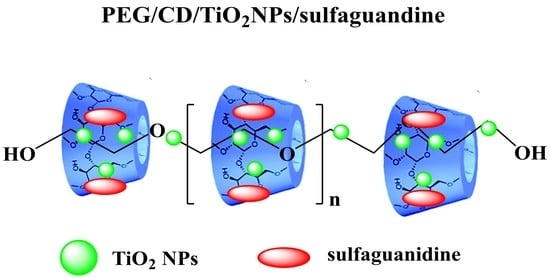
Design, Synthesis and Evaluation of Novel Antimicrobial Polymers Based on the Inclusion of Polyethylene Glycol/TiO2 Nanocomposites in Cyclodextrin as Drug Carriers for Sulfaguanidine
Abstract
:1. Introduction
2. Experimental
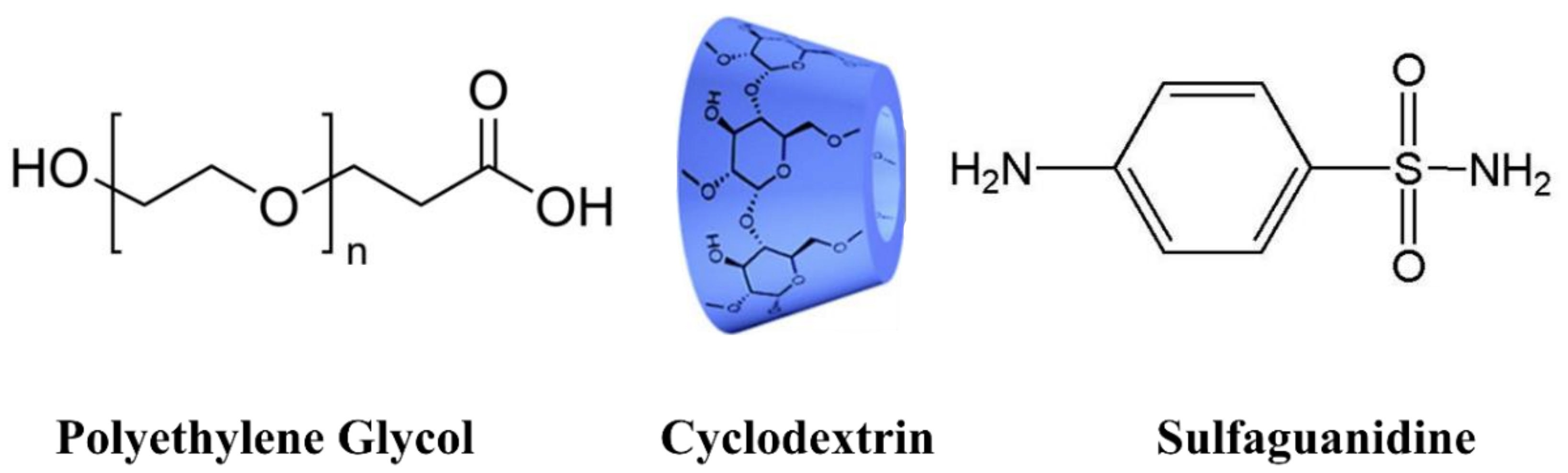
2.1. Chemicals and Reagents
2.2. Preparation of TiO2 Nanorods
2.3. General Procedure for the Synthesis of PEG-Based Polymers
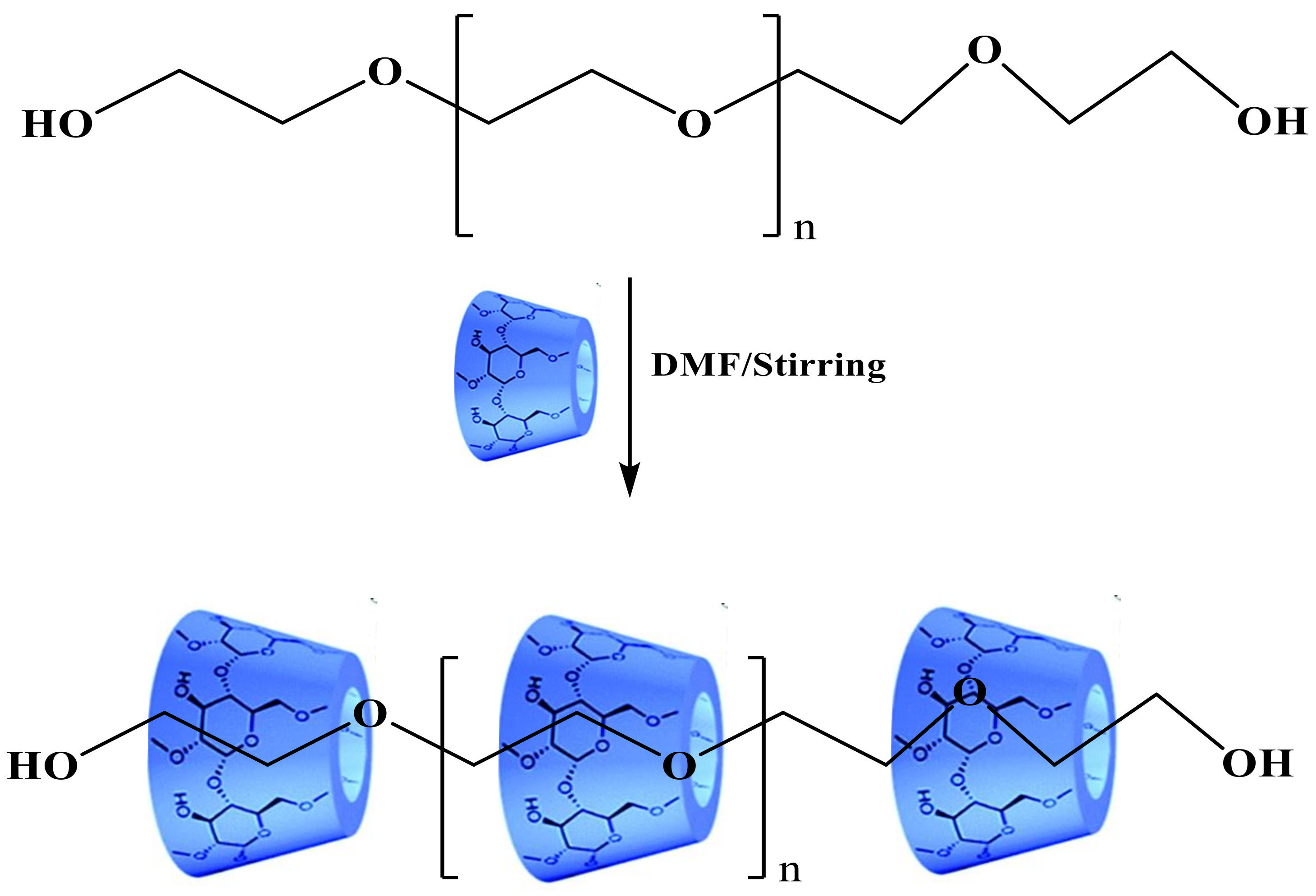
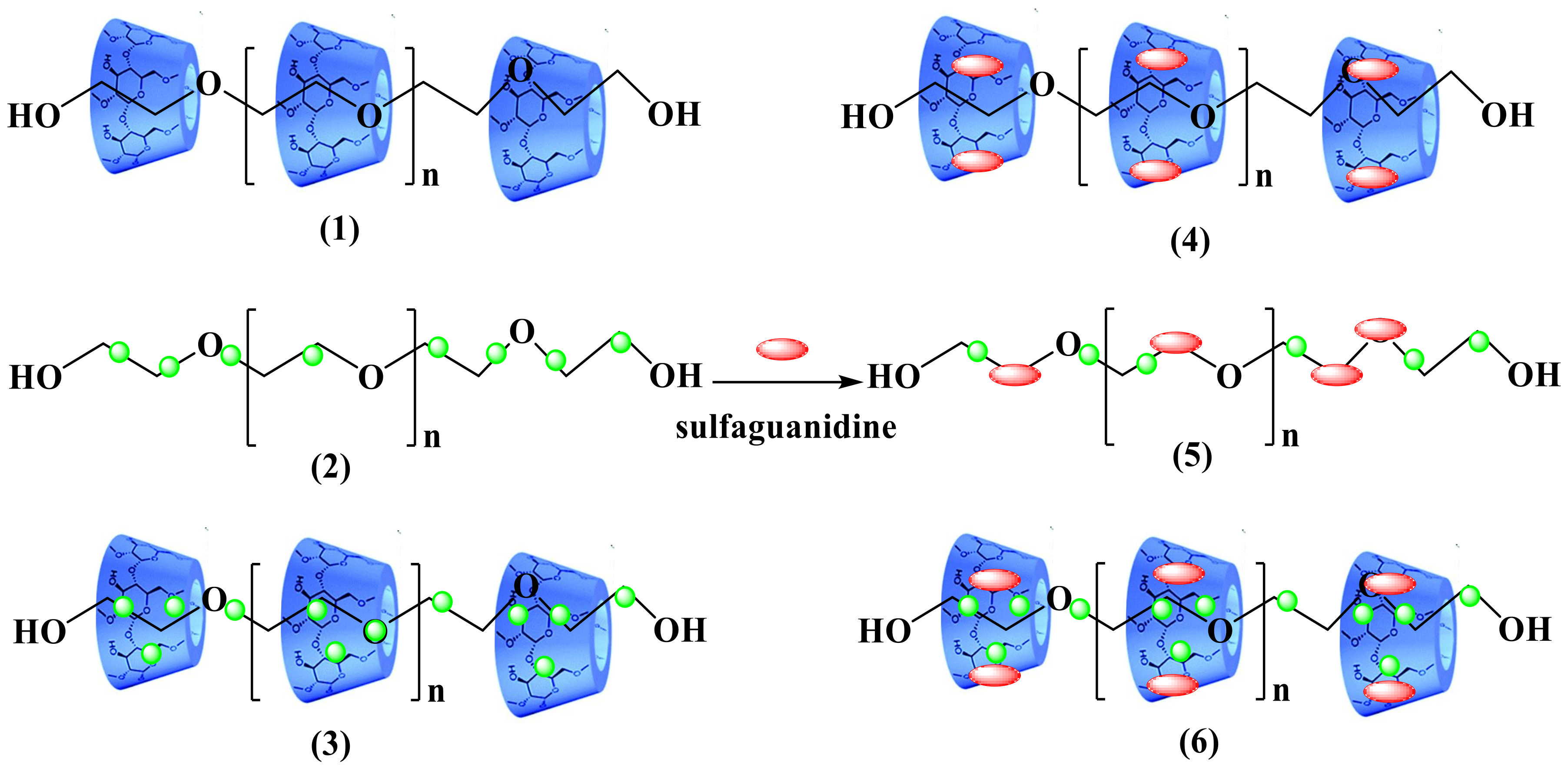
2.3.1. Synthesis of Pseudopolyrotaxane PEG/α-CD (1)
2.3.2. Synthesis of PEG/TiO2 NPs Composite (2)
2.3.3. Synthesis of Pseudopolyrotaxane Composite PEG/α-CD/TiO2 (3)
2.3.4. Preparation of PEG/α-CD/Drug (4)
2.3.5. Preparation of PEG/TiO2/Drug (5)
2.3.6. Preparation of PEG/α-CD/TiO2/Drug (6)
2.4. Characterization
2.5. Antibacterial Tests
2.6. Static Biofilm Assay
3. Results and Discussion
3.1. Chemistry
3.2. FT-IR-Studies
3.3. 1H-NMR Analysis
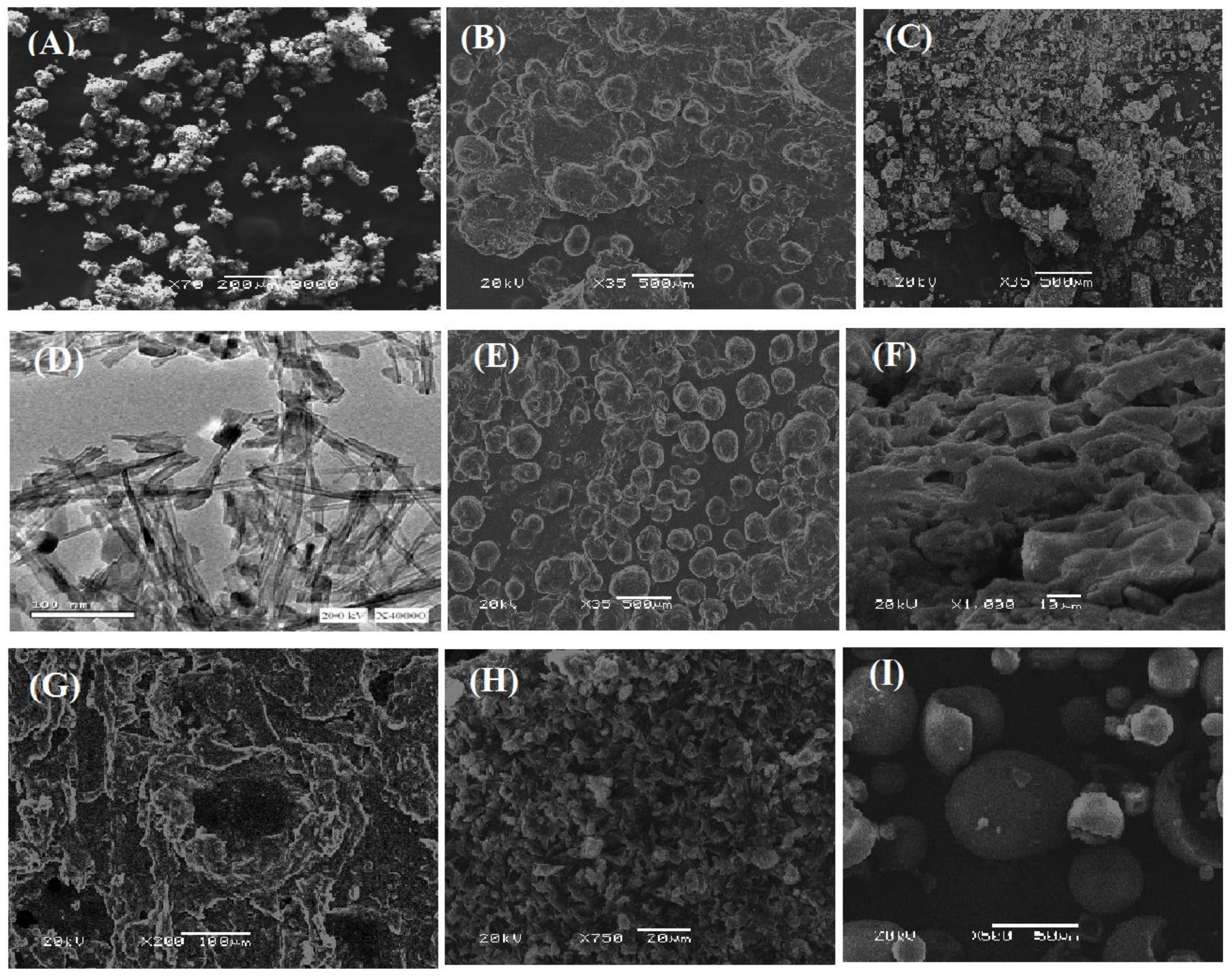
3.4. Surface Morphology
3.5. Optical Properties
3.6. Antimicrobial Performance Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, R.; Hwang, Y.-C.; Liu, I.; Lee, C.; Tsai, H.; Li, H.; Wu, H.J. Development of therapeutic antibodies for the treatment of diseases. Biomed. Sci. 2020, 27, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, A.; Toghan, A. Unprecedented Treatment Strategy of Aquatic Environments: Oxidative Degradation of Penicillin G by Chromium Trioxide in Acidic Media and the Impact of Metal Ion Catalysts: Kinetics and Mechanistic Insights. ACS Omega 2020, 5, 32781–32791. [Google Scholar] [CrossRef] [PubMed]
- Alamro, F.S.; Mostafa, A.M.; Al-Ola, K.A.A.; Ahmed, H.A.; Toghan, A. Synthesis of Ag Nanoparticles-Decorated CNTs via Laser Ablation Method for the Enhancement the Photocatalytic Removal of Naphthalene from Water. Nanomaterials 2021, 11, 2142. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J.J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K.J. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. Control. Release 2000, 65, 271–287. [Google Scholar] [CrossRef]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Schiffelers, R.M.; Ansari, A.; Xu, J.; Zhou, Q.; Tang, Q.; Storm, G.; Molema, G.; Lu, P.Y.; Scaria, P.V.; Woodle, M.C. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res. 2004, 32, e149. [Google Scholar] [CrossRef]
- Lee, E.S.; Gao, Z.; Kim, D.; Park, K.; Kwon, I.C.; Bae, Y.H. Super pH-sensitive Multifunctional Polymeric Micelle for Tumor pH(e) Specific TAT Exposure and Multidrug Resistance. J. Control. Release 2008, 129, 228–236. [Google Scholar] [CrossRef]
- Qiu, L.; Li, Z.; Qiao, M.; Long, M.; Wang, M.; Zhang, X.; Tian, C.; Chen, D. Self-assembled pH-responsive hyaluronic acid-g-poly((L)-histidine) copolymer micelles for targeted intracellular delivery of doxorubicin. Acta Biomater. 2014, 10, 2024–2035. [Google Scholar] [CrossRef]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; Lleonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Zhu, W.; Yi, X.; Dong, Z.; Xu, X.; Chen, M.; Yang, K.; Lu, G.; Jiang, L.; et al. Nanoscale metal-organic frameworks for combined photodynamic & radiation therapy in cancer treatment. Biomaterials 2016, 97, 1–9. [Google Scholar]
- Crohns, M.; Saarelainen, S.; Erhola, M.; Alho, H.; Kellokumpu-Lehtinen, P. Impact of radiotherapy and chemotherapy on biomarkers of oxidative DNA damage in lung cancer patients. Clin. Biochem. 2009, 42, 1082–1090. [Google Scholar]
- Loftsson, T. Cyclodextrins and the Biopharmaceutics Classification System of Drugs. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 63–67. [Google Scholar] [CrossRef]
- Kang, T.; Gao, S.; Zhao, L.-X.; Zhai, Y.; Ye, F.; Fu, Y. Design, Synthesis, and SAR of Novel 1,3-Disubstituted Imidazolidine or Hexahydropyrimidine Derivatives as Herbicide Safeners. J. Agric. Food Chem. 2021, 69, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Li, X.; Yang, G.; Feng, W.; Zong, L.; Zhao, L.; Ye, F.; Fu, Y. Antibacterial perillaldehyde/hydroxypropyl-γ-cyclodextrin inclusion complex electrospun polymer-free nanofiber: Improved water solubility, thermostability, and antioxidant activity. Ind Crops Prod. 2022, 176, 114300. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Fast Dissolving Oral Drug Delivery System Based on Electrospun Nanofibrous Webs of Cyclodextrin/Ibuprofen Inclusion Complex Nanofibers. Mol. Pharm. 2019, 16, 4387–4398. [Google Scholar] [CrossRef]
- Belica-Pacha, S.; Miłowska, K.; Ionov, M.; Bryszewska, M.; Buczkowski, A.; Budryn, G.; Oracz, J.; Zaczyńska, D.; Wróblewska, A.; Urbaniak, P.; et al. The impact of β-cyclodextrin on biological and chemical properties of mianserin hydrochloride in aqueous solution. J. Mol. Liq. 2020, 314, 113589. [Google Scholar] [CrossRef]
- Aiassa, V.; Garnero, C.; Longhi, M.R.; Zoppi, A. Cyclodextrin Multicomponent Complexes: Pharmaceutical Applications. Pharmaceutics 2021, 13, 1099. [Google Scholar] [CrossRef]
- Ncube, P.; Krause, R.W.; Mamba, B.B. Fluorescent Sensing of Chlorophenols in Water Using an Azo Dye Modified β-Cyclodextrin Polymer. Sensors 2011, 11, 4598–4608. [Google Scholar] [CrossRef]
- Ogoshi, T.; Harada, A. Chemical Sensors Based on Cyclodextrin Derivatives. Sensors 2008, 8, 4961–4982. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Zhao, X.; Song, Z. Exploring host-guest interactions of sulfobutylether-β-cyclodextrin and phenolic acids by chemiluminescence and site-directed molecular docking. Anal Biochem. 2014, 460, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Loh, X.J. Cyclodextrin-based supramolecular architectures: Syntheses, structures, and applications for drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1000–1017. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Shende, P. Cyclodextrins-modified metallic nanoparticles for effective cancer therapy. J. Control. Release 2021, 339, 41–50. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Reza, M.S.; Quadir, M.A.; Haider, S.S. Comparative evaluation of plastic, hydrophobic and hydrophilic polymers as matrices for controlledrelease drug delivery. J. Pharm. Pharm. Sci. 2003, 6, 282–291. [Google Scholar]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Vilar, G.; Tulla-Puche, J.; Albericio, F. Polymers and drug delivery systems. Curr. Drug Deliv. 2012, 9, 367–394. [Google Scholar] [CrossRef]
- Harada, A.; Li, J.; Kamachi, M. Synthesis of a tubular polymer from threaded cyclodextrins. Nature 1993, 364, 516–518. [Google Scholar] [CrossRef]
- Taylor, P.N.; O’Connell, M.J.; McNeill, L.A.; Hall, M.J.; Aplin, R.T.; Anderson, H.L. Insulated Molecular Wires: Synthesis of Conjugated Polyrotaxanes by Suzuki Coupling in Water We are grateful to Carol A. Stanier for valuable discussion and to Professor Christopher J. Schofield for providing facilities for gel electrophoresis. Disodium 1-aminonaphthalene-3,6-disulfonate was generously provided by Dr. M. G. Hutchings of BASF plc (Cheadle Hulme, UK). This project is funded by the Engineering and Physical Sciences Research Council (UK). Angew. Chem. Int. Ed. Engl. 2000, 39, 3456–3460. [Google Scholar] [PubMed]
- Yu, G.; Yang, Z.; Fu, X.; Yung, B.C.; Yang, J.; Mao, Z.; Shao, L.; Hua, B.; Liu, Y.; Zhang, F.; et al. Polyrotaxane-based supramolecular theranostics. Nat. Commun. 2018, 9, 766. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Morita, K.; Song, X.; Zhu, J.; Tamura, A.; Yui, N.; Li, J. One-pot synthesis of cyclodextrin-based radial poly[n]catenanes. Commun. Chem. 2019, 2, 78. [Google Scholar] [CrossRef]
- Steiner, T.; Saenger, W. Crystal structure of anhydrous hexakis-(2,3,6-tri-O-methyl)-cyclomaltohexaose (permethyl-alpha-cyclodextrin) grown from hot water and from cold NaCl solutions. Carbohydr. Res. 1996, 282, 53–63. [Google Scholar] [CrossRef]
- Araki, J.; Ito, K. Polyrotaxane derivatives. I. Preparation of modified polyrotaxanes with nonionic functional groups and their solubility in organic solvents. J Polym Sci Part A Polym Chem 2006, 44, 6312–6323. [Google Scholar] [CrossRef]
- Yui, N.; Ooya, T.; Kumeno, T. Effect of Biodegradable Polyrotaxanes on Platelet Activation. Bioconjugate Chem. 1998, 9, 118–125. [Google Scholar] [CrossRef]
- Ooya, T.; Arizono, K.; Yui, N. Synthesis and characterization of an oligopeptide - terminated polyrotaxane as a drug carrier. Polym. Adv. Technol. 2000, 11, 642–651. [Google Scholar] [CrossRef]
- Abidi, S.S.A.; Azim, Y.; Khan, S.N.; Khan, A.U. Sulfaguanidine cocrystals: Synthesis, structural characterization and their antibacterial and hemolytic analysis. J. Pharm. Biomed. Anal. 2018, 149, 351–357. [Google Scholar] [CrossRef]
- Verma, S.K.; Verma, R.; Xue, F.; Thakur, P.K.; Girish, Y.R.; Rakesh, K.P. Antibacterial activities of sulfonyl or sulfonamide containing heterocyclic derivatives and its structure-activity relationships (SAR) studies: A critical review. Bioorg. Chem. 2020, 105, 104400. [Google Scholar] [CrossRef] [PubMed]
- Meanwell, N.A. Synopsis of some recent tactical application of bioisosteres in drug design. J. Med. Chem. 2011, 54, 2529–2591. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Mondal, S. Recent Developments in the Synthesis of Fused Sultams. Chem. Rev. 2011, 111, 7749–7773. [Google Scholar] [CrossRef]
- Scozzafava, A.; Menabuoni, L.; Mincione, F.; Briganti, F.; Mincione, G.; Supuran, C.T. Carbonic Anhydrase Inhibitors. Synthesis of Water-Soluble, Topically Effective, Intraocular Pressure-Lowering Aromatic/Heterocyclic Sulfonamides Containing Cationic or Anionic Moieties: Is the Tail More Important than the Ring? J. Med. Chem. 1999, 42, 2641–2650. [Google Scholar] [CrossRef]
- Konda, S.; Raparthi, S.; Bhaskar, K.; Munaganti, R.K.; Guguloth, V.; Nagarapu, L.; Akkewar, D.M. Synthesis and antimicrobial activity of novel benzoxazine sulfonamide derivatives. Bioorg. Med. Chem. Lett. 2015, 25, 1643–1646. [Google Scholar]
- Liu, H.; Long, S.; Rakesh, K.P.; Zha, G.-F. Structure-activity relationships (SAR) of triazine derivatives: Promising antimicrobial agents. Eur. J. Med. Chem. 2020, 185, 111804. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Wan, K.; Zhou, C.-H. Synthesis of novel sulfanilamide-derived 1,2,3-triazoles and their evaluation for antibacterial and antifungal activities. Eur. J. Med. Chem. 2010, 45, 4631–4639. [Google Scholar] [CrossRef] [PubMed]
- Naaz, F.; Srivastava, R.; Singh, A.; Singh, N.; Verma, R.; Singh, V.K.; Singh, R.K. Molecular modeling, synthesis, antibacterial and cytotoxicity evaluation of sulfonamide derivatives of benzimidazole, indazole, benzothiazole and thiazole. Bioorg. Med. Chem. 2018, 26, 3414–3428. [Google Scholar] [CrossRef]
- Aday, B.; Sola, P.; Çolak, F.; Kaya, M. Synthesis of novel sulfonamide analogs containing sulfamerazine/sulfaguanidine and their biological activities. J. Enzyme Inhib. Med. Chem. 2016, 31, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, N.; Nishikata, M.; Morita, E.; Miyake, K. Gastrointestinal absorption of sulfaguanidine in neonatal and adult rats. J. Pharmacobiodyn. 1986, 9, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, N.; Horta, E.; Guerra, R.; Pérez-Santiago, E. Poorly Absorbed Sulfonamides in the Treatment of Tropical Sprue. Gastroenterology 1969, 57, 559–568. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Samanta, S.; Mukherjee, A.; Santra, C.R.; Ghosh, A.N.; Niyogi, S.K.; Karmakar, P. Antibacterial Activities of Polyethylene Glycol, Tween 8o and Sodium Dodecyl Sulphate Coated Silver Nanoparticles in Normal and Multi-Drug Resistant Bacteria. J. Nanosci. Nanotechnol. 2012, 12, 2513–2521. [Google Scholar]
- Fatima, A.; Khanum, G.; Srivastava, S.K.; Verma, I.; Siddiqui, N.; Javed, S. Synthesis, computational, spectroscopic, hirshfeld surface, electronic state and molecular docking studies on diethyl-5-amino-3-methylthiophene-2,4-dicarboxylate. Chem. Phys. Lett. 2021, 783, 139049. [Google Scholar] [CrossRef]
- Mutavdžić Pavlović, D.; Nikšić, K.; Livazović, S.; Brnardić, I.; Anžlovar, A. Preparation and application of sulfaguanidine-imprinted polymer on solid-phase extraction of pharmaceuticals from water. Talanta 2015, 131, 99–107. [Google Scholar] [CrossRef]
- Mondal, S.; Mandal, S.M.; Ojha, D.; Chattopadhyay, D.; Sinha, C. Water soluble sulfaguanidine based Schiff base as a “Turn-on” fluorescent probe for intracellular recognition of Zn2+ in living cells and exploration for biological activities. Polyhedron 2019, 172, 28–38. [Google Scholar] [CrossRef]
- El Alami El Hassani, N.; Llobet, E.; Popescu, L.-M.; Ghita, M.; Bouchikhi, B.; El Bari, N. Development of a highly sensitive and selective molecularly imprinted electrochemical sensor for sulfaguanidine detection in honey samples. J. Electroanal. Chem. 2018, 823, 647–655. [Google Scholar] [CrossRef]
- Elamary, R.B.; Albarakaty, F.M.; Salem, W.M. Efficacy of Acacia nilotica aqueous extract in treating biofilm-forming and multidrug resistant uropathogens isolated from patients with UTI syndrome. Sci. Rep. 2020, 10, 11125. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Bobillier, F.; Canales, D.; Sepúlveda, F.A.; Cament, A.; Amigo, N.; Rivas, L.M.; Ulloa, M.T.; Reyes, P.; Ortiz, J.A.; et al. Mechanical and Antimicrobial Polyethylene Composites with CaO Nanoparticles. Polymers 2020, 12, 2132. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, M.M.; El-Lateef, H.M.A.; Mohamed, I.M.A.; Zaki, M.E.A.; Toghan, A. Facile synthesis of gold-nanoparticles by different capping agents and their anticancer performance against liver cancer cells. Colloids Interface Sci. Commun. 2021, 44, 100482. [Google Scholar] [CrossRef]
- Toghan, A.; Modwi, A. Boosting unprecedented Indigo carmine dye photodegradation via Mesoporous MgO@g-C3N4 nanocomposite. J. Photochem. Photobiol. A Chem. 2021, 419, 113467. [Google Scholar] [CrossRef]
- Mehl, S.; Toghan, A.; Bauer, T.; Brummel, O.; Taccardi, N.; Wasserscheid, P.; Libuda, J. Pd Nanoparticle Formation in Ionic Liquid Thin Films Monitored by in situ Vibrational Spectroscopy. Langmuir 2015, 31, 12126–12139. [Google Scholar] [CrossRef]
- Gouda, M.H.; Elessawy, N.A.; Toghan, A. Novel Crosslinked Sulfonated PVA/PEO Doped with Phosphated Titanium Oxide Nanotubes as Effective Green Cation Exchange Membrane for Direct Borohydride Fuel Cells. Polymers 2021, 13, 2050. [Google Scholar] [CrossRef] [PubMed]
- ElFaham, M.M.; Mostafa, A.M.; Toghan, A. Facile synthesis of Cu2O nanoparticles using pulsed laser ablation method for optoelectronic applications. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127562. [Google Scholar] [CrossRef]
- Elsawy, H.; El-Lateef, H.M.A.; Khalaf, M.M.; Mohamed, I.M.A.; Touny, A.H.; Toghan, A. Synthesis and antimicrobial activity assessment of calcium and iron phosphate nanoparticles prepared by a facile and cost-effective method. Chem. Phys. Lett. 2021, 779, 138839. [Google Scholar] [CrossRef]
- Gouda, M.H.; Elessawy, N.A.; Al-Hussain, S.A.; Toghan, A. Design of Promising Green Cation-Exchange-Membranes-Based Sulfonated PVA and Doped with Nano Sulfated Zirconia for Direct Borohydride Fuel Cells. Polymers 2021, 13, 4205. [Google Scholar] [CrossRef] [PubMed]
- Hanemann, T.; Szabó, D.V. Polymer-Nanoparticle Composites: From Synthesis to Modern Applications. Materials 2010, 3, 3468–3517. [Google Scholar] [CrossRef]
- Hashemzadeh, H.; Allahverdi, A.; Ertl, P.; Naderi-Manesh, H. Comparison Between Three-Dimensional Spheroid And Two-Dimensional Monolayer in A549 Lung Cancer and Pc9 Normal Cell Lines Under Treatment of Silver Nanoparticles. J. Biotechnol. 2019, 10, 573–580. [Google Scholar]
- McCullagh, C.; Robertson, J.M.C.; Bahnemann, D.W.; Robertson, P.K.J. The application of TiO2 photocatalysis for disinfection of water contaminated with pathogenic micro-organisms: A review. Res. Chem. Intermed. 2007, 33, 359–375. [Google Scholar] [CrossRef]
- Bonetta, S.; Bonetta, S.; Motta, F.; Strini, A.; Carraro, E. Photocatalytic bacterial inactivation by TiO2-coated surfaces. AMB Express. 2013, 3, 59. [Google Scholar] [CrossRef] [PubMed]
- Toghan, A.; Taha, K.K.; Modwi, A. TiO2–ZnO composites fabricated via sonication assisted with gelatin for potential use in Rhodamine B degradation. J. Mater. Sci. Mater. Electron. 2021, 32, 2471–2485. [Google Scholar] [CrossRef]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical Applications of TiO2 Nanostructures: Recent Advances. Int. J. Nanomed. 2020, 15, 3447–3470. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, Z.I.; Millar, B.C.; Downey, D.G.; Moore, J.E. Antimicrobial effect of dimethyl sulfoxide and N, N-Dimethylformamide on Mycobacterium abscessus: Implications for antimicrobial susceptibility testing. Int. J. Mycobacteriol. 2018, 7, 134–136. [Google Scholar] [PubMed]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta. Med. 1998, 46, 711–713. [Google Scholar] [CrossRef]
- Salem, W.; Leitner, D.R.; Zingl, F.G.; Schratter, G.; Prassl, R.; Goessler, W.; Reidl, J.; Schild, S. Antibacterial activity of silver and zinc nanoparticles against Vibrio cholerae and enterotoxic Escherichia coli. Int. J. Med. Microbiol. 2015, 305, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, M. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nanomicro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Seper, A.; Fengler, V.H.; Roier, S.; Wolinski, H.; Kohlwein, S.D.; Bishop, A.L.; Camilli, A.; Reidl, J.; Schild, S. Extracellular nucleases and extracellular DNA play important roles in Vibrio cholerae biofilm formation. Int. J. Med. Microbiol. 2011, 82, 1015–1037. [Google Scholar] [CrossRef] [PubMed]
- Dardeer, H.M. Synthesis, characterization of novel rotaxanes depend on cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2018, 91, 105–114. [Google Scholar] [CrossRef]
- Dardeer, H.M.; Hassan, M.A. Synthesis of [2] Rotaxanes Derived from Host-Guest Interaction. Int. J. Chem. 2015, 7, 161–167. [Google Scholar] [CrossRef]
- Dardeer, H.M.; Toghan, A. A novel route for the synthesis of pseudopolyrotaxane containing γ-Cyclodextrin based on environmental waste recycling. J. Mol. Struct. 2021, 1227, 129707. [Google Scholar] [CrossRef]
- Dardeera, H.M.; Ebnalwaled, A.A. On improving the spectral response of organic dyes sensitizer based on β-cyclodextrin inclusion complex. Optik 2019, 178, 197–209. [Google Scholar] [CrossRef]
- Rajakumar, G.; Rahuman, A.A.; Roopan, S.M.; Khanna, V.G.; Elango, G.; Kamaraj, C.; Zahir, A.A.; Velayutham, K. Fungus-mediated biosynthesis and characterization of TiO2 nanoparticles and their activity against pathogenic bacteria. Spectrochim. Acta Part A 2012, 91, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.V.R.; Oliveira, J.L.; Fraceto, L.F. Poly(ethylene glycol) and Cyclodextrin-Grafted Chitosan: From Methodologies to Preparation and Potential Biotechnological Applications. Front. Chem. 2017, 5, 93. [Google Scholar] [CrossRef]
- Garay, R.P.; El-Gewely, R.; Armstrong, J.K.; Garratty, G.; Richette, P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin. Drug Deliv. 2012, 9, 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Zaas, D.W.; Duncan, M.; Wright, J.R.; Abraham, S.N. The role of lipid rafts in the pathogenesis of bacterial infections. Biochim. Biophys. Acta Mol. Cell Res. 2005, 1746, 305–313. [Google Scholar] [CrossRef]
- Barnaby, R.; Koeppen, K.; Stanton, B.A. Cyclodextrins reduce the ability of Pseudomonas aeruginosa outer-membrane vesicles to reduce CFTR Cl– secretion. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L206–L215. [Google Scholar] [CrossRef]
- Morohoshi, T.; Tokita, K.; Ito, S.; Saito, Y.; Maeda, S.; Kato, N.; Ikeda, T. Inhibition of quorum sensing in gram-negative bacteria by alkylamine-modified cyclodextrins. J. Biosci. Bioeng. 2013, 116, 175–179. [Google Scholar] [CrossRef]
- Karginov, V.A. Cyclodextrin derivatives as anti-infectives. Curr. Opin. Pharmacol. 2013, 13, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Singh, A.; Gautam, P.K.; Verma, A.; Singh, V.; Shivapriya, P.M.; Shivalkar, S.; Sahoo, A.K.; Samanta, S.K. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol. Rep. 2020, 25, e00427. [Google Scholar] [CrossRef] [PubMed]
- Jihad, M.A.; Noori, F.T.M.; Jabir, M.S.; Albukhaty, S.; AlMalki, F.A.; Alyamani, A.A. Polyethylene Glycol Functionalized Graphene Oxide Nanoparticles Loaded with Nigella sativa Extract: A Smart Antibacterial Therapeutic Drug Delivery System. Molecules 2021, 26, 3067. [Google Scholar] [CrossRef] [PubMed]
- Elamary, R.; Salem, W.M. Optimizing and purifying extracellular amylase from soil bacteria to inhibit clinical biofilm-forming bacteria. Peer J 2020, 8, e10288. [Google Scholar] [CrossRef]
- Rodrigues, W.F.; Miguel, C.B.; Nogueira, A.P.O.; Ueira-Vieira, C.; Paulino, T.D.P.; Soares, S.D.C.; DeResende, E.A.M.R.; Lazo-Chica, J.E.; Araújo, M.C.; Oliveira, C.J. Antibiotic Resistance of Bacteria Involved in Urinary Infections in Brazil: A Cross-Sectional and Retrospective Study. Int. J. Environ. Res. Public Health 2016, 13, 918. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Misba, L.D.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Parsek, M.R.; Singh, P.K. Bacterial biofilms: An emerging link to disease pathogenesis. Annu. Rev. Microbiol. 2003, 57, 677–701. [Google Scholar] [CrossRef]
- Shakibaie, M.R. Bacterial Biofilm and its Clinical Implications. Ann. Microbiol. Res. 2018, 2, 45–50. [Google Scholar]


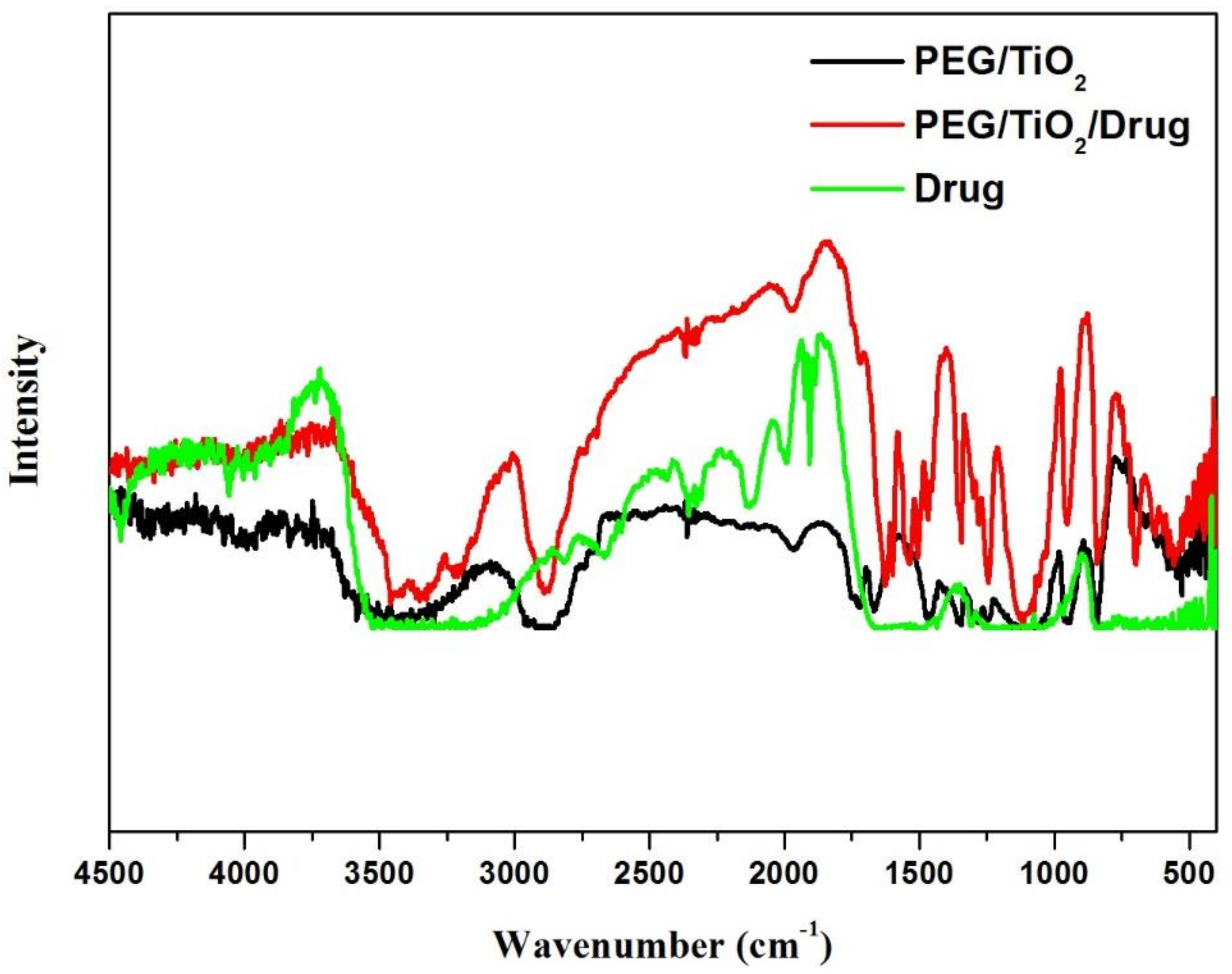
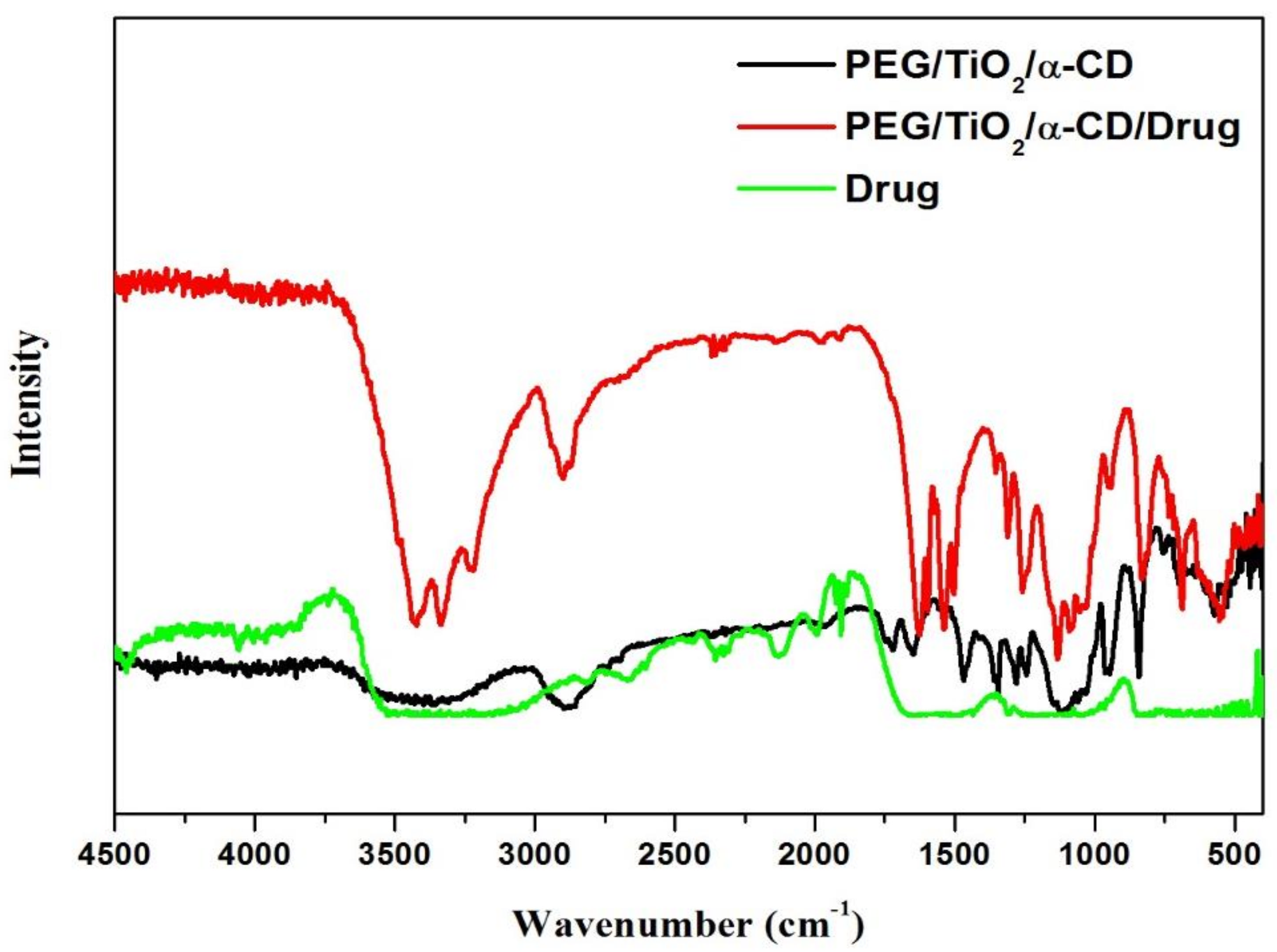
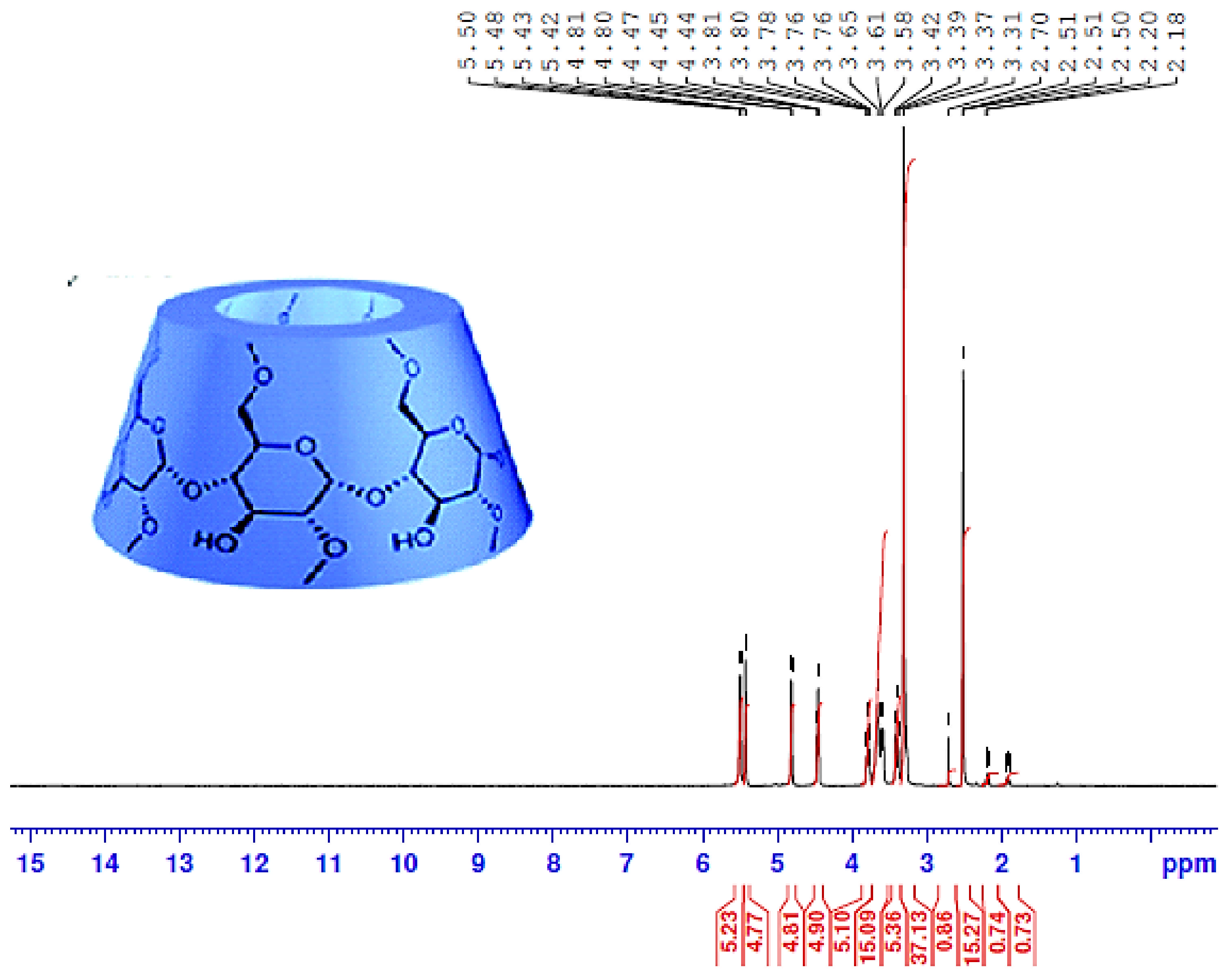

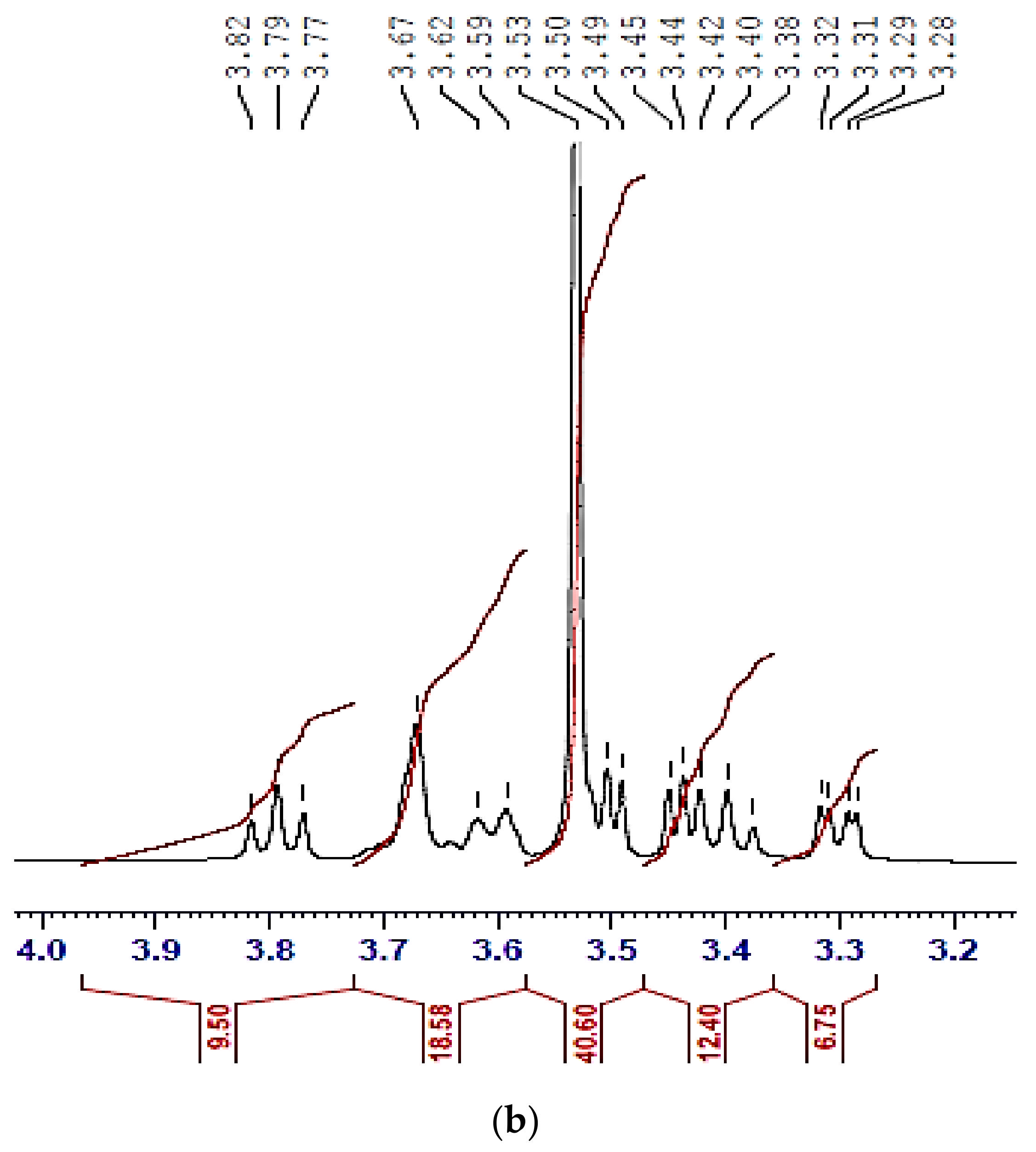

| Functional Group | Wavenumber, cm−1 | Δδ | |
|---|---|---|---|
| α-CD | PEG/α-CD (1) | ||
| ν[OH] symmetric | 3407.64 | 3401.81 | −5.83 |
| ν[CH-aliphatic] | 2927.41 | 2937.05 | +9.64 |
| ν[C–O–C] | 1157.08 | 1159.97 | +2.89 |
| ν[OH] bending | 1078.01 | 1080.90 | +2.89 |
| Functional Group | Wavenumber, cm−1 | Δν | |
|---|---|---|---|
| Drug | PEG/α-CD/Drug (4) | ||
| ν[NH2] symmetric | 3144.37 | 3129.90 | −14.47 |
| ν[CH-aromatic] | 3010.33 | 3016.06 | +5.73 |
| ν[SO2] | 1367.28 | 1374.99 | +7.71 |
| ν[C=N] | 1567.84 | 1550.37 | −17.47 |
| Functional Group | Wavenumber, cm−1 | Δν | |
|---|---|---|---|
| Drug | PEG/TiO2/Drug (3) | ||
| ν[NH2] symmetric | 3144.37 | 3130.86 | −13.51 |
| ν[CH-aromatic] | 3010.33 | 3019.97 | +9.64 |
| ν[SO2] | 1367.28 | 1397.17 | +29.89 |
| ν[C=N] | 1567.84 | 1579.41 | +11.57 |
| Functional Group | Wavenumber, cm−1 | Δν | |
|---|---|---|---|
| Drug | PEG/α-CD/TiO2/Drug (6) | ||
| ν[NH2] symmetric | 3144.37 | 3136.56 | −7.81 |
| ν[CH-aromatic] | 3010.33 | 3005.51 | −4.62 |
| ν[SO2] | 1367.28 | 1382.71 | +15.43 |
| ν[C=N] | 1567.84 | 1577.48 | +9.64 |
| Functional Group | Wavenumber, cm−1 | Δδ | |
|---|---|---|---|
| PEG/α-CD (1) | PEG/TiO2/α-CD (3) | ||
| ν[OH] symmetric | 3401.81 | 3417.24 | +15.43 |
| ν[CH-aliphatic] | 2937.05 | 2898.15 | −38.9 |
| ν[C–O–C] | 1159.97 | 1161.90 | +1.93 |
| ν[OH] bending | 1080.90 | 1079.94 | −0.96 |
| Compound | H-1 | H-2 | H-3 | H-4 | H-5 | H-6 |
|---|---|---|---|---|---|---|
| α-CD | 4.81 | 3.65 | 3.78 | 3.61 | 3.76 | 3.81 |
| PEG/α-CD (1) | 4.82 | 3.44 | 3.62 | 3.28 | 3.49 | 3.79 |
| Δδ | 0.01 | 0.21 | 0.16 | 0.33 | 0.27 | 0.02 |
| Strains | E. coli | Ps. aeruginosa | S. aureus | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | MIC | MBC | BIP | MIC | MBC | BIP | MIC | MBC | BIP |
| PEG/αCD (1) | 17 ± 1 | 17 ± 1 | 52.5 | 17± | 17± | 41.2 | 8.5 ± 0.5 | 8.5 ± 0.5 | 15.5 |
| PEG/αCD/Drug (4) | -ve | -ve | -ve | -ve | -ve | -ve | -ve | -ve | -ve |
| PEG/TiO2 (2) | 20 ± 2 | 20 ± 2 | 39.5 | 20 ± 2 | 20 ± 2 | 34.8 | 10 ± 1 | 10 ± 1 | 9.5 |
| PEG/TiO2/Drug (5) | 14 ± 2 | 14 ± 2 | 53.9 | 14 ± 2 | 14 ± 2 | 44.5 | 7 ± 1 | 7 ± 1 | 13.8 |
| PEG/αCD/TiO2 (3) | 6 ± 0.5 | 6 ± 0.5 | 73.2 | 6 ± 0.5 | 6 ± 0.5 | 60.7 | -ve | -ve | -ve |
| PEG/αCD/TiO2/Drug (6) | 20 + 1 | 20 + 1 | 33.3 | 20 + 1 | 20 + 1 | 37.3 | -ve | -ve | -ve |
| Pure Drug (SULFA) | -ve | -ve | -ve | -ve | -ve | -ve | -ve | -ve | −ve |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dardeer, H.M.; Toghan, A.; Zaki, M.E.A.; Elamary, R.B. Design, Synthesis and Evaluation of Novel Antimicrobial Polymers Based on the Inclusion of Polyethylene Glycol/TiO2 Nanocomposites in Cyclodextrin as Drug Carriers for Sulfaguanidine. Polymers 2022, 14, 227. https://doi.org/10.3390/polym14020227
Dardeer HM, Toghan A, Zaki MEA, Elamary RB. Design, Synthesis and Evaluation of Novel Antimicrobial Polymers Based on the Inclusion of Polyethylene Glycol/TiO2 Nanocomposites in Cyclodextrin as Drug Carriers for Sulfaguanidine. Polymers. 2022; 14(2):227. https://doi.org/10.3390/polym14020227
Chicago/Turabian StyleDardeer, Hemat M., Arafat Toghan, Magdi E. A. Zaki, and Rokaia B. Elamary. 2022. "Design, Synthesis and Evaluation of Novel Antimicrobial Polymers Based on the Inclusion of Polyethylene Glycol/TiO2 Nanocomposites in Cyclodextrin as Drug Carriers for Sulfaguanidine" Polymers 14, no. 2: 227. https://doi.org/10.3390/polym14020227
APA StyleDardeer, H. M., Toghan, A., Zaki, M. E. A., & Elamary, R. B. (2022). Design, Synthesis and Evaluation of Novel Antimicrobial Polymers Based on the Inclusion of Polyethylene Glycol/TiO2 Nanocomposites in Cyclodextrin as Drug Carriers for Sulfaguanidine. Polymers, 14(2), 227. https://doi.org/10.3390/polym14020227