electrospinning Using Benign Solvents. Nanomaterials 2016, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Van der Schueren, L.; de Schoenmaker, B.; Kalaoglu, Ö.I.; de Clerck, K. An alternative solvent system for the steady state electrospinning of polycaprolactone. Eur. Polym. J. 2011, 47, 1256–1263. [Google Scholar] [CrossRef]
- Pires, L.R.; Guarino, V.; Oliveira, M.J.; Ribeiro, C.C.; Barbosa, M.A.; Ambrosio, L.; Pêgo, A.P. Ibuprofen-loaded poly(trimethylene carbonate-co-ε-caprolactone) electrospun fibres for nerve regeneration. J. Tissue Eng. Regen. Med. 2016, 10, E154–E166. [Google Scholar] [CrossRef] [PubMed]
- Altobelli, R.; Guarino, V.; Ambrosio, L. Micro- and nanocarriers by electrofludodynamic technologies for cell and molecular therapies. Process Biochem. 2016, 51, 2143–2154. [Google Scholar] [CrossRef]
- Jaworek, A. Micro- and nanoparticle production by electrospraying. Powder Technol. 2007, 176, 18–35. [Google Scholar] [CrossRef]
- Guarino, V.; Khodir, W.K.W.A.; Ambrosio, L. Biodegradable microparticles and nanoparticles by electrospraying techniques. J. Appl. Biomater. Funct. Mater. 2012, 10, 191–196. [Google Scholar] [CrossRef]
- Smeets, A.; Clasen, C.; van den Mooter, G. Electrospraying of polymer solutions: Study of formulation and process parameters. Eur. J. Pharm. Biopharm. 2017, 119, 114–124. [Google Scholar] [CrossRef]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef]
- Guarino, V.; Altobelli, R.; Cirillo, V.; Cummaro, A.; Ambrosio, L. Additive electrospraying: A route to process electrospun scaffolds for controlled molecular release. Polym. Adv. Technol. 2015, 26, 1359–1369. [Google Scholar] [CrossRef]
- Enayati, M.; Chang, M.W.; Bragman, F.; Edirisinghe, M.; Stride, E. Electrohydrodynamic preparation of particles, capsules and bubbles for biomedical engineering applications. Colloids Surf. A Physicochem. Eng. Asp. 2011, 382, 154–164. [Google Scholar] [CrossRef]
- Guarino, V.; Ambrosio, L.; Bellini, D. Process for the Preparation of Microspheres Comprising Semisynthetic. Polymers. Patent WO/2009/143947, 3 December 2009. [Google Scholar]
- Mayol, L.; Borzacchiello, A.; Guarino, V.; Serri, C.; Biondi, M.; Ambrosio, L. Design of electrospayed non-spherical poly (L-lactide-co-glicolide) microdevices for sustained drug delivery. J. Mater. Sci. Mater. Med. 2014, 25, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; D’Albore, M.; Altobelli, R.; Ambrosio, L. Polymer Bioprocessing to Fabricate 3D Scaffolds for Tissue Engineering. Int. Polym. Process. 2016, 31, 587–597. [Google Scholar] [CrossRef]
- Zhang, L.-H.; Duan, X.P.; Yan, X.; Yu, M.; Ning, X.; Zhao, Y.; Long, Y.Z. Recent advances in melt electrospinning. RSC Adv. 2016, 6, 53400–53414. [Google Scholar] [CrossRef]
- Qin, C.-C.; Duan, X.P.; Wang, L.; Zhang, L.H.; Yu, M.; Dong, R.H.; Long, Y.Z. Melt electrospinning of poly(lactic acid) and polycaprolactone microfibers by using a hand-operated Wimshurst generator. Nanoscale 2015, 7, 16611–16615. [Google Scholar] [CrossRef] [PubMed]
- Bubakir, M.M.; Li, H.; Barhoum, A.; Yang, W. Advances in Melt Electrospinning. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Sarwar, Z.; Krugly, E.; Danilovas, P.P.; Ciuzas, D.; Kauneliene, V.; Martuzevicius, D. Fabrication and characterization of PEBA fibers by melt and solution electrospinning. J. Mater. Res. Technol. 2019, 8, 6074–6085. [Google Scholar] [CrossRef]
- Morikawa, K.; Green, M.; Naraghi, M. A Novel Approach for Melt Electrospinning of Polymer Fibers. Procedia Manuf. 2018, 26, 205–208. [Google Scholar] [CrossRef]
- Lyons, J.; Li, C.; Ko, F. Melt-electrospinning part I: Processing parameters and geometric properties. Polymer 2004, 45, 7597–7603. [Google Scholar] [CrossRef]
- Goldstein, A.S.; Thayer, P.S. Chapter 11—Fabrication of complex biomaterial scaffolds for soft tissue engineering by electrospinning. In Nanobiomaterials in Soft Tissue Engineering; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 299–330. [Google Scholar]
- Pillay, V.; Dott, C.; Choonara, Y.E.; Tyagi, C.; Tomar, L.; Kumar, P.; Ndesendo, V.M. A Review of the Effect of Processing Variables on the Fabrication of Electrospun Nanofibers for Drug Delivery Applications. J. Nanomater. 2013, 2013, 789289. [Google Scholar] [CrossRef]
- Shao, P.; Niu, B.; Chen, H.; Sun, P. Fabrication and characterization of tea polyphenols loaded pullulan-CMC electrospun nanofiber for fruit preservation. Int. J. Biol. Macromol. 2018, 107, 1908–1914. [Google Scholar] [CrossRef]
- Shao, H.; Fang, J.; Wang, H.; Lin, T. Effect of electrospinning parameters and polymer concentrations on mechanical-to-electrical energy conversion of randomly-oriented electrospun poly(vinylidene fluoride) nanofiber mats. RSC Adv. 2015, 5, 14345–14350. [Google Scholar] [CrossRef]
- Herrero-Herrero, M.; Gómez-Tejedor, J.A.; Vallés-Lluch, A. PLA/PCL electrospun membranes of tailored fibres diameter as drug delivery systems. Eur. Polym. J. 2018, 99, 445–455. [Google Scholar] [CrossRef]
- Nasouri, K.; Bahrambeygi, H.; Rabbi, A.; Shoushtari, A.M.; Kaflou, A. Modeling and optimization of electrospun PAN nanofiber diameter using response surface methodology and artificial neural networks. J. Appl. Polym. Sci. 2012, 126, 127–135. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.D.; Hirvonen, J.K.; Tan, N.C.B. Controlled deposition of electrospun poly(ethylene oxide) fibers. Polymer 2001, 42, 8163–8170. [Google Scholar] [CrossRef]
- Mosher, C.Z.; Brudnicki, P.A.; Gong, Z.; Childs, H.R.; Lee, S.W.; Antrobus, R.M.; Lu, H.H. Green electrospinning for biomaterials and biofabrication. Biofabrication 2021, 13, 35049. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jeong, Y.J.; Park, W.H. Poly(vinyl alcohol) nanofibrous membranes via green electrospinning and tannin coating for selective removal of Pb(II) ion. Chemosphere 2022, 307, 135719. [Google Scholar] [CrossRef] [PubMed]
- Tonda-Turo, C.; Ruini, F.; Ceresa, C.; Gentile, P.; Varela, P.; Ferreira, A.M.; Ciardelli, G. Nanostructured scaffold with biomimetic and antibacterial properties for wound healing produced by ‘green electrospinning’. Colloids Surf. B Biointerfaces 2018, 172, 233–243. [Google Scholar] [CrossRef]
- Celebioglu, A.; Saporito, A.F.; Uyar, T. Green Electrospinning of Chitosan/Pectin Nanofibrous Films by the Incorporation of Cyclodextrin/Curcumin Inclusion Complexes: pH-Responsive Release and Hydrogel Features. ACS Sustain. Chem. Eng. 2022, 10, 4758–4769. [Google Scholar] [CrossRef]
- Zhu, M.; Hua, D.; Zhong, M.; Zhang, L.; Wang, F.; Gao, B.; Huang, C. Antibacterial and Effective Air Filtration Membranes by ‘Green’ Electrospinning and Citric Acid Crosslinking. Colloid Interface Sci. Commun. 2018, 23, 52–58. [Google Scholar] [CrossRef]
- Zhong, T.; Liu, W.; Liu, H. Green electrospinning of chitin propionate to manufacture nanofiber mats. Carbohydr. Polym. 2021, 273, 118593. [Google Scholar] [CrossRef]
- Robinson, T.M.; Hutmacher, D.W.; Dalton, P.D. The Next Frontier in Melt Electrospinning: Taming the Jet. Adv. Funct. Mater. 2019, 29, 1904664. [Google Scholar] [CrossRef]
- Gajjar, C.R.; Stallrich, J.W.; Pasquinelli, M.A.; King, M.W. Process–Property Relationships for Melt-Spun Poly(lactic acid) Yarn. ACS Omega 2021, 6, 15920–15928. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, M.; Ye, Q.; Chen, D.; Liu, K.; Bai, H. Spinning from Nature: Engineered Preparation and Application of High-Performance Bio-Based Fibers. Engineering 2022, 14, 100–112. [Google Scholar] [CrossRef]
- Yu, L.; Dean, K.; Li, L. Polymer blends and composites from renewable resources. Prog. Polym. Sci. 2006, 31, 576–602. [Google Scholar] [CrossRef]
- Ghajarieh, A.; Habibi, S.; Talebian, A. Biomedical Applications of Nanofibers. Russ. J. Appl. Chem. 2021, 94, 847–872. [Google Scholar] [CrossRef]
- Liu, L.; Xu, W.; Ding, Y.; Agarwal, S.; Greiner, A.; Duan, G. A review of smart electrospun fibers toward textiles. Compos. Commun. 2020, 22, 100506. [Google Scholar] [CrossRef]
- Angel, N.; Li, S.; Yan, F.; Kong, L. Recent advances in electrospinning of nanofibers from bio-based carbohydrate polymers and their applications. Trends Food Sci. Technol. 2022, 120, 308–324. [Google Scholar] [CrossRef]
- Sionkowska, A. Collagen blended with natural polymers: Recent advances and trends. Prog. Polym. Sci. 2021, 122, 101452. [Google Scholar] [CrossRef]
- Hanumantharao, S.N.; Rao, S. Multi-Functional Electrospun Nanofibers from Polymer Blends for Scaffold Tissue Engineering. Fibers 2019, 7, 66. [Google Scholar] [CrossRef]
- Renkler, N.Z.; Ergene, E.; Gokyer, S.; Ozturk, M.T.; Huri, P.Y.; Tuzlakoglu, K. Facile modification of polycaprolactone nanofibers with egg white protein. J. Mater. Sci. Mater. Med. 2021, 32, 34. [Google Scholar] [CrossRef]
- Cirillo, V.; Clements, B.A.; Guarino, V.; Bushman, J.; Kohn, J.; Ambrosio, L. A comparison of the performance of mono- and bi-component electrospun conduits in a rat sciatic model. Biomaterials 2014, 35, 8970–8982. [Google Scholar] [CrossRef]
- Guarino, V.; Cirillo, V.; Ambrosio, L. Bicomponent electrospun scaffolds to design extracellular matrix tissue analogs. Expert Rev. Med. Devices 2016, 13, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Wehlage, D.; Böttjer, R.; Grothe, T.; Ehrmann, A. Electrospinning water-soluble/insoluble polymer blends. AIMS Mater. Sci. 2018, 5, 190–200. [Google Scholar] [CrossRef]
- Huang, C.; Chen, R.; Ke, Q.; Morsi, Y.; Zhang, K.; Mo, X. Electrospun collagen–chitosan–TPU nanofibrous scaffolds for tissue engineered tubular grafts. Colloids Surf. B Biointerfaces 2011, 82, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Fasolino, I.; Guarino, V.; Cirillo, V.; Ambrosio, L. 5-Azacytidine-mediated hMSC behavior on electrospun scaffolds for skeletal muscle regeneration. J. Biomed. Mater. Res. A 2017, 105, 2551–2561. [Google Scholar] [CrossRef]
- Miele, D.; Catenacci, L.; Rossi, S.; Sandri, G.; Sorrenti, M.; Terzi, A.; Bonferoni, M.C. Collagen/PCL Nanofibers Electrospun in Green Solvent by DOE Assisted Process. An Insight into Collagen Contribution. Materials 2020, 13, 4698. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Chagala, Y.E.; Altuzar, V.; León-Sarabia, E.; Tinoco-Magaña, J.C.; Yañez-Limón, J.M.; Mendoza-Barrera, C. Physicochemical properties of polycaprolactone/collagen/elastin nanofibers fabricated by electrospinning. Mater. Sci. Eng. C 2017, 76, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.O.S.; Cruz-Maya, I.; Vineis, C.; Guarino, V.; Tonetti, C.; Varesano, A. Wool Keratin-Based Nanofibres-In Vitro Validation. Bioengineering 2021, 8, 224. [Google Scholar] [CrossRef]
- Ramirez, D.O.S.; Cruz-Maya, I.; Vineis, C.; Tonetti, C.; Varesano, A.; Guarino, V. Design of Asymmetric Nanofibers-Membranes Based on Polyvinyl Alcohol and Wool-Keratin for Wound Healing Applications. J. Funct. Biomater. 2021, 12, 76. [Google Scholar] [CrossRef]
- Vineis, C.; Maya, I.C.; Mowafi, S.; Varesano, A.; Ramírez, D.S.; Abou Taleb, M.; El-Sayed, H. Synergistic effect of sericin and keratin in gelatin based nanofibers for in vitro applications. Int. J. Biol. Macromol. 2021, 190, 375–381. [Google Scholar] [CrossRef]
- Xanthos, M. Functional Fillers for Plastics; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Pleşa, I.; Noţingher, P.V.; Schlögl, S.; Sumereder, C.; Muhr, M. Properties of Polymer Composites Used in High-Voltage Applications. Polymers 2016, 8, 173. [Google Scholar] [CrossRef]
- Ghosal, K.; Agatemor, C.; Špitálsky, Z.; Thomas, S.; Kny, E. Electrospinning tissue engineering and wound dressing scaffolds from polymer-titanium dioxide nanocomposites. Chem. Eng. J. 2019, 358, 1262–1278. [Google Scholar] [CrossRef]
- Lee, J.K.Y.; Chen, N.; Peng, S.; Li, L.; Tian, L.; Thakor, N.; Ramakrishna, S. Polymer-based composites by electrospinning: Preparation & functionalization with nanocarbons. Prog. Polym. Sci. 2018, 86, 40–84. [Google Scholar] [CrossRef]
- Watanabe, K.; Maeda, T.; Hotta, A. Uniformly dispersed polymeric nanofiber composites by electrospinning: Poly(vinyl alcohol) nanofibers/polydimethylsiloxane composites. Compos. Sci. Technol. 2018, 165, 18–23. [Google Scholar] [CrossRef]
- Ni, Q.Q.; Jin, X.D.; Xia, H.; Liu, F. 7—Electrospinning, processing and characterization of polymer-based nano-composite fibers. In Advances in Filament Yarn Spinning of Textiles and Polymers; Zhang, D., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 128–148. [Google Scholar]
- Kallu, S.; Kowalski, R.J.; Ganjyal, G.M. Impacts of Cellulose Fiber Particle Size and Starch Type on Expansion During Extrusion Processing. J. Food Sci. 2017, 82, 1647–1656. [Google Scholar] [CrossRef]
- Bates, I.I.C.; Loranger, É.; Mathew, A.P.; Chabot, B. Cellulose reinforced electrospun chitosan nanofibers bio-based composite sorbent for water treatment applications. Cellulose 2021, 28, 4865–4885. [Google Scholar] [CrossRef]
- Vasile, C.; Baican, M. Progresses in Food Packaging, Food Quality, and Safety-Controlled-Release Antioxidant and/or Antimicrobial Packaging. Molecules 2021, 26, 1263. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L. Superhydrophilic and underwater superoleophobic nanofibrous membrane for separation of oil/water emulsions. J. Mater. Res. 2020, 35, 1504–1513. [Google Scholar] [CrossRef]
- Elkady, M.; Salama, E.; Amer, W.A.; Ebeid, E.-Z.M.; Ayad, M.M.; Shokry, H. Novel eco-friendly electrospun nanomagnetic zinc oxide hybridized PVA/alginate/chitosan nanofibers for enhanced phenol decontamination. Environ. Sci. Pollut. Res. Int. 2020, 27, 43077–43092. [Google Scholar] [CrossRef]
- Hu, J.; Xiong, Z.; Liu, Y.; Lin, J. A biodegradable composite filter made from electrospun zein fibers underlaid on the cellulose paper towel. Int. J. Biol. Macromol. 2022, 204, 419–428. [Google Scholar] [CrossRef]
- Lv, D.; Wang, R.; Tang, G.; Mou, Z.; Lei, J.; Han, J.; Huang, C. Ecofriendly Electrospun Membranes Loaded with Visible-Light-Responding Nanoparticles for Multifunctional Usages: Highly Efficient Air Filtration, Dye Scavenging, and Bactericidal Activity. ACS Appl. Mater. Interfaces 2019, 11, 12880–12889. [Google Scholar] [CrossRef]
- Zhou, W.; Gong, X.; Li, Y.; Si, Y.; Zhang, S.; Yu, J.; Ding, B. Environmentally friendly waterborne polyurethane nanofibrous membranes by emulsion electrospinning for waterproof and breathable textiles. Chem. Eng. J. 2022, 427, 130925. [Google Scholar] [CrossRef]
- Han, W.; Rao, D.; Gao, H.; Yang, X.; Fan, H.; Li, C.; Meng, H. Green-solvent-processable biodegradable poly(lactic acid) nanofibrous membranes with bead-on-string structure for effective air filtration: ‘Kill two birds with one stone’. Nano Energy 2022, 97, 107237. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, X.; Gong, X.; Ding, M.; Yu, J.; Zhang, S.; Ding, B. Environmentally Friendly Polyamide Nanofiber Membranes with Interconnective Amphiphobic Channels for Seawater Desalination. ACS Appl. Mater. Interfaces 2022, 14, 35287–35296. [Google Scholar] [CrossRef] [PubMed]
- Adebajo, M.O.; Frost, R.L.; Kloprogge, J.T.; Carmody, O.; Kokot, S. Porous Materials for Oil Spill Cleanup: A Review of Synthesis and Absorbing Properties. J. Porous Mater. 2003, 10, 159–170. [Google Scholar] [CrossRef]
- Zhu, H.; Qiu, S.; Jiang, W.; Wu, D.; Zhang, C. Evaluation of Electrospun Polyvinyl Chloride/Polystyrene Fibers as Sorbent Materials for Oil Spill Cleanup. Environ. Sci. Technol. 2011, 45, 4527–4531. [Google Scholar] [CrossRef]
- Alnaqbi, M.A.; Greish, Y.E.; Mohsin, M.A.; Elumalai, E.J.; al Blooshi, A. Morphological variations of micro-nanofibrous sorbents prepared by electrospinning and their effects on the sorption of crude oil. J. Environ. Chem. Eng. 2016, 4, 1850–1861. [Google Scholar] [CrossRef]
- Oliveira, M.B.; Mano, J.F. Polymer-based microparticles in tissue engineering and regenerative medicine. Biotechnol. Prog. 2011, 27, 897–912. [Google Scholar] [CrossRef]
- Barua, S.; Mitragotri, S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef]
- Bock, N.; Dargaville, T.R.; Woodruff, M.A. Electrospraying of polymers with therapeutic molecules: State of the art. Prog. Polym. Sci. 2012, 37, 1510–1551. [Google Scholar] [CrossRef]
- Parhizkar, M.; Reardon, P.J.; Knowles, J.C.; Browning, R.J.; Stride, E.; Barbara, P.R.; Edirisinghe, M. Electrohydrodynamic encapsulation of cisplatin in poly (lactic-co-glycolic acid) nanoparticles for controlled drug delivery. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1919–1929. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Marijnissen, J.C.M.; Wang, C.-H. Microparticles developed by electrohydrodynamic atomization for the local delivery of anticancer drug to treat C6 glioma in vitro. Biomaterials 2006, 27, 3321–3332. [Google Scholar] [CrossRef] [PubMed]
- Gurler, E.B.; Ergul, N.M.; Ozbek, B.; Ekren, N.; Oktar, F.N.; Haskoylu, M.E.; Gunduz, O. Encapsulated melatonin in polycaprolactone (PCL) microparticles as a promising graft material. Mater. Sci. Eng. C 2019, 100, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.M.D.; Siqueira, N.M.; Prabhakaram, M.P.; Ramakrishna, S. Electrospinning and electrospray of bio-based and natural polymers for biomaterials development. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Altobelli, R.; Ambrosio, L. Chitosan Microgels and Nanoparticles via Electrofluidodynamic Techniques for Biomedical Applications. Gels 2016, 2, 2. [Google Scholar] [CrossRef]
- Başpinar, Y.; Akbaba, H.; Bayraktar, O. Encapsulation of paclitaxel in electrosprayed chitosan nanoparticles. J. Res. Pharm. 2019, 23, 886–896. [Google Scholar] [CrossRef]
- Guarino, V.; Altobelli, R.; Caputo, T.; Ambrosio, L.; Caserta, S.; Calcagnile, P.; Demitri, C. Mono- and Bi-Phasic Cellulose Acetate Micro-Vectors for Anti-Inflammatory Drug Delivery. Pharmaceutics 2019, 11, 87. [Google Scholar] [CrossRef]
- Zuppolini, S.; Maya, I.C.; Diodato, L.; Guarino, V.; Borriello, A.; Ambrosio, L. Self-associating cellulose-graft-poly(ε-caprolactone) to design nanoparticles for drug release. Mater. Sci. Eng. C 2020, 108, 110385. [Google Scholar] [CrossRef]
- Gandhimathi, C. Controlled Release of Dexamethasone in PCL/Silk Fibroin/Ascorbic Acid Nanoparticles for the Initiation of Adipose Derived Stem Cells into Osteogenesis. J. Drug Metab. Toxicol. 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Xu, H.; Sun, M.; Wang, C.; Xia, K.; Xiao, S.; Wang, Y.; Chen, L. Growth differentiation factor-5–gelatin methacryloyl injectable microspheres laden with adipose-derived stem cells for repair of disc degeneration. Biofabrication 2020, 13, 15010. [Google Scholar] [CrossRef]
- Hasan, M.N.; Shahriar, S.M.S.; Mondal, J.; Nurunnabi, M.; Lee, Y. Chapter Six—Bioinspired and biomimetic materials for oral drug delivery. In Bioinspired and Biomimetic Materials for Drug Delivery; Woodhead Publishing Series in Biomaterials; Nurunnabi, M., Ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 89–104. [Google Scholar]
- Guarino, V.; Caputo, T.; Calcagnile, P.; Altobelli, R.; Demitri, C.; Ambrosio, L. Core/shell cellulose-based microspheres for oral administration of Ketoprofen Lysinate. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2636–2644. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, X.; He, Y.; Ma, J.; Ni, G.; Zhou, S. From nano to micro to macro: Electrospun hierarchically structured polymeric fibers for biomedical applications. Prog. Polym. Sci. 2018, 81, 80–113. [Google Scholar] [CrossRef]
- Khodir, W.W.A.; Guarino, V.; Alvarez-Perez, M.; Cafiero, C.; Ambrosio, L. Trapping tetracycline-loaded nanoparticles into polycaprolactone fiber networks for periodontal regeneration therapy. J. Bioact. Compat. Polym. 2013, 28, 258–273. [Google Scholar] [CrossRef]
- Guarino, V.; Cruz-Maya, I.; Altobelli, R.; Khodir, W.A.; Ambrosio, L.; Pèrez, M.A.A.; Flores, A.A. Electrospun polycaprolactone nanofibres decorated by drug loaded chitosan nano-reservoirs for antibacterial treatments. Nanotechnology 2017, 28, 505103. [Google Scholar] [CrossRef]
- Saadipour, M.; Karkhaneh, A.; Nazarpak, M.H. An investigation into curcumin release from PLA particles loaded in PCL-GELATIN fibers for skin application. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 386–394. [Google Scholar] [CrossRef]
- Muthukumaran, P.; Babu, P.S.; Karthikeyan, S.; Kamaraj, M.; Aravind, J. Tailored natural polymers: A useful eco-friendly sustainable tool for the mitigation of emerging pollutants: A review. Int. J. Environ. Sci. Technol. 2021, 18, 2491–2510. [Google Scholar] [CrossRef]
- Xu, M.; Qin, M.; Cheng, Y.; Niu, X.; Kong, J.; Zhang, X.; Wang, H. Alginate microgels as delivery vehicles for cell-based therapies in tissue engineering and regenerative medicine. Carbohydr. Polym. 2021, 266, 118128. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Sharma, S. Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Funct. Polym. 2004, 59, 129–140. [Google Scholar] [CrossRef]
- Nikoo, A.M.; Kadkhodaee, R.; Ghorani, B.; Razzaq, H.; Tucker, N. Electrospray-assisted encapsulation of caffeine in alginate microhydrogels. Int. J. Biol. Macromol. 2018, 116, 208–216. [Google Scholar] [CrossRef]
- Cruz-Maya, I.; Altobelli, R.; Marrese, M.; Guarino, V. Design of alginate based micro-gels via electro fluid dynamics to construct microphysiological cell culture systems. Polym. Adv. Technol. 2021, 32, 2981–2989. [Google Scholar] [CrossRef]
- Murphy, A.R.; Laslett, A.; O’Brien, C.M.; Cameron, N.R. Scaffolds for 3D in vitro culture of neural lineage cells. Acta Biomater. 2017, 54, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, M.T.; Di Lisa, D.; Massobrio, P.; Colistra, N.; Pesce, M.; Catelani, T.; Pastorino, L. Soft chitosan microbeads scaffold for 3D functional neuronal networks. Biomaterials 2018, 156, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Chen, G.; Zhao, C.; Gan, J.; Alip, M.; Zhao, Y.; Sun, L. Bio-inspired adhesive porous particles with human MSCs encapsulation for systemic lupus erythematosus treatment. Bioact. Mater. 2021, 6, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Varesano, A. Electrospinning Technology for Filtering Membranes Fabrication. In Filtering Media by Electrospinning: Next Generation Membranes for Separation Applications; Focarete, M.L., Gualandi, C., Ramakrishna, S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–24. [Google Scholar]
- De Falco, F.; Guarino, V.; Gentile, G.; Cocca, M.; Ambrogi, V.; Ambrosio, L.; Avella, M. Design of functional textile coatings via non-conventional electrofluidodynamic processes. J. Colloid Interface Sci. 2019, 541, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, J.; Fang, L.; Lin, H.; Liu, F.; Tang, C.Y. Electrosprayed polyamide nanofiltration membrane with intercalated structure for controllable structure manipulation and enhanced separation performance. J. Membr. Sci. 2020, 602, 117971. [Google Scholar] [CrossRef]
- Qian, X.; Ravindran, T.; Lounder, S.J.; Asatekin, A.; McCutcheon, J.R. Printing zwitterionic self-assembled thin film composite membranes: Tuning thickness leads to remarkable permeability for nanofiltration. J. Membr. Sci. 2021, 635, 119428. [Google Scholar] [CrossRef]
- Huang, Y.; Qian, X.; Wang, X.; Wang, T.; Lounder, S.J.; Ravindran, T.; Li, B. Electrospraying Zwitterionic Copolymers as an Effective Biofouling Control for Accurate and Continuous Monitoring of Wastewater Dynamics in a Real-Time and Long-Term Manner. Environ. Sci. Technol. 2022, 56, 8176–8186. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Peng, Q.; Venkataraman, M.; Novotna, J.; Karpiskova, J.; Mullerova, J.; Militky, J. Hydrophobicity, water moisture transfer and breathability of PTFE-coated viscose fabrics prepared by electrospraying technology and sintering process. Prog. Org. Coat. 2022, 165, 106775. [Google Scholar] [CrossRef]
- Chalapud, M.C.; Baümler, E.R.; Carelli, A.A.; Salgado-Cruz MD, L.P.; Morales-Sánchez, E.; Rentería-Ortega, M.; Calderón-Domínguez, G. Pectin Films with Recovered Sunflower Waxes Produced by Electrospraying. Membranes 2022, 12, 560. [Google Scholar] [CrossRef]
- Hu, Z.; Hao, J.; Shen, D.; Gao, C.; Liu, Z.; Zhao, J.; Lu, B. Electro-spraying/spinning: A novel battery manufacturing technology. Green Energy Environ. 2022; in press. [Google Scholar] [CrossRef]







| Polymer State | HV | Size | Main Benefits | Limitations | Refs. |
|---|---|---|---|---|---|
| Melt | + | 10 µm to 100 µm | -High structural control at the microscale; -No solvent use; | -Time-consuming to build. -Few polymers to test (thermal stability required) -High equipment costs -Hard to reach the nanoscale | [61] |
| Solution | + | <100 nm to 10 µm | -Highly customizable setup; -Low costs; -Suitable for many polymers; -Sub-micrometric scale | -High solvent volatility is required (few green solvent) -Low control of structural order (no direct writing) | [55] |
| Melt | - | 50 µm to 500 µm | -High mass production -Weaving tech combination -Large-scale applicability | -High production cost -No diameter homogeneity -No nanometric scale | [62] |
| Solution | - | 500 nm to 50 µm | -Thermally unstable polymer use; -Several configurations -Industrial use | -Solvent removal -Lack in morphological order (high defect occurrence) | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renkler, N.Z.; Cruz-Maya, I.; Bonadies, I.; Guarino, V. Electro Fluid Dynamics: A Route to Design Polymers and Composites for Biomedical and Bio-Sustainable Applications. Polymers 2022, 14, 4249. https://doi.org/10.3390/polym14194249
Renkler NZ, Cruz-Maya I, Bonadies I, Guarino V. Electro Fluid Dynamics: A Route to Design Polymers and Composites for Biomedical and Bio-Sustainable Applications. Polymers. 2022; 14(19):4249. https://doi.org/10.3390/polym14194249
Chicago/Turabian StyleRenkler, Nergis Zeynep, Iriczalli Cruz-Maya, Irene Bonadies, and Vincenzo Guarino. 2022. "Electro Fluid Dynamics: A Route to Design Polymers and Composites for Biomedical and Bio-Sustainable Applications" Polymers 14, no. 19: 4249. https://doi.org/10.3390/polym14194249
APA StyleRenkler, N. Z., Cruz-Maya, I., Bonadies, I., & Guarino, V. (2022). Electro Fluid Dynamics: A Route to Design Polymers and Composites for Biomedical and Bio-Sustainable Applications. Polymers, 14(19), 4249. https://doi.org/10.3390/polym14194249







