Construction of a (NNN)Ru-Incorporated Porous Organic Polymer with High Catalytic Activity for β-Alkylation of Secondary Alcohols with Primary Alcohols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
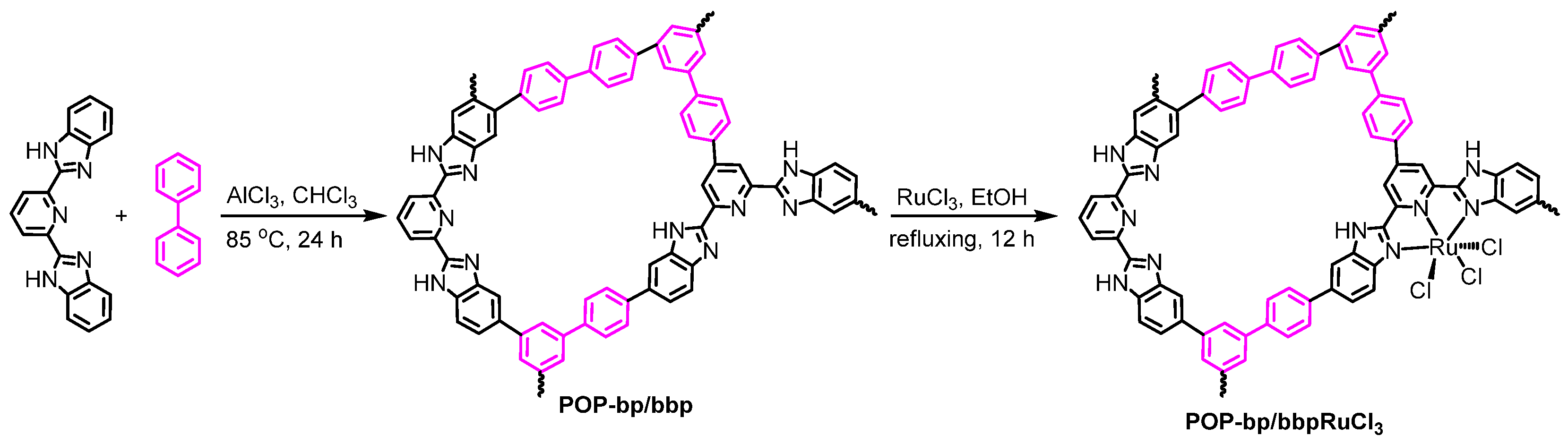
2.2. Synthesis of Porous Organic Polymer (POP)-bp/bbp
2.3. Synthesis of POP-bp/bbpRuCl3
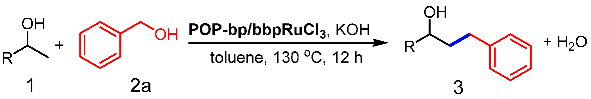
2.4. Typical Procedure for Syntheses of β-Alkylated Secondary Alcohols
2.5. Characterizations
3. Results and Discussion
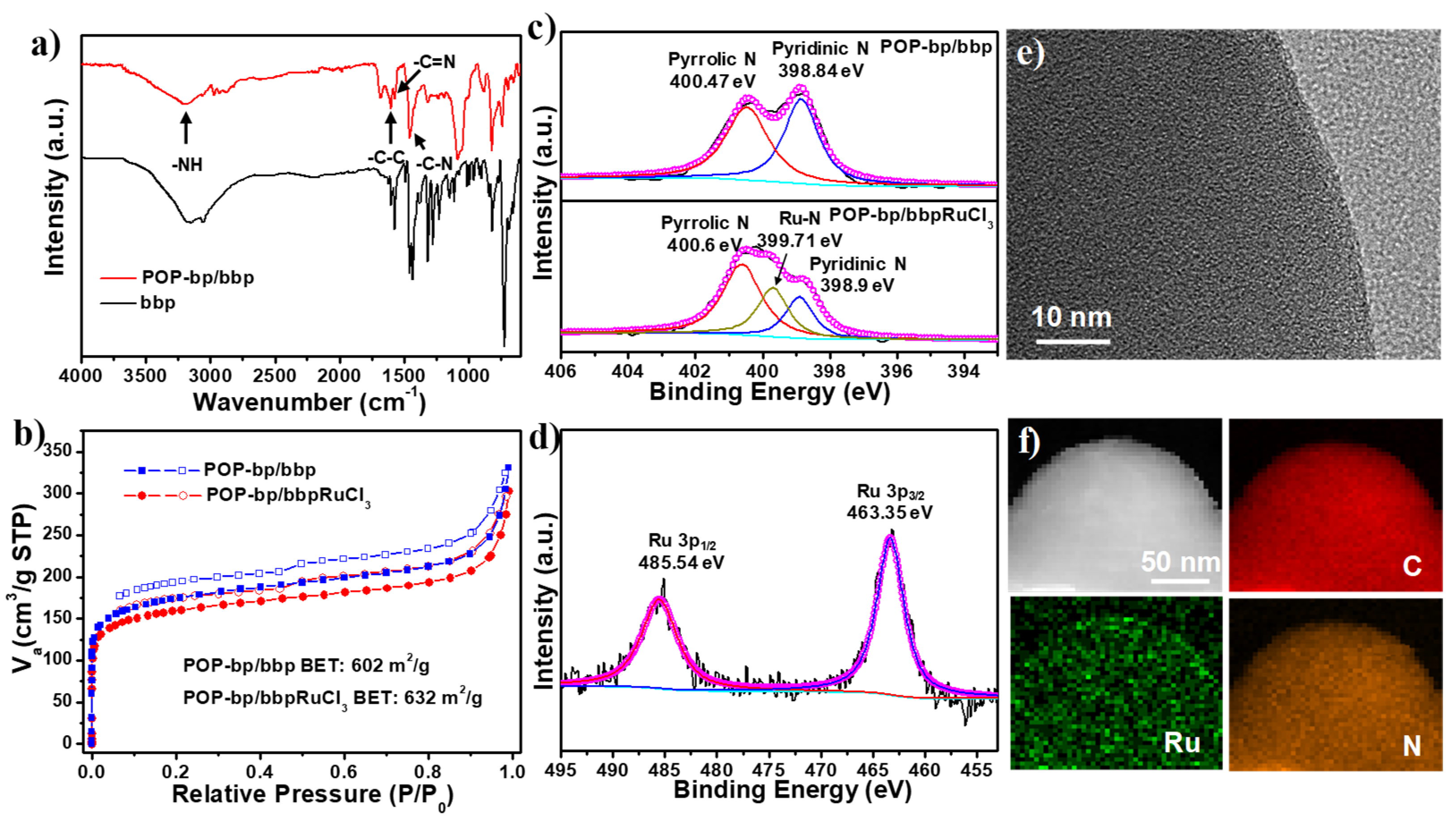
3.1. Subsection Synthesis and Characterization of POP-bp/bbpRuCl3
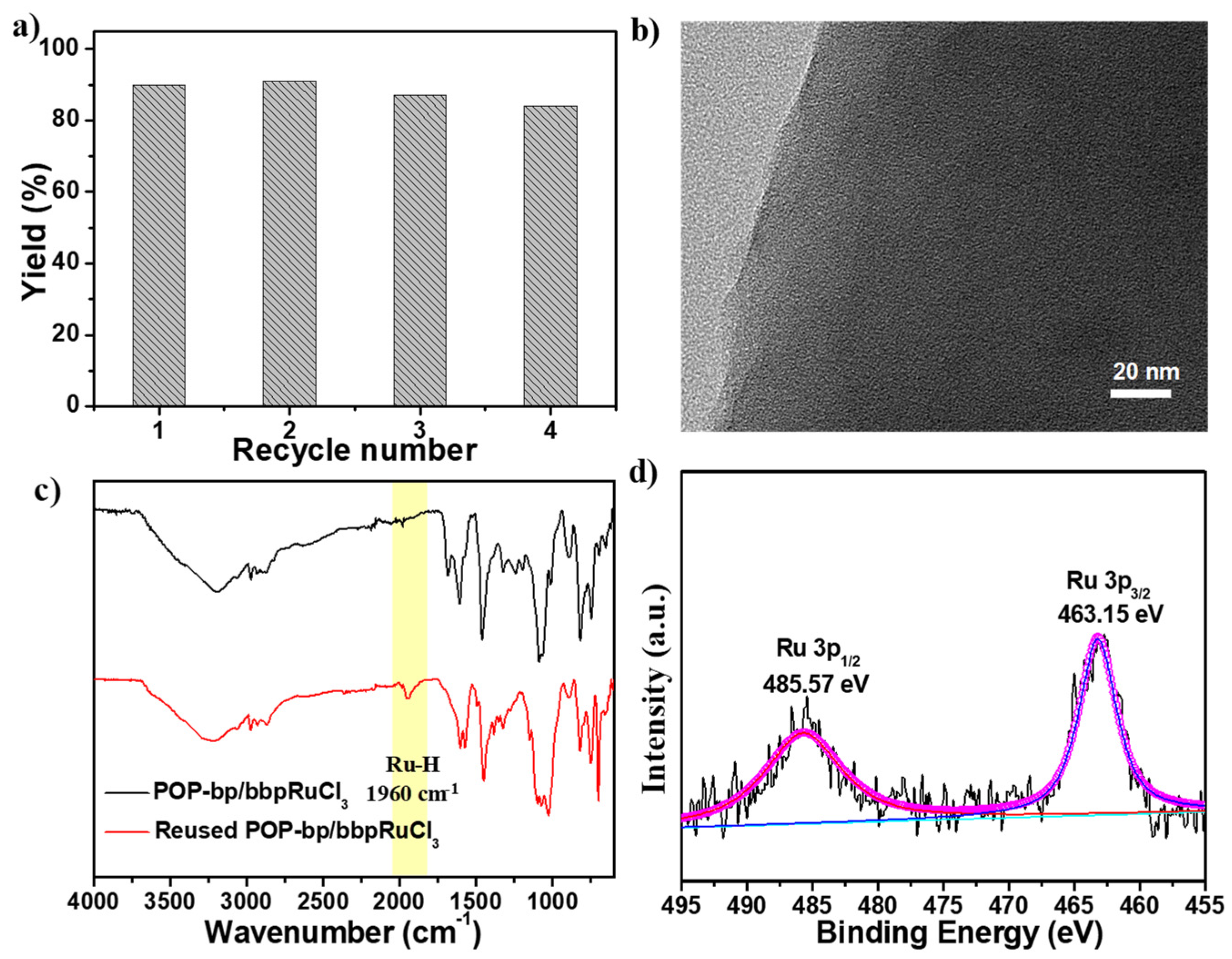
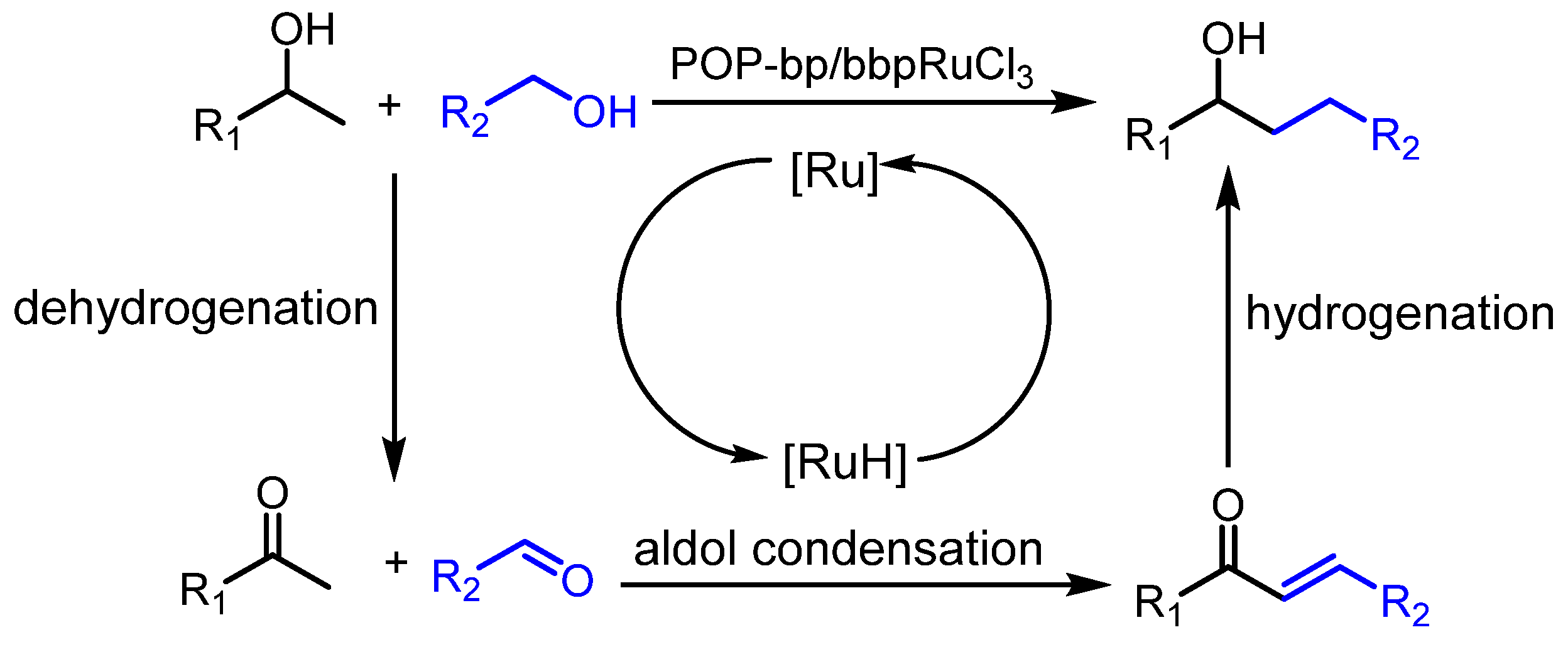
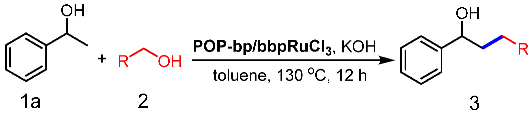
3.2. Catalytic β-Alkylation of Secondary Alcohols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Irrgang, T.; Kempe, R. 3d-Metal catalyzed N- and C-alkylation reactions via borrowing hydrogen or hydrogen autotransfer. Chem. Rev. 2019, 119, 2524–2549. [Google Scholar] [CrossRef]
- Shevick, S.L.; Wilson, C.V.; Kotesova, S.; Kim, D.; Holland, P.L.; Shenvi, R.A. Catalytic hydrogen atom transfer to alkenes: A roadmap for metal hydrides and radicals. Chem. Sci. 2020, 11, 12401–12422. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Shi, Z.J. Upgrading cross-coupling reactions for biaryl syntheses. Acc. Chem. Res. 2019, 52, 161–169. [Google Scholar] [CrossRef]
- Li, H.; Zheng, B.; Huang, K.-W. A new class of PN3-pincer ligands for metal–ligand cooperative catalysis. Coord. Chem. Rev. 2015, 293–294, 116–138. [Google Scholar] [CrossRef] [Green Version]
- Nixon, T.D.; Whittlesey, M.K.; Williams, J.M.J. Transition metal catalysed reactions of alcohols using borrowing hydrogen methodology. Dalton Trans. 2009, 753–762. [Google Scholar] [CrossRef]
- Wang, D.; Astruc, D. The golden age of transfer hydrogenation. Chem. Rev. 2015, 115, 6621–6686. [Google Scholar]
- Gunanathan, C.; Milstein, D. Bond activation and catalysis by ruthenium pincer complexes. Chem. Rev. 2014, 114, 12024–12087. [Google Scholar] [CrossRef] [PubMed]
- Valdés, H.; García-Eleno, M.A.; Canseco-Gonzalez, D.; Morales-Morales, D. Recent advances in catalysis with transition-metal pincer compounds. ChemCatChem 2018, 10, 3136–3172. [Google Scholar] [CrossRef]
- Iuchi, Y.; Obora, Y.; Ishii, Y. Iridium-catalyzed α-alkylation of acetates with primary alcohols and diols. J. Am. Chem. Soc. 2010, 132, 2536–2537. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.Q.; Junge, H.; Beller, M. Selective ruthenium-catalyzed methylation of 2-arylethanols using methanol as C1 feedstock. Chem. Commun. 2014, 50, 14991–14994. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Li, F.; Li, Y.Q.; Zhang, W.B.; Liu, F.H.; Shi, S.L. Base metal-catalyzed alcohol C–C couplings under hydrogen transfer conditions. Tetrahedron Lett. 2018, 59, 1073–1079. [Google Scholar] [CrossRef]
- Allen, L.J.; Crabtree, R.H. Green alcohol couplings without transition metal catalysts: Base-mediated β-alkylation of alcohols in aerobic conditions. Green Chem. 2010, 12, 1362–1364. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, J.; Liu, Q. Aldehyde-catalyzed transition metal-free dehydrative β-alkylation of methyl carbinols with alcohols. Adv. Synth. Catal. 2013, 355, 697–704. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Sarmah, B.K.; Nandi, S.; Srivastava, H.K.; Das, A. Selective catalytic synthesis of α-alkylated ketones and β-disubstituted ketones via acceptorless dehydrogenative cross-coupling of alcohols. Org. Lett. 2021, 23, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.R.; Lalitha, G.; Murugesh, V.; Bruneau, C.; Sharma, G.V.M.; Suresh, S.; Achard, M. Ruthenium phosphine–pyridone catalyzed cross-coupling of alcohols to form α-alkylated ketones. J. Org. Chem. 2017, 82, 10727–10731. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.S.; Kim, B.T.; Kim, H.-S.; Kim, T.-J.; Shim, S.C. Ruthenium-catalyzed one-pot β-alkylation of secondary alcohols with primary alcohols. Organometallics 2003, 22, 3608–3610. [Google Scholar] [CrossRef]
- Shee, S.; Paul, B.; Panja, D.; Roy, B.C.; Chakrabarti, K.; Ganguli, K.; Das, A.; Das, G.K.; Kundu, S. Tandem cross coupling reaction of alcohols for sustainable synthesis of β-alkylated secondary alcohols and flavan derivatives. Adv. Synth. Catal. 2017, 359, 3888–3893. [Google Scholar] [CrossRef]
- Ng, T.W.; Liao, G.; Lau, K.K.; Pan, H.-J.; Zhao, Y. Room-temperature Guerbet reaction with unprecedented catalytic efficiency and enantioselectivity. Angew. Chem. Int. Ed. 2020, 59, 11384–11389. [Google Scholar] [CrossRef]
- Genc, S.; Gulcemal, S.; Gunnaz, S.; Cetinkaya, B.; Gulcemal, D. Iridium-catalyzed alkylation of secondary alcohols with primary alcohols: A route to access branched ketones and alcohols. J. Org. Chem. 2020, 85, 9139–9152. [Google Scholar] [CrossRef]
- Fujita, K.; Asai, C.; Yamaguchi, T.; Hanasaka, F.; Yamaguchi, R. Direct β-alkylation of secondary alcohols with primary alcohols catalyzed by a Cp*Ir complex. Org. Lett. 2005, 7, 4017–4019. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, K.; Xu, C.; Miao, H.; Ding, Y. Synthesis, structures of benzoxazolyl iridium(III) complexes, and applications on C–C and C–N bond formation reactions under solvent-free conditions: Catalytic activity enhanced by noncoordinating anion without silver effect. ACS Catal. 2014, 4, 3910–3918. [Google Scholar] [CrossRef]
- Filonenko, G.A.; van Putten, R.; Hensen, E.J.M.; Pidko, E.A. Catalytic (de)hydrogenation promoted by non-precious metals—Co, Fe and Mn: Recent advances in an emerging field. Chem. Soc. Rev. 2018, 47, 1459–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, A.; Milstein, D. Homogeneous catalysis by cobalt and manganese pincer complexes. ACS Catal. 2018, 8, 11435–11469. [Google Scholar] [CrossRef]
- Pandey, B.; Xu, S.; Ding, K. Selective ketone formations via cobalt-catalyzed β-alkylation of secondary alcohols with primary alcohols. Org. Lett. 2019, 21, 7420–7423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, A.; Wang, W.; Huang, Y.; Liu, X. Co–N–C catalyst for C–C coupling reactions: On the catalytic performance and active sites. ACS Catal. 2015, 5, 6563–6572. [Google Scholar] [CrossRef]
- Alonso, F.; Riente, P.; Yus, M. Nickel nanoparticles in hydrogen transfer reactions. Acc. Chem. Res. 2011, 44, 379–391. [Google Scholar] [CrossRef]
- Tang, G.; Cheng, C.-H. Synthesis of α-hydroxy carboxylic acids via a nickel(II)-catalyzed hydrogen transfer process. Adv. Synth. Catal. 2011, 353, 1918–1922. [Google Scholar] [CrossRef]
- Shimura, K.; Kon, K.; Hakim Siddiki, S.M.A.; Shimizu, K.-I. Self-coupling of secondary alcohols by Ni/CeO2 catalyst. Appl. Catal. A-Gen. 2013, 462–463, 137–142. [Google Scholar] [CrossRef]
- Babu, R.; Subaramanian, M.; Midya, S.P.; Balaraman, E. Nickel-catalyzed Guerbet type reaction: C-Alkylation of secondary alcohols via double (de)hydrogenation. Org. Lett. 2021, 23, 3320–3325. [Google Scholar] [CrossRef]
- Alanthadka, A.; Bera, S.; Vellakkaran, M.; Banerjee, D. Nickel-catalyzed double dehydrogenative coupling of secondary alcohols and β-amino alcohols to access substituted pyrroles. J. Org. Chem. 2019, 84, 13557–13564. [Google Scholar] [CrossRef]
- Liu, T.; Wang, L.; Wu, K.; Yu, Z. Manganese-catalyzed β-alkylation of secondary alcohols with primary alcohols under phosphine-free conditions. ACS Catal. 2018, 8, 7201–7207. [Google Scholar] [CrossRef]
- Lan, X.-B.; Ye, Z.; Liu, J.; Huang, M.; Shao, Y.; Cai, X.; Liu, Y.; Ke, Z. Sustainable and selective alkylation of deactivated secondary alcohols to ketones by non-bifunctional pincer N-heterocyclic carbene manganese. ChemSusChem 2020, 13, 2557–2563. [Google Scholar] [CrossRef]
- El-Sepelgy, O.; Matador, E.; Brzozowska, A.; Rueping, M. C-Alkylation of secondary alcohols by primary alcohols through manganese-catalyzed double hydrogen autotransfer. ChemSusChem 2019, 12, 3099–3102. [Google Scholar] [CrossRef] [PubMed]
- Kaithal, A.; van Bonn, P.; Hçlscher, M.; Leitner, W. Manganese(I)-catalyzed β-methylation of alcohols using methanol as C1 source. Angew. Chem. Int. Ed. 2020, 59, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, S.; Shao, Z.; Wang, Y.; Liu, Q. Manganese-catalyzed upgrading of ethanol into 1-butanol. J. Am. Chem. Soc. 2017, 139, 11941–11948. [Google Scholar] [CrossRef]
- Kulkarni, N.V.; Brennessel, W.W.; Jones, W.D. Catalytic upgrading of ethanol to n-butanol via manganese-mediated Guerbet reaction. ACS Catal. 2018, 8, 997–1002. [Google Scholar] [CrossRef]
- Cano, R.; Yus, M.; Ramon, D.J. First practical cross-alkylation of primary alcohols with a new and recyclable impregnated iridium on magnetite catalyst. Chem. Commun. 2012, 48, 7628–7630. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, W.; Wu, C.; Gong, Y.; Zhang, J.; Shen, C. Upgrading n-butanol to branched alcohols over Ni/CaxMgyO. ACS Sustain. Chem. Eng. 2020, 8, 16960–16967. [Google Scholar] [CrossRef]
- Wang, D.; Guo, X.-Q.; Wang, C.-X.; Wang, Y.-N.; Zhong, R.; Zhu, X.-H.; Cai, L.-H.; Gao, Z.-W.; Hou, X.-F. An efficient and recyclable catalyst for N-alkylation of amines and β-alkylation of secondary alcohols with primary alcohols: SBA-15 supported N-heterocyclic carbene iridium complex. Adv. Synth. Catal. 2013, 355, 1117–1125. [Google Scholar] [CrossRef]
- Wu, K.; He, W.; Sun, C.; Yu, Z. Bimetallic Pt–Sn/γ-Al2O3 catalyzed β-alkylation of secondary alcohols with primary alcohols under solvent-free conditions. Tetrahedron Lett. 2016, 57, 4017–4020. [Google Scholar] [CrossRef]
- Oikawa, K.; Itoh, S.; Yano, H.; Kawasaki, H.; Obora, Y. Preparation and use of DMF-stabilized iridium nanoclusters as methylation catalysts using methanol as the C1 source. Chem. Commun. 2017, 53, 1080–1083. [Google Scholar] [CrossRef]
- Zhang, Y.; Riduan, S.N. Functional porous organic polymers for heterogeneous catalysis. Chem. Soc. Rev. 2012, 41, 2083–2094. [Google Scholar] [CrossRef]
- Tan, L.; Tan, B. Hypercrosslinked porous polymer materials: Design, synthesis, and applications. Chem. Soc. Rev. 2017, 46, 3322–3356. [Google Scholar] [CrossRef]
- Enjamuri, N.; Sarkar, S.; Reddy, B.M.; Mondal, J. Design and catalytic application of functional porous organic polymers: Opportunities and challenges. Chem. Rec. 2019, 19, 1782–1792. [Google Scholar] [CrossRef]
- Sun, Q.; Dai, Z.; Meng, X.; Wang, L.; Xiao, F.-S. Task-specific design of porous polymer heterogeneous catalysts beyond homogeneous counterparts. ACS Catal. 2015, 5, 4556–4567. [Google Scholar] [CrossRef]
- Giri, A.; Hussain, M.D.W.; Sk, B.; Patra, A. Connecting the dots: Knitting C-phenylresorcin[4]arenes with aromatic linkers for task-specific porous organic polymers. Chem. Mater. 2019, 31, 8440–8450. [Google Scholar] [CrossRef]
- Lee, J.M.; Briggs, M.E.; Hasell, T.; Cooper, A.I. Hyperporous carbons from hypercrosslinked polymers. Adv. Mater. 2016, 28, 9804–9810. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.; Molla, R.A.; Kumari, V.; Islam, S.M.; Bhaumik, A. Zn(ii) assisted synthesis of porous salen as an efficient heterogeneous scaffold for capture and conversion of CO2. Chem. Commun. 2015, 51, 15732–15735. [Google Scholar] [CrossRef]
- Li, L.-H.; Feng, X.-L.; Cui, X.-H.; Ma, Y.-X.; Ding, S.-Y.; Wang, W. Salen-based covalent organic framework. J. Am. Chem. Soc. 2017, 139, 6042–6045. [Google Scholar] [CrossRef]
- Li, B.; Guan, Z.; Wang, W.; Yang, X.; Hu, J.; Tan, B.; Li, T. Highly dispersed Pd catalyst locked in knitting aryl network polymers for Suzuki–Miyaura coupling reactions of aryl chlorides in aqueous media. Adv. Mater. 2012, 24, 3390–3395. [Google Scholar] [CrossRef]
- Song, K.; Liu, P.; Wang, J.; Pang, L.; Chen, J.; Hussain, I.; Tan, B.; Li, T. Controlled synthesis of uniform palladium nanoparticles on novel micro-porous carbon as a recyclable heterogeneous catalyst for the Heck reaction. Dalton Trans. 2015, 44, 13906–13913. [Google Scholar] [CrossRef] [PubMed]
- Mondal, J.; Biswas, A.; Chiba, S.; Zhao, Y. Cu0 nanoparticles deposited on nanoporous polymers: A recyclable heterogeneous nanocatalyst for Ullmann coupling of aryl halides with amines in water. Sci. Rep. 2015, 5, 8294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhanja, P.; Modak, A.; Bhaumik, A. Porous organic polymers for CO2 storage and conversion reactions. ChemCatChem 2019, 11, 244–257. [Google Scholar] [CrossRef]
- Whiteoak, C.J.; Henseler, A.H.; Ayats, C.; Kleij, A.W.; Pericàs, M.A. Conversion of oxiranes and CO2 to organic cyclic carbonates using a recyclable, bifunctional polystyrene-supported organocatalyst. Green Chem. 2014, 16, 1552–1559. [Google Scholar] [CrossRef]
- Lan, L.X.; Du, C.; Cao, L.; She, T.; Li, Y.; Bai, G. Ultrafine Ag nanoparticles encapsulated by covalent triazine framework nanosheets for CO2 conversion. ACS Appl. Mater. Interfaces 2018, 10, 38953–38962. [Google Scholar] [CrossRef]
- Dang, Q.Q.; Liu, C.Y.; Wang, X.M.; Zhang, X.M. Novel covalent triazine framework for high-performance CO2 capture and alkyne carboxylation reaction. ACS Appl. Mater. Interfaces 2018, 10, 27972–27978. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Xu, Z.; Li, H.-Y.; Young, D.J.; Ren, Z.-G.; Li, H.-X. Synthesis of a pyrazole-based microporous organic polymer for high-performance CO2 capture and alkyne carboxylation. ACS Appl. Polym. Mater. 2020, 2, 4512–4520. [Google Scholar] [CrossRef]
- Dhanalaxmi, K.; Yadav, R.; Kundu, S.K.; Reddy, B.M.; Amoli, V.; Sinha, A.K.; Mondal, J. MnFe2O4 nanocrystals wrapped in a porous organic polymer: A designed architecture for water-splitting photocatalysis. Chem. Eur. J. 2016, 22, 15639–15644. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Wang, R. In situ formation of well-dispersed palladium nanoparticles immobilized in imidazolium-based organic ionic polymers. Chem. Commun. 2014, 50, 10871–10874. [Google Scholar] [CrossRef]
- Mondal, J.; Kundu, S.K.; Hung Ng, W.K.; Singuru, R.; Borah, P.; Hirao, H.; Zhao, Y.; Bhaumik, A. Fabrication of ruthenium nanoparticles in porous organic polymers: Towards advanced heterogeneous catalytic nanoreactors. Chem. Eur. J. 2015, 21, 19016–19027. [Google Scholar] [CrossRef]
- He, J.; Razzaque, S.; Jin, S.; Hussain, I.; Tan, B. Efficient synthesis of Ultrafine gold nanoparticles with tunable sizes in a hyper-cross-linked polymer for nitrophenolreduction. ACS Appl. Nano Mater. 2019, 2, 546–553. [Google Scholar] [CrossRef]
- Jiang, M.; Ding, Y.; Yan, L.; Song, X.; Lin, R. Rh catalysts supported on knitting aryl network polymers for the hydroformylation of higher olefins. Chin. J. Catal. 2014, 35, 1456–1464. [Google Scholar] [CrossRef]
- Chetia, B.; Goutam, P.J.; Chipem, F.A.S.; Iyer, P.K. Thiourea recognition by 2,6-bis(2-benzimidazolyl)pyridine using spectroscopic techniques and DFT. J. Mol. Struct. 2013, 1042, 32–36. [Google Scholar] [CrossRef]
- Tan, D.-W.; Li, H.-X.; Zhu, D.-L.; Li, H.-Y.; Young, D.J.; Yao, J.-L.; Lang, J.-P. Ligand-controlled copper(I)-catalyzed cross-coupling of secondary and primary alcohols to α-alkylated ketones, pyridines, and quinolines. Org. Lett. 2018, 20, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Li, H.-X.; Zha, C.-H.; Young, D.J.; Li, H.-Y.; Lang, J.-P. Visible-light-enhanced Suzuki–Miyaura reactions of aryl chlorides in water with Pd NPs supported on a conjugated nanoporous polycarbazole. ChemSusChem 2019, 12, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Padmanaban, S.; Gunasekar, G.H.; Lee, M.; Yoon, S. Recyclable covalent triazine framework-based Ru catalyst for transfer hydrogenation of carbonyl compounds in water. ACS Sustain. Chem. Eng. 2019, 7, 8893–8899. [Google Scholar] [CrossRef]
- Wang, S.; Hou, S.; Wu, C.; Zhao, Y.; Ma, X. RuCl3 anchored onto post-synthetic modification MIL-101(Cr)-NH2 as heterogeneous catalyst for hydrogenation of CO2 to formic acid. Chin. Chem. Lett. 2019, 30, 398–402. [Google Scholar] [CrossRef]
- Morgan, B.D.J. Resolving ruthenium: XPS studies of common ruthenium materials. Surf. Interface Anal. 2015, 47, 1072–1079. [Google Scholar] [CrossRef]
- Park, K.; Padmanaban, S.; Kim, S.-H.; Jung, K.-D.; Yoon, S. NNN Pincer-functionalized porous organic polymer supported Ru(III) as a heterogeneous catalyst for Levulinic acid hydrogenation to γ-valerolactone. ChemCatChem 2021, 13, 695–703. [Google Scholar] [CrossRef]
- Genç, S.; Arslan, B.; Gülcemal, S.; Günnaz, S.; Çetinkaya, B.; Gülcemal, D. Iridium(I)-catalyzed C–C and C–N bond formation reactions via the borrowing hydrogen strategy. J. Org. Chem. 2019, 84, 6286–6297. [Google Scholar] [CrossRef]
- Wang, Q.F.; Wu, K.K.; Yu, Z.K. Ruthenium(III)-catalyzed β-alkylation of secondary alcohols with primary alcohols. Organometallics 2016, 35, 1251–1256. [Google Scholar] [CrossRef]
- Roy, B.C.; Debnath, S.; Chakrabarti, K.; Paul, B.; Maji, M.; Kundu, S. ortho-Amino group functionalized 2,2′-bipyridine based Ru(ii) complex catalysed alkylation of secondary alcohols, nitriles and amines using alcohols. Org. Chem. Front. 2018, 5, 1008–1018. [Google Scholar] [CrossRef]
- Zhang, M.-J.; Li, H.-X.; Young, D.J.; Li, H.-Y.; Lang, J.-P. Reaction condition controlled nickel(II)-catalyzed C-C cross coupling of alcohols. Org. Biomol. Chem. 2019, 17, 3567–3574. [Google Scholar] [CrossRef] [PubMed]

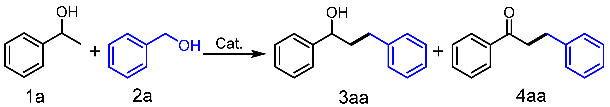
| Entry a | Catalyst | Base (equiv.) | Temperature (°C) | Time(h) | Yield (%) 3aa | 4aa |
|---|---|---|---|---|---|---|
| 1 | POP-bp/bbpRuCl3 | KOH/1.0 | 130 | 4 | 68 | <5 |
| 2 | POP-bp/bbpRuCl3 | KOtBu/1.0 | 130 | 4 | 33 | trace |
| 3 | POP-bp/bbpRuCl3 | Cs2CO3/1.0 | 130 | 4 | 32 | <5 |
| 4 | POP-bp/bbpRuCl3 | NaOH/1.0 | 130 | 4 | 11 | trace |
| 5 | POP-bp/bbpRuCl3 | CsOH/1.0 | 130 | 4 | 45 | <5 |
| 6 | POP-bp/bbpRuCl3 | KOH/1.0 | 100 | 4 | 7 | 25 |
| 7 | POP-bp/bbpRuCl3 | KOH/1.0 | 110 | 4 | 20 | 14 |
| 8 | POP-bp/bbpRuCl3 | KOH/1.0 | 120 | 4 | 49 | 20 |
| 9 | POP-bp/bbpRuCl3 | KOH/1.0 | 140 | 4 | 70 | trace |
| 10 | POP-bp/bbpRuCl3 | KOH/0.3 | 130 | 4 | 60 | 12 |
| 11 | POP-bp/bbpRuCl3 | KOH/0.5 | 130 | 4 | 76 | 8 |
| 12 | POP-bp/bbpRuCl3 | KOH/0.7 | 130 | 4 | 75 | 6 |
| 13 | POP-bp/bbpRuCl3 | KOH/1.5 | 130 | 4 | 69 | <5 |
| 14 | POP-bp/bbpRuCl3 | KOH/1.0 | 130 | 8 | 82 | <5 |
| 15 | POP-bp/bbpRuCl3 | KOH/0.5 | 130 | 12 | 93 | trace |
| 16 | POP-bp/bbp | KOH/0.5 | 130 | 12 | 0 | trace |
| 17 | RuCl3 | KOH/0.5 | 130 | 12 | 18 | trace |
| Entry a | Primary Alcohol | Product | Yield (%) | ||
|---|---|---|---|---|---|
| 1 |  | 2a |  | 3aa | 88 |
| 2 | 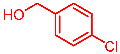 | 2b | 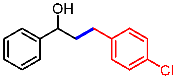 | 3ab | 85 |
| 3 |  | 2c |  | 3ac | 73 |
| 4 |  | 2d | 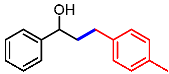 | 3ad | 85 |
| 5 | 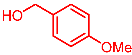 | 2e | 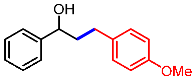 | 3ae | 87 |
| 6 | 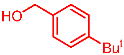 | 2f | 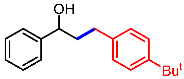 | 3af | 87 |
| 7 | 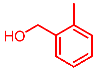 | 2g | 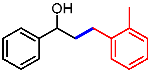 | 3ag | 89 |
| 8 | 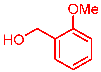 | 2h |  | 3ah | 79 |
| 9 |  | 2i | 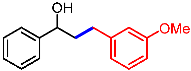 | 3ai | 86 |
| 10 |  | 2j | 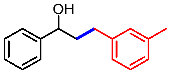 | 3aj | 81 |
| 11 | 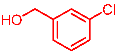 | 2k | 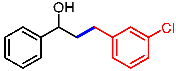 | 3ak | 85 |
| 12 | 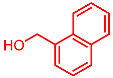 | 2l | 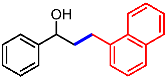 | 3al | 88 |
| 13 |  | 2m | 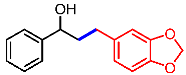 | 3am | 80 |
| 14 |  | 2n | 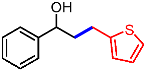 | 3an | 59 |
| Entry a | Secondary Alcohol | Product | Yield (%) | ||
|---|---|---|---|---|---|
| 1 | 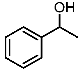 | 1a |  | 3aa | 88 |
| 2 |  | 1b | 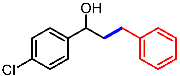 | 3ba | 80 |
| 3 | 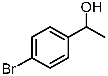 | 1c |  | 3ca | 89 |
| 4 |  | 1d | 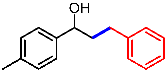 | 3da | 81 |
| 5 |  | 1e | 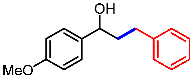 | 3ea | 88 |
| 6 |  | 1f | 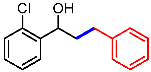 | 3fa | 53 |
| 7 | 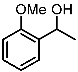 | 1g | 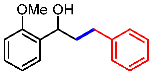 | 3ga | 76 |
| 8 | 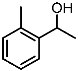 | 1h | 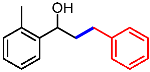 | 3ha | 79 |
| 9 | 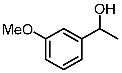 | 1i | 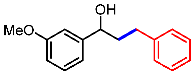 | 3ia | 88 |
| 10 |  | 1j | 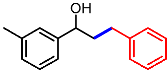 | 3ja | 85 |
| 11 |  | 1k | 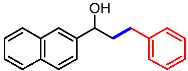 | 3ka | 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Wang, J.; Yu, L.; Xu, Y.; Young, D.J.; Li, H.; Li, H. Construction of a (NNN)Ru-Incorporated Porous Organic Polymer with High Catalytic Activity for β-Alkylation of Secondary Alcohols with Primary Alcohols. Polymers 2022, 14, 231. https://doi.org/10.3390/polym14020231
Cui Y, Wang J, Yu L, Xu Y, Young DJ, Li H, Li H. Construction of a (NNN)Ru-Incorporated Porous Organic Polymer with High Catalytic Activity for β-Alkylation of Secondary Alcohols with Primary Alcohols. Polymers. 2022; 14(2):231. https://doi.org/10.3390/polym14020231
Chicago/Turabian StyleCui, Yao, Jixian Wang, Lei Yu, Ying Xu, David J. Young, Haiyan Li, and Hongxi Li. 2022. "Construction of a (NNN)Ru-Incorporated Porous Organic Polymer with High Catalytic Activity for β-Alkylation of Secondary Alcohols with Primary Alcohols" Polymers 14, no. 2: 231. https://doi.org/10.3390/polym14020231
APA StyleCui, Y., Wang, J., Yu, L., Xu, Y., Young, D. J., Li, H., & Li, H. (2022). Construction of a (NNN)Ru-Incorporated Porous Organic Polymer with High Catalytic Activity for β-Alkylation of Secondary Alcohols with Primary Alcohols. Polymers, 14(2), 231. https://doi.org/10.3390/polym14020231







