Synthesis and Study of Thermoresponsive Amphiphilic Copolymers via RAFT Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. RAFT Polymerization of Styrene
2.3. RAFT Polymerization of p(St-co-PEO5MEMA)
2.4. Analysis and Characterization
3. Results and Discussion
3.1. Study of Styrene Polymerization
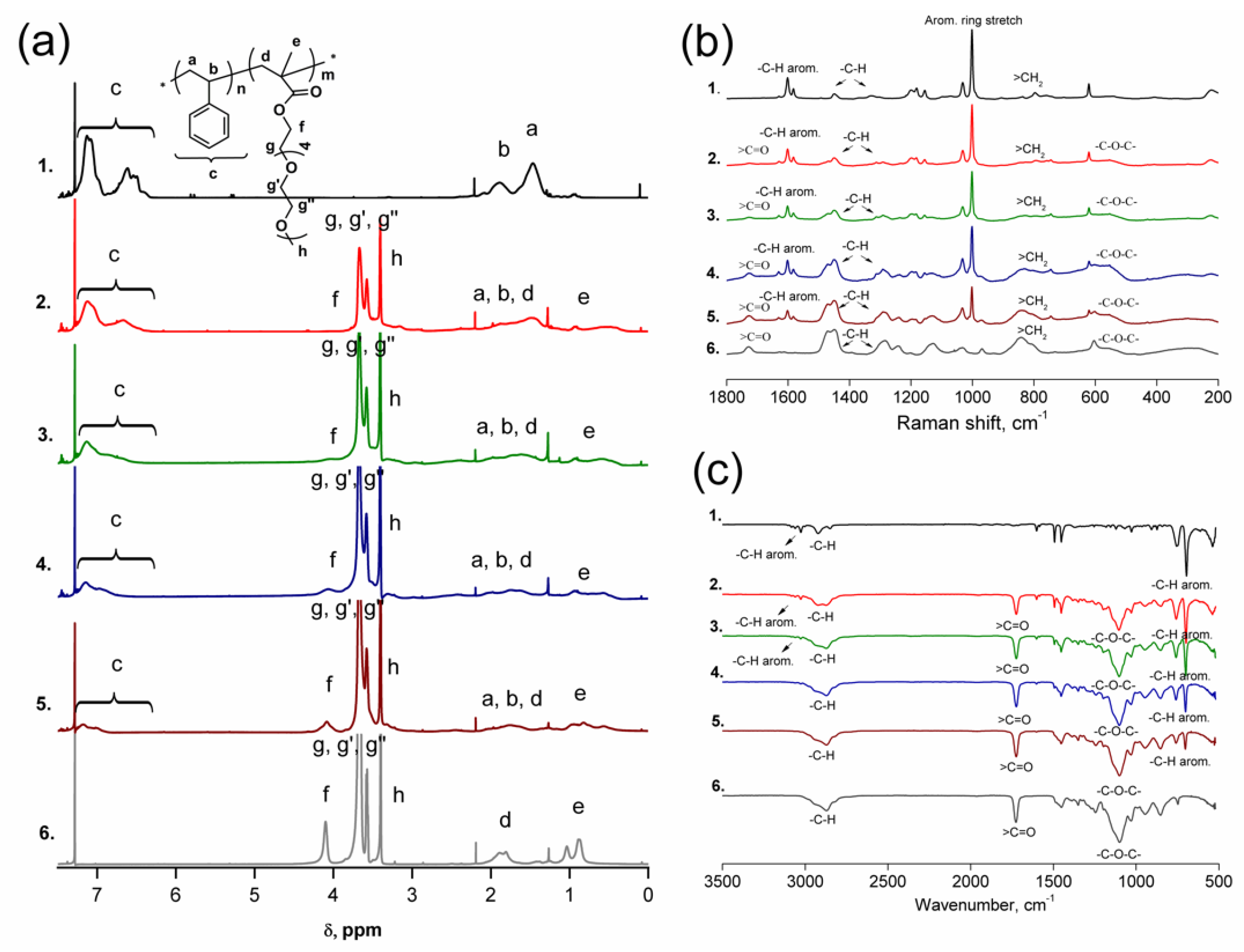
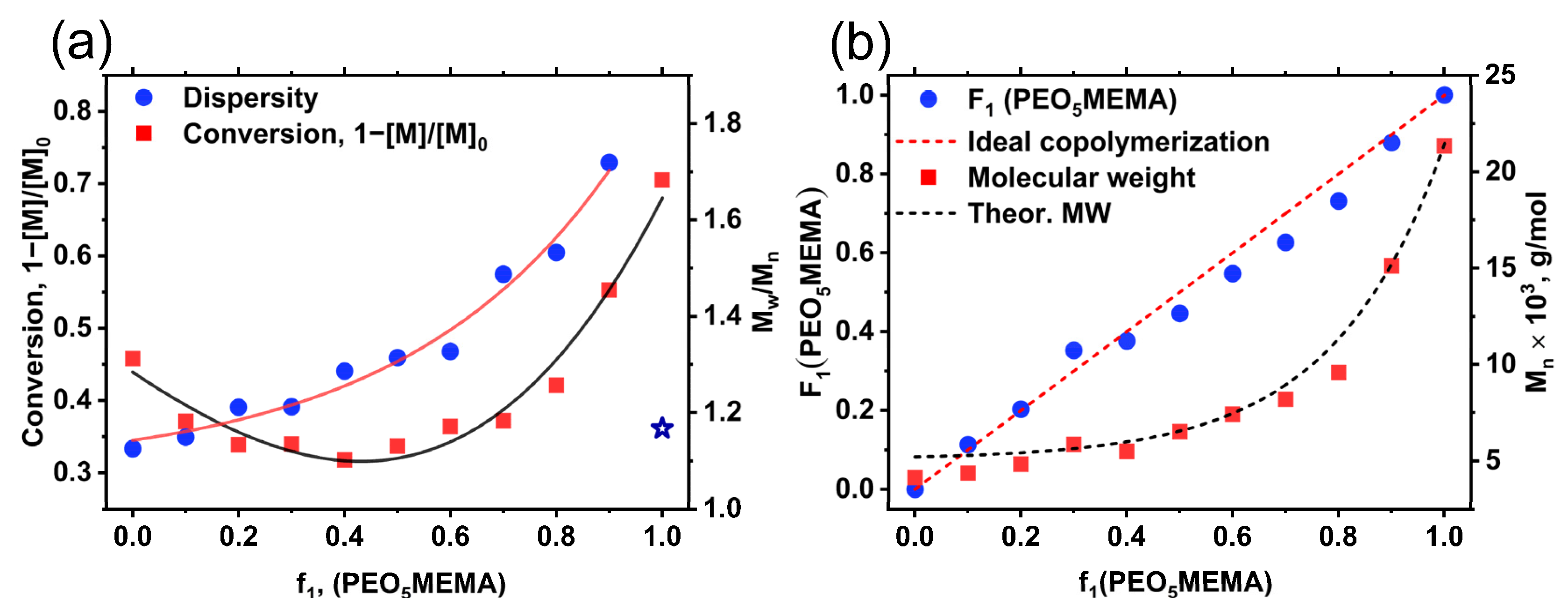
3.2. Synthesis of p(St-co-PEO5MEMA) Copolymers
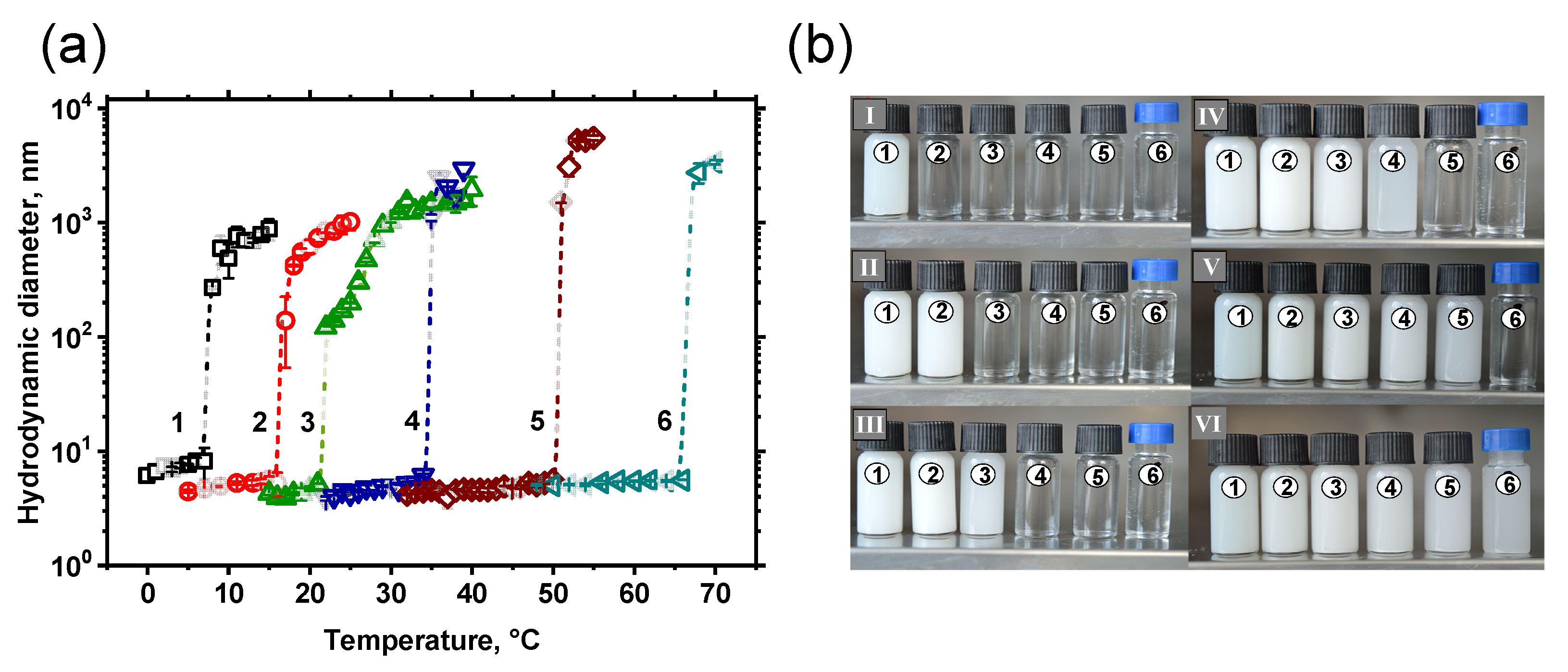
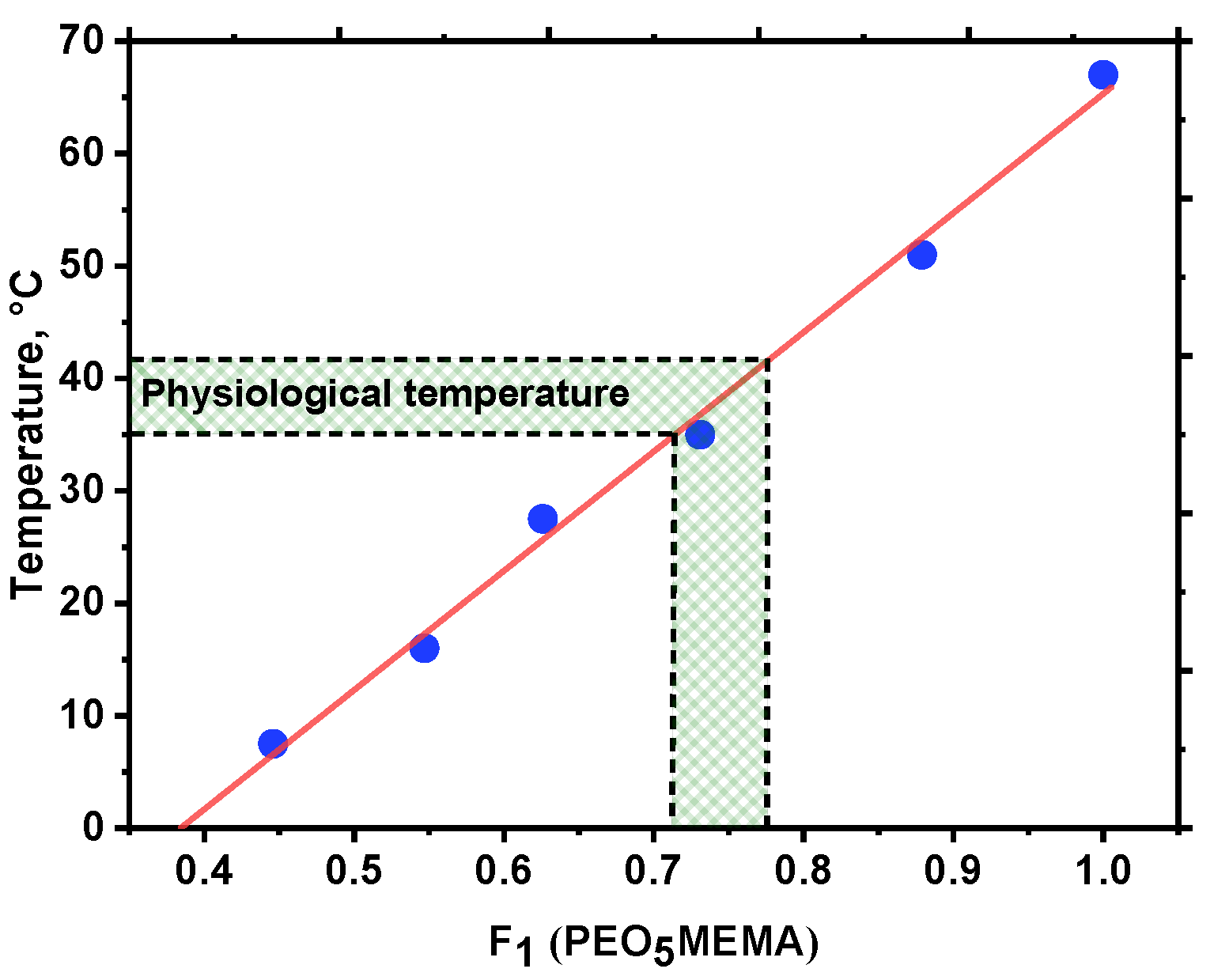
3.3. Thermoresponsivity of Amphiphilic Copolymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-Responsive Polymers and Their Applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef] [Green Version]
- Indermun, S.; Govender, M.; Kumar, P.; Choonara, Y.E.; Pillay, V. Stimuli-Responsive Polymers as Smart Drug Delivery Systems: Classifications Based on Carrier Type and Triggered-Release Mechanism. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2018; Volume 1, pp. 43–58. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Wan, Y.; Carvalho, W.; Hu, L.; Serpe, M.J. Stimuli-Responsive Polymers for Sensing and Reacting to Environmental Conditions. Prog. Polym. Sci. 2021, 116, 101386. [Google Scholar] [CrossRef]
- Patil, A.O.; Schulz, D.N.; Novak, B.M. Functional Polymers: Modern Synthetic Methods and Novel Structures; American Chemical Society: Washington, DC, USA, 1998. [Google Scholar] [CrossRef] [Green Version]
- Shu, T.; Hu, L.; Shen, Q.; Jiang, L.; Zhang, Q.; Serpe, M.J. Stimuli-Responsive Polymer-Based Systems for Diagnostic Applications. J. Mater. Chem. B 2020, 8, 7042–7061. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Li, Y. Strategies To Design and Synthesize Polymer-Based Stimuli-Responsive Drug-Delivery Nanosystems. ChemBioChem 2020, 21, 1236–1253. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.L. Review of Stimuli-Responsive Polymers in Drug Delivery and Textile Application. Molecules 2019, 24, 2547. [Google Scholar] [CrossRef] [Green Version]
- Mirvakili, S.M.; Hunter, I.W. Artificial Muscles: Mechanisms, Applications, and Challenges. Adv. Mater. 2018, 30, 1704407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.M.; Serpe, M.J. Stimuli-Responsive Polymers for Actuation. ChemPhysChem 2017, 18, 1451–1465. [Google Scholar] [CrossRef] [Green Version]
- Moad, G. RAFT Polymerization to Form Stimuli-Responsive Polymers. Polym. Chem. 2017, 8, 177–219. [Google Scholar] [CrossRef]
- Gaynor, S.G.; Matyjaszewski, K. Functionalized Polymers by Atom Transfer Radical Polymerization. ACS Symp. Ser. 2000, 768, 347–360. [Google Scholar] [CrossRef]
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-Mediated Polymerization. Prog. Polym. Sci. 2013, 38, 63–235. [Google Scholar] [CrossRef]
- Sun, H.; Kabb, C.P.; Sims, M.B.; Sumerlin, B.S. Architecture-Transformable Polymers: Reshaping the Future of Stimuli-Responsive Polymers. Prog. Polym. Sci. 2019, 89, 61–75. [Google Scholar] [CrossRef]
- Corrigan, N.; Jung, K.; Moad, G.; Hawker, C.J.; Matyjaszewski, K.; Boyer, C. Reversible-Deactivation Radical Polymerization (Controlled/Living Radical Polymerization): From Discovery to Materials Design and Applications. Prog. Polym. Sci. 2020, 111, 101311. [Google Scholar] [CrossRef]
- Chernikova, E.V.; Zaitsev, S.D.; Plutalova, A.V.; Mineeva, K.O.; Zotova, O.S.; Vishnevetsky, D.V. Control over the Relative Reactivities of Monomers in RAFT Copolymerization of Styrene and Acrylic Acid. RSC Adv. 2018, 8, 14300–14310. [Google Scholar] [CrossRef] [Green Version]
- Sponchioni, M.; Capasso Palmiero, U.; Moscatelli, D. Thermo-Responsive Polymers: Applications of Smart Materials in Drug Delivery and Tissue Engineering. Mater. Sci. Eng. C 2019, 102, 589–605. [Google Scholar] [CrossRef]
- Kim, Y.J.; Matsunaga, Y.T. Thermo-Responsive Polymers and Their Application as Smart Biomaterials. J. Mater. Chem. B 2017, 5, 4307–4321. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, G.; Tsitsilianis, C. LCST Polymers: Thermoresponsive Nanostructured Assemblies towards Bioapplications. Polymer 2020, 211, 123146. [Google Scholar] [CrossRef]
- De Oliveira, T.E.; Mukherji, D.; Kremer, K.; Netz, P.A. Effects of Stereochemistry and Copolymerization on the LCST of PNIPAm. J. Chem. Phys. 2017, 146, 034904. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Deshmukh, S.A.; Kamath, G.; Sankaranarayanan, S.K.R.S.; Balasubramanian, G. Controlling the Aqueous Solubility of PNIPAM with Hydrophobic Molecular Units. Comput. Mater. Sci. 2017, 126, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Schild, H.G. Poly(N-Isopropylacrylamide): Experiment, Theory and Application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Wu, J.Y.; Liu, S.Q.; Heng, P.W.S.; Yang, Y.Y. Evaluating Proteins Release from, and Their Interactions with, Thermosensitive Poly (N-Isopropylacrylamide) Hydrogels. J. Control. Release 2005, 102, 361–372. [Google Scholar] [CrossRef]
- Fournier, D.; Hoogenboom, R.; Thijs, H.M.L.; Paulus, R.M.; Schubert, U.S. Tunable PH- and Temperature-Sensitive Copolymer Libraries by Reversible Addition-Fragmentation Chain Transfer Copolymerizations of Methacrylates. Macromolecules 2007, 40, 915–920. [Google Scholar] [CrossRef]
- Butun, V.; Top, R.B.; Ufuklar, S. Synthesis and Characterization of Novel “Schizophrenic” Water-Soluble Triblock Copolymers and Shell Cross-Linked Micelles. Macromolecules 2006, 39, 1216–1225. [Google Scholar] [CrossRef]
- Liu, L.; Wu, C.; Zhang, J.; Zhang, M.; Liu, Y.; Wang, X.; Fu, G. Controlled Polymerization of 2-(Diethylamino)Ethyl Methacrylate and Its Block Copolymer with N-Isopropylacrylamide by RAFT Polymerization. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3294–3305. [Google Scholar] [CrossRef]
- Xian, Y.; Shui, Y.; Li, M.; Pei, C.; Zhang, Q.; Yao, Y. PH-Dependent Thermoresponsive Poly[2-(Diethylamino)Ethyl Acrylamide]-Grafted PVDF Membranes with Switchable Wettability for Efficient Emulsion Separation. J. Appl. Polym. Sci. 2020, 137, 49032. [Google Scholar] [CrossRef]
- Kasza, G.; Stumphauser, T.; Bisztrán, M.; Szarka, G.; Hegedüs, I.; Nagy, E.; Iván, B. Thermoresponsive Poly(N,N-Diethylacrylamide-Co-Glycidyl Methacrylate) Copolymers and Its Catalytically Active α-Chymotrypsin Bioconjugate with Enhanced Enzyme Stability. Polymers 2021, 13, 987. [Google Scholar] [CrossRef]
- Ramírez-Jiménez, A.; Montoya-Villegas, K.A.; Licea-Claverie, A.; Gónzalez-Ayón, M.A. Tunable Thermo-Responsive Copolymers from DEGMA and OEGMA Synthesized by RAFT Polymerization and the Effect of the Concentration and Saline Phosphate Buffer on Its Phase Transition. Polymers 2019, 11, 1657. [Google Scholar] [CrossRef] [Green Version]
- Lutz, J.F.; Hoth, A. Preparation of Ideal PEG Analogues with a Tunable Thermosensitivity by Controlled Radical Copolymerization of 2-(2-Methoxyethoxy)Ethyl Methacrylate and Oligo(Ethylene Glycol) Methacrylate. Macromolecules 2006, 39, 893–896. [Google Scholar] [CrossRef]
- Lutz, J.F. Polymerization of Oligo(Ethylene Glycol) (Meth)Acrylates: Toward New Generations of Smart Biocompatible Materials. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3459–3470. [Google Scholar] [CrossRef]
- Klimkevicius, V.; Steponaviciute, M.; Makuska, R. Kinetics of RAFT Polymerization and Copolymerization of Vinyl Monomers by Size Exclusion Chromatography. Eur. Polym. J. 2020, 122, 109356. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living Radical Polymerization by the RAFT Process A Second Update. Aust. J. Chem. 2009, 62, 1402–1472. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living Radical Polymerization by the RAFT Process a Third Update. Aust. J. Chem. 2012, 65, 985–1076. [Google Scholar] [CrossRef]
- Klimkevicius, V.; Makuska, R. Successive RAFT Polymerization of Poly(Ethylene Oxide) Methyl Ether Methacrylates with Different Length of PEO Chains Giving Diblock Brush Copolymers. Eur. Polym. J. 2017, 86, 94–105. [Google Scholar] [CrossRef]
- Arita, T.; Buback, M.; Vana, P. Cumyl Dithiobenzoate Mediated RAFT Polymerization of Styrene at High Temperatures. Macromolecules 2005, 38, 7935–7943. [Google Scholar] [CrossRef]
- Ponnusamy, K.; Babu, R.P.; Dhamodharan, R. Synthesis of Block and Graft Copolymers of Styrene by Raft Polymerization, Using Dodecyl-Based Trithiocarbonates as Initiators and Chain Transfer Agents. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 1066–1078. [Google Scholar] [CrossRef]
- Kim, B.S.; Lee, H.K.; Jeong, S.; Lee, J.O.; Paik, H.J. Amphiphilic Gradient Copolymer of [Poly(Ethylene Glycol) Methyl Ether] Methacrylate and Styrene via Atom Transfer Radical Polymerization. Macromol. Res. 2011, 19, 1257–1263. [Google Scholar] [CrossRef]
- Obata, M.; Tanaka, S.; Mizukoshi, H.; Ishihara, E.; Takahashi, M.; Hirohara, S. RAFT Synthesis of Polystyrene-Block-Poly(Polyethylene Glycol Monomethyl Ether Acrylate) for Zinc Phthalocyanine-Loaded Polymeric Micelles as Photodynamic Therapy Photosensitizers. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 560–570. [Google Scholar] [CrossRef]
- Lewis, F.M.; Walling, C.; Cummings, W.; Briggs, E.R.; Mayo, F.R. Copolymerization. IV. Effects of Temperature and Solvents on Monomer Reactivity Ratios. J. Am. Chem. Soc. 1948, 70, 1519–1523. [Google Scholar] [CrossRef]
- Meyer, V.E. Reactivity Ratios of Styrene and Methyl Methacrylate at 90 °C. J. Polym. Sci. Part A-1 Polym. Chem. 1967, 5, 1289–1296. [Google Scholar] [CrossRef]
- Boulding, N.A.; Millican, J.M.; Hutchings, L.R. Understanding Copolymerisation Kinetics for the Design of Functional Copolymers: Via Free Radical Polymerisation. Polym. Chem. 2019, 10, 5665–5675. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef] [Green Version]
- Thanh, T.; Thi, H.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S. The Importance of Poly (Ethylene Glycol) Alternatives for Overcoming PEG Immunogenicity in Drug. Polymers 2020, 3, 298. [Google Scholar] [CrossRef] [Green Version]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abuchowski, A.; McCoy, J.R.; Palczuk, N.C.; van Es, T.; Davis, F.F. Effect of Covalent Attachment of Polyethylene Glycol on Immunogenicity and Circulating Life of Bovine Liver Catalase. J. Biol. Chem. 1977, 252, 3582–3586. [Google Scholar] [CrossRef]
- Sheleg, S.V.; Zhavrid, E.A.; Khodina, T.V.; Kochubeev, G.A.; Istomin, Y.P.; Chalov, V.N.; Zhuravkin, I.N. Photodynamic Therapy with Chlorin E6 for Skin Metastases of Melanoma. Photodermatol. Photoimmunol. Photomed. 2004, 20, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, S.; Hlil, A.R.; Lepiller, C.; Gaudet, J.; Guay, D.; Shi, Z.; Holdcroft, S.; Hay, A.S. Stability and Utility of Pyridyl Disulfide Functionality in RAFT and Conventional Radical Polymerizations. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7207–7224. [Google Scholar] [CrossRef]



| Method | r1 (Styrene) | r2 (PEO5MEMA) | r1 × r2 |
|---|---|---|---|
| Non-linear least squares | 0.83 ± 0.06 | 0.66 ± 0.06 | 0.55 |
| Kelen–Tüdös | 0.81 ± 0.03 | 0.62 ± 0.05 | 0.50 |
| Kelen–Tüdös (high q) | 0.76 ± 0.03 | 0.51 ± 0.02 | 0.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavaliauskaite, M.; Steponaviciute, M.; Kievisaite, J.; Katelnikovas, A.; Klimkevicius, V. Synthesis and Study of Thermoresponsive Amphiphilic Copolymers via RAFT Polymerization. Polymers 2022, 14, 229. https://doi.org/10.3390/polym14020229
Kavaliauskaite M, Steponaviciute M, Kievisaite J, Katelnikovas A, Klimkevicius V. Synthesis and Study of Thermoresponsive Amphiphilic Copolymers via RAFT Polymerization. Polymers. 2022; 14(2):229. https://doi.org/10.3390/polym14020229
Chicago/Turabian StyleKavaliauskaite, Marija, Medeina Steponaviciute, Justina Kievisaite, Arturas Katelnikovas, and Vaidas Klimkevicius. 2022. "Synthesis and Study of Thermoresponsive Amphiphilic Copolymers via RAFT Polymerization" Polymers 14, no. 2: 229. https://doi.org/10.3390/polym14020229







